Abstract
Context:
Obese women have poorer in vitro fertilization outcomes, but underlying mechanisms remain unclear.
Objective:
The objectives of the study were to compare the pharmacokinetics of human chorionic gonadotropin (hCG) and ovarian steroid hormone production, after subcutaneous (sc) and intramuscular (im) injection of hCG in obese and normal-weight women.
Design and Setting:
This was a randomized, experimental study.
Patients or Other Participants:
Twenty-two women aged 18–42 years with body mass index of 18.5–24.9 (normal) or 30–40 kg/m2 (obese).
Interventions:
Participants received im urinary hCG or sc recombinant hCG and returned for a second injection type after a 4-week washout. Intramuscular injections were performed under ultrasound guidance. Blood was taken 0, 0.5, 1, 2, 4, 6, 8, 12, 24, and 36 hours after injection.
Main Outcome Measures:
hCG was measured at each time point; estradiol, progesterone, 17-hydroxyprogesterone (17-OHP), testosterone (T), dehydroepiandrosterone, and SHBG were measured at 0 and 36 hours.
Results:
Twenty-two women completed the study. In both normal-weight and obese women, peak serum concentration (Cmax), area under the curve (AUC), and average hCG concentration were higher after im injection as compared with sc injection (all P < .003). Obese women had markedly lower Cmax, AUC, and average hCG concentration after sc injection as compared with normal-weight women (P = .02, P = .009, and P = .008, respectively). After im injection, Cmax, AUC, and average concentration were similar for normal-weight and obese women (P = .31, P = .25, and P = .18, respectively). Thirty-six percent of obese women had muscular layers beyond the reach of a standard 1.5 inch needle. hCG caused a significant rise in 17-OHP in both obese and normal-weight women and an increase in T in obese but not normal-weight women (all P < .04).
Conclusions:
Subcutaneous injection yields lower hCG levels in obese women. Standard-length needles are insufficient to administer im injections in many obese women.
Human chorionic gonadotropin (hCG) is widely used as a surrogate for luteinizing hormone (LH) during in vitro fertilization (IVF) cycles, in which its administration induces the resumption of meiosis and triggers the final maturation of the cumulus-oocyte complex. hCG has traditionally been extracted from the urine of pregnant women and formulated for im injection. Although many groups have demonstrated equal (1–4) or greater (5) efficacy with sc injection of the urinary formulation (u-hCG), current formulations of u-hCG are approved by the US Food and Drug Administration (FDA) for im injection only. A recombinant form of hCG (r-hCG) specifically formulated for sc injection has also been FDA approved, and a significant body of literature supports equivalency between the recombinant and urinary formulations with respect to oocyte and embryo parameters as well as implantation and pregnancy rates (6–9).
The deleterious effects of obesity on female reproduction are well recognized and have resulted in a large proportion of obese women seeking treatment for infertility. The underlying mechanisms by which obesity exerts this detrimental impact on IVF outcome, however, remain poorly elucidated. Drug metabolism and distribution is known to depend on the volume of adipose tissue (10), and body mass index (BMI) is widely used in epidemiological studies as an indicator of adiposity (11). A study of women undergoing IVF found a significant negative correlation between BMI and serum hCG levels 12 hours after injection of u-hCG, regardless of the route of administration (1). Additionally, the bioavailability of hCG has been shown to be greater after im injection of u-hCG as compared with sc injection of u-hCG in overweight women with a mean BMI of 29 kg/m2 as compared with those with normal BMI (12). There are few data directly comparing the pharmacokinetics of hCG in obese and nonobese women, and no studies have reported on the use of r-hCG in the obese population.
The primary aim of this study was to compare the pharmacokinetics of hCG after sc injection of r-hCG and im injection of u-hCG in obese and normal-weight women. A secondary aim was to determine whether ovarian hormone production, as reflected by the levels of estradiol, progesterone, 17-hydroxyprogesterone (17-OHP), total testosterone (T), dehydroepiandrosterone (DHEA), and sex hormone binding globulin (SHBG), differs after sc injection of r-hCG and im injection of u-hCG in obese and normal-weight women. We hypothesized that obese women would have lower serum levels of hCG after both sc and im injection and that higher serum hCG levels would correlate with higher levels of ovarian steroids.
Materials and Methods
Study participants
Women aged 18–42 years were eligible to participate. BMI was calculated as weight in kilograms over height in meters squared. Participants were required to have a BMI categorized as either normal weight (18.5–24.9 kg/m2) or obese (>30.0 kg/m2) based on World Health Organization cut points. The upper BMI of 40 kg/m2 was chosen because many infertility programs in the United States do not accept women with BMI above 40 for treatment (13). Participants were age matched to ensure equal age distributions in each weight class. Women who reported a history of irregular menses, polycystic ovary syndrome (PCOS), liver or kidney disease, hypertension, thyroid dysfunction, or diabetes were ineligible. Current smokers, pregnant women, and women who had used hormonal contraception in the last 30 days were also excluded. Study participants were recruited using the Partners Research Study Volunteer Program as well as via advertisements in free local online media. Recruitment began in January 2010 and concluded in March 2012.
Study design and procedures
This was a randomized, experimental, pilot study in which obese and normal-weight women underwent administration of 10 000 IU of im u-hCG (Novarel; Ferring Pharmaceuticals Inc) or 250 μg of sc r-hCG (Ovidrel; Serono Inc) according to FDA-approved labeling, followed by serial blood draws. These two hCG formulations have been previously found to be equivalent at the selected doses with respect to numbers of retrieved oocytes and normally fertilized embryos (14).
Initial eligibility screening took place via structured telephone interviews. Eligible subjects presented for an intake visit that included history and physical examination, serum β-hCG to exclude pregnancy, complete blood count, and baseline creatinine. Racial demographics were based on self-report. The study was conducted in the Harvard Catalyst Clinical Research Center (HCCRC) and was approved by the Partners Healthcare Institutional Review Board. Written informed consent was obtained from all participants at the intake visit before formal entry into the study.
Participants were admitted to the HCCRC inpatient unit during the follicular phase of their menstrual cycle (cycle d 1–10). Weight was repeated on admission, and an iv was placed to facilitate serial blood draws. Participants were randomly assigned to receive im u-hCG or sc r-hCG during their first admission; each woman served as her own control and returned for the second injection type after a 4-week washout period. Computer-generated randomization sequences were created and held by the Partners Healthcare Investigational Drug Service pharmacist, who dispensed the appropriate hCG injection according to the randomization sequence. Equal numbers of obese and normal-weight participants were assigned to begin with either the sc or im injection.
Subcutaneous injections of 250 μg r-hCG were administered in the midline lower abdominal wall by HCCRC personnel. Intramuscular injections of 10 000 IU u-hCG were administered to the upper outer quadrant of the buttock (above the midpoint of a line connecting the posterior superior iliac spine to the greater trochanter) by a single investigator (D.K.S.) using a 22-gauge, 1.5-in. (3.8 cm) needle (BD Medical). All im injections were performed under ultrasound guidance to confirm im placement. In participants in whom the thickness of the sc layer was noted to exceed the reach of the 1.5-in. needle, a 22-gauge, 5-in. Quincke spinal needle (BD Medical) was used.
Serial blood samples were taken immediately prior to and 0.5, 1, 2, 4, 6, 8, 12, 24, and 36 hours after the hCG injection. Study participants remained in the HCCRC for the duration of the study to ensure timely collection of all samples. Samples were immediately processed by the HCCRC laboratory and aliquots of serum and plasma from each specimen were reserved. Samples were labeled by number only and were frozen at −80°C to avoid degradation until assays were run.
Following a 4-week washout period to allow clearance of hCG, participants returned in the follicular phase of their next menstrual cycle for a second inpatient admission. Weights were again repeated on admission and an iv needle placed to facilitate blood draws. Participants received hCG injection by the second route according the randomization scheme, and blood was taken at the same intervals as mentioned above.
Sample analysis
Serum hCG from each time point (baseline and 0.5, 1, 2, 4, 6, 8, 12, 24, 36 h after injection) was measured using an electrochemiluminescence immunoassay (ECLIA) by the Harvard Catalyst Clinical Laboratory (Boston, Massachusetts). All serum samples from an individual woman were batched together for the hCG assay. The freezing of serum samples at −80°C until the time of assay does not impact ECLIA results. Two blinded homogenous quality control (QC) samples from pregnant women expected to have detectable hCG levels were included with each participant batch to assess laboratory precision. The interassay coefficient of variation (CV) of these samples was 3.7% and the intraassay CV was less than 8.6% for all batches.
All other ovarian steroid hormones were measured at two time points: baseline and 36 hours after the administration of hCG. Estradiol, progesterone, T, and SHBG were measured via an ECLIA in the Harvard Catalyst Clinical Laboratory. 17-OHP and DHEA were measured at Labcorp using liquid-chromotography-tandem mass spectrometry and an ECLIA, respectively. For each hormonal assay, all normal-weight participant samples were run in a single batch, and all obese participant samples were run in a single batch. The freezing of serum samples at −80°C until the time of assay does not impact the ECLIA or liquid-chromotography-tandem mass spectrometry results. Two blinded homogenous QC samples were included with each batch to assess laboratory precision. The interassay CV of these QC samples was 12.9% for estradiol, 9.7% for T, 3.5% for 17-OHP, 2.6% for progesterone, and 2.1% for SHBG. The interassay CV for DHEA was 29.7%. The intraassay CVs remained less than 5.3% for all hormonal assays.
Power calculation
A prior study comparing overweight women with a mean BMI of 29 kg/m2 with normal-weight women showed a 39% reduction in the median peak serum concentration of hCG (Cmax) after an im administration of 10 000 IU u-hCG and a 43% reduction in median Cmax after an sc administration of the same drug (12). Using the nonparametric Mann-Whitney U test, comparison of the median Cmax between obese and nonobese women after the sc injection required a total of 22 women (11 in each group); comparison of the median Cmax between obese and nonobese women after the im injection required a total of 11 women. All calculations assumed a type I error probability of 0.05 and 80% power. To allow for the most conservative sample size calculation as well as a potential dropout rate of 15%, we aimed to recruit 12 women in each group.
Statistical analysis
Because data were not normally distributed, nonparametric tests were used for all comparisons. Paired comparisons of pharmacokinetic parameters after the im vs the sc injection of hCG in a given woman were performed using Wilcoxon signed rank tests. Unpaired comparisons of pharmacokinetic parameters after hCG injection between normal-weight and obese women were made using Wilcoxon rank sum (Mann-Whitney U) tests.
Nonparametric tests were similarly performed to assess the impact of hCG injection on ovarian hormone levels. Paired comparisons of hormone responses from baseline to 36 hours within a given woman were performed using Wilcoxon signed rank tests. Unpaired comparisons of ovarian responsiveness in normal and obese women were calculated using Wilcoxon rank sum (Mann Whitney U) tests. Spearman correlation coefficients were used to identify associations between ovarian steroids and hCG levels.
Wilcoxon rank sum (Mann-Whitney U) tests were used to compare the depth of the muscular layer between normal and obese women.
Results
Two hundred two women responded to recruitment efforts and underwent phone screening. One hundred thirty-six women declined participation or were disqualified based on the phone screen, and the remaining 66 were enrolled. Of these, 46 women presented for the first intake visit, at which time an additional 18 were either disqualified or declined participation. Of 28 eligible women, 22 (11 normal weight and 11 obese) completed the study. The remaining six participants were not enrolled because goal participation had been reached.
The demographics of the study population are shown in Table 1. Participants in the normal-weight study group had a mean BMI of 22.2 kg/m2; those in the obese study group had a mean BMI of 34.7 kg/m2. Both groups had similar numbers of Caucasian participants but there were more obese participants who self-identified as Hispanic. Normal-weight participants were younger and more frequently nulliparous. Two women in each group were noted to have progesterone levels above 3 ng/mL at baseline, indicating inadvertent assessment in the luteal phase. Results were unchanged when these participants were excluded (data not shown).
Table 1.
Demographics of Study Population
| Normal Weight (n = 11) | Obese (n = 11) | |
|---|---|---|
| Age, ya | 32.5 (7.6) | 34.8 (4.1) |
| BMI, kg/m2a | 22.2 (1.9) | 34.7 (3.8) |
| Waist to hip ratioa | 0.79 (0.06) | 0.83 (0.05) |
| Never gravidb | 8 (72.7%) | 7 (63.6%) |
| Nulliparousb | 10 (91.9%) | 8 (72.7%) |
| Raceb | ||
| White | 7 (63.6%) | 6 (54.5%) |
| Asian/Pacific Islander | 2 (18.2%) | 1 (9.1%) |
| African-American | 2 (18.2%) | 2 (27.3%) |
| Hispanic | 0 (0.0%) | 4 (36.4%) |
| Current smokerb | 3 (27.2%) | 4 (36.4%) |
| Age at menarche, yb | ||
| <11 | 1 (9.1%) | 1 (9.1%) |
| 11–14 | 8 (36.3%) | 8 (36.3%) |
| >14 | 2 (18.2%) | 2 (18.2%) |
Expressed as mean (SD).
Expressed as number (percentage).
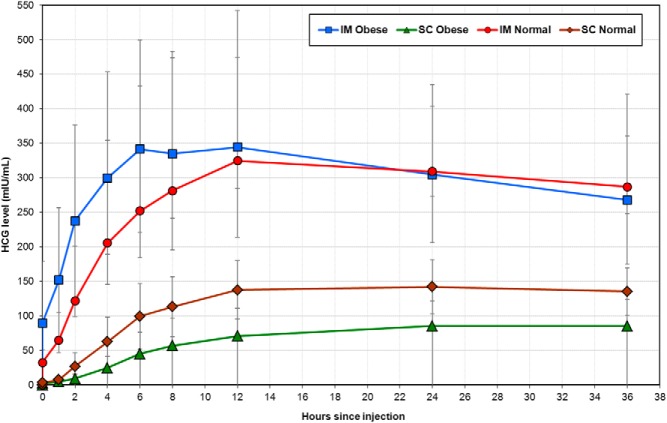
In both normal-weight and obese women, peak serum concentration of hCG (Cmax), area under the curve (AUC), and average hCG concentration were higher after the im as compared with the sc injection (P < .003 for all comparisons) (Table 2 and Figure 1). The proportion of women who reached Cmax by the 12-hour time point [percentage time to reach Cmax (Tmax) by 12 h] after the im vs the sc injection was similar in normal-weight women (45.5% vs 54.6%, P > .99) but significantly higher after the im as compared with the sc injection in obese women (81.8% vs 18.2%, P = .008) (Table 2). Median Tmax values were not reflective because all groups reached Tmax between the discrete time points of 12 and 24 hours.
Table 2.
Pharmacokinetics of hCG After SC vs IM Injection in Normal and Obese Womena
| Normal Weight (n = 11) |
Obese (n = 11) |
|||||
|---|---|---|---|---|---|---|
| SC | IM | P Valueb | SC | IM | P Valueb | |
| Tmax by 12 h, % | 54.6 | 45.5 | >.99 | 18.2 | 81.8 | .008 |
| Cmax, mIU/mL | 142 (115–192) | 291 (225–380) | .002 | 89 (58–123) | 436 (246–526) | .001 |
| AUC, mIU/h·mL | 4152 (3410–5395) | 9586 (5945–11 625) | .003 | 2352 (1593–3655) | 13327 (7736–14 863) | .001 |
| Average concentration, mIU/mL | 72 (59–96) | 189 (96–220) | .003 | 34 (25–55) | 277 (168–344) | .001 |
Proportion of women who reached Tmax by the 12-hour time point is expressed as a percentage; all other data are expressed as median (interquartile range).
P values comparing responses within woman after an im vs sc injection of hCG were calculated using Wilcoxon signed rank tests.
Figure 1.
Median serum levels of hCG after im vs sc injection in normal and obese women. Data points represent the median serum hCG level at the respective time point. Error bars indicate SD.
Obese women had significantly lower Cmax (89 vs 142 mIU/mL, P = .02; Table 3), AUC (2352 vs 4152 mIU/h·mL, P = .009; data not shown), and average hCG concentration over time (34 vs 72 mIU/mL, P = .008; Table 3) after sc injection as compared with those of normal weight. After im injection of hCG, Cmax, AUC, and average concentrations were similar for normal-weight and obese women (P = .31, P = .25, and P = .18, respectively; data not shown).
Table 3.
Pharmacokinetics of hCG in Normal and Obese Women After SC vs IM Injectiona
| SC |
IM |
|||||
|---|---|---|---|---|---|---|
| Normal (n = 11) | Obese (n = 11) | P Valueb | Normal (n = 11) | Obese (n = 11) | P Valueb | |
| Cmax, mIU/mL | 142 (115–192) | 89 (58–123) | .02 | 291 (225–380) | 436 (246–526) | .31 |
| AUC, mIU/h·mL | 4152 (3410–5395) | 2352 (1593–3655) | .009 | 9586 (5945–11 625) | 13 327 (7736–14 863) | .25 |
| Average concentration, mIU/mL | 72 (59–96) | 34 (25–55) | .008 | 189 (96–220) | 277 (168–344) | .18 |
Proportion of women who reached Tmax by the 12-hour time point is expressed as a percentage; all other data are expressed as median (interquartile range).
P value comparing responses after either sc or im injection in normal vs obese women were calculated using Wilcoxon rank sum tests.
There were no differences in baseline levels of estradiol, progesterone, T, 17-OHP, and DHEA between normal and obese participants (P > .11 for all comparisons) (Tables 4 and 5). As expected, the median baseline SHBG was significantly lower in obese participants (47.3 vs 68.8 nmol/L, P = .03). 17-OHP levels demonstrated a statistically significant increase from baseline to 36 hours after the im and sc injections of hCG in obese women; a similar trend was identified in normal-weight women, although the increase after im injection did not reach statistical significance (P = .07). Significant increases in T levels were also noted 36 hours after im and sc injections of hCG in obese (P = .04 for both) but not normal-weight women (P ≥ .31). Median progesterone levels were higher in obese women 36 hours after im u-hCG (0.7 vs 2.6 ng/mL) but did not differ after sc injection or in normal-weight women. Estradiol levels were found to decrease in normal-weight women (76.9 vs 149.3 pg/mL, P = .01) after im injection of u-hCG. Clinically insignificant changes were noted in estradiol, SHBG, and DHEA levels in obese women after sc injection of r-hCG. Despite the few discrete changes in steroid levels between the 0- and 36-hour time points noted above, there were no significant correlations between any analyte and serum levels of hCG (Spearman correlation coefficients all < 0.36; data not shown).
Table 4.
Ovarian Steroids After Intramuscular hCG Injection in Normal and Obese Womena
| Analyte | Time Point (hours) | Normal (n = 11) | Obese (n = 11) | P Valueb |
|---|---|---|---|---|
| Estradiol, pg/mL | 0 | 149.3 (86.4–244.5) | 79.3 (56.3–112.8) | .11 |
| 36 | 76.9 (51.7–105.5) | 81.6 (76.2–102.0) | ||
| P valuec | .01 | .91 | ||
| Progesterone, ng/mL | 0 | 0.7 (0.4–2.3) | 0.7 (0.5–2.0) | .18 |
| 36 | 0.7 (0.4–3.3) | 2.6 (0.6–8.6) | ||
| P valuec | .28 | .02 | ||
| T, ng/dL | 0 | 30.0 (21.2–51.2) | 35.5 (27.0–46.2) | .32 |
| 36 | 37.4 (25.6–41.9) | 51.3 (21.9–56.0) | ||
| P valuec | .36 | .04 | ||
| SHBG, nmol/L | 0 | 72.2. (54.9–96.9) | 47.0 (28.6–59.3) | .03 |
| 36 | 64.5 (54.4–108.1) | 54.8 (28.8–66.2) | ||
| P valuec | .97 | .51 | ||
| 17-OHP, ng/dL | 0 | 37.0 (22.0–117.5) | 38.0 (20.0–97.5) | .71 |
| 36 | 83.0 (66.0–146.5) | 145.5 (84.8–190.5) | ||
| P valuec | .07 | .004 | ||
| DHEA, ng/dL | 0 | 139.0 (104.0–232.0) | 207.0 (85.5–274.0) | .24 |
| 36 | 124.0 (90.0–149.0) | 111.5 (85.3–207.3) | ||
| P valuec | .52 | .61 |
Results are expressed as median (interquartile range).
P values comparing normal with obese women were calculated using Wilcoxon rank sum (Mann-Whitney U) tests.
P values comparing responses from baseline with 36 hours within a weight class were calculated using Wilcoxon signed rank tests.
Table 5.
Ovarian Steroids After Subcutaneous hCG Injection in Normal and Obese Womena
| Analyte | Time Point (hours) | Normal (n = 11) | Obese (n = 11) | P Valueb |
|---|---|---|---|---|
| Estradiol, pg/mL | 0 | 107.5 (55.7–205.3) | 79.2 (60.8–101.4) | .11 |
| 36 | 67.4 (58.5–106.2) | 74.7 (71.5–132.2) | ||
| P valuec | .16 | .03 | ||
| Progesterone, ng/mL | 0 | 0.4 (0.2–0.5) | 0.8 (0.4–2.4) | .18 |
| 36 | 0.5 (0.3–0.9) | 0.6 (0.4–6.1) | ||
| P valuec | .08 | .10 | ||
| T, ng/dL | 0 | 31.8 (17.7–45.1) | 35.8 (21.0–42.6) | .32 |
| 36 | 38.5 (33.6–43.4) | 41.6 (26.0–49.9) | ||
| P valuec | .31 | .04 | ||
| SHBG, nmol/L | 0 | 58.8 (43.6–102.4) | 47.7 (27.0–68.9) | .03 |
| 36 | 75.2 (52.4–113.4) | 51.7 (28.5–78.0) | ||
| P valuec | .06 | .02 | ||
| 17-OHP, ng/dL | 0 | 25.0 (20.0–47.5) | 31.0 (12.5–43.5) | .71 |
| 36 | 89.0 (57.0–121.0) | 78.0 (64.0–156.0) | ||
| P valuec | .001 | .001 | ||
| DHEA, ng/dL | 0 | 92.0 (66.0–181.5) | 204.0 (129.5–267.0) | .24 |
| 36 | 124.5 (65.0–151.5) | 158.0 (107.0–193.5) | ||
| P valuec | .64 | .03 |
Results are expressed as median (interquartile range).
P values comparing normal with obese women were calculated using Wilcoxon rank sum (Mann-Whitney U) tests.
P values comparing responses from baseline with 36 hours within a weight class were calculated using Wilcoxon signed rank tests.
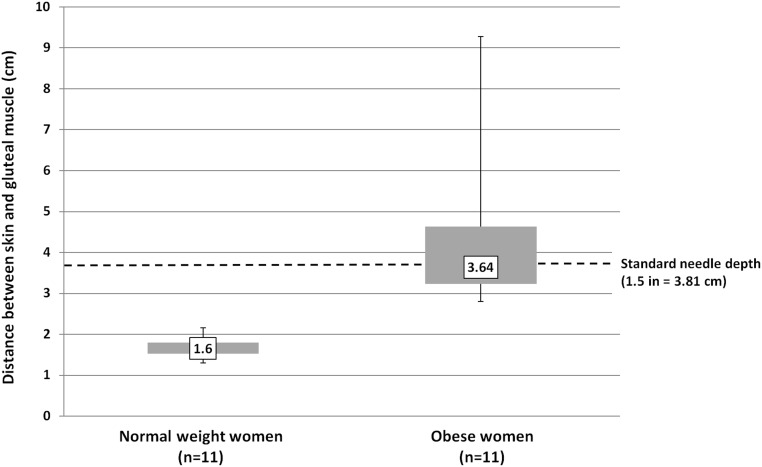
Normal-weight women had significantly less depth between their skin and gluteal muscle as compared with obese women (mean depth 1.6 vs 3.64 cm, P < .001) (Figure 2). The thickness of the sc layer ranged from 1.4 to 2.2 cm in normal-weight women and 2.8 cm to 9.3 cm in obese women. The distance between the skin and the gluteal muscle was beyond the reach of a standard needle (1.5 in or 3.81 cm), in 4 of 11 obese participants, and all 11 obese participants had a sc fat thickness of greater than 2.8 cm. The muscular layer depth was less than the length of a standard needle in all 11 normal-weight participants.
Figure 2.
Distance between skin and gluteal muscle in normal and obese participants. Shaded boxes represent the interquartile range (Q1–Q3). Whiskers indicate minimum and maximum values. Median values are marked, and the difference is statistically significant (P < .001) using the Wilcoxon rank sum (Mann-Whitney U) test. Depth penetrated by a standard 1.5-in. needle is indicated with a dotted line.
Discussion
This is the only study examining the pharmacokinetics of hCG in an obese population with both the recombinant and urinary formulations administered according to FDA-approved labeling. When compared with those of normal weight, obese women had significantly lower serum hCG levels after sc injection of r-hCG but comparable levels after im injection of u-hCG. Baseline concentrations of ovarian steroids other than SHBG did not significantly differ between normal and obese women. Significant increases in 17-OHP and T levels were noted 36 hours after hCG injection; no systematic changes in the level of other ovarian steroids were observed. Notably, the depth of the sc layer in 36% of obese women would have precluded self-administration of an im injection using a standard 1.5-in. needle.
It has been proposed that the less favorable IVF outcome in obese women is linked in part to a lesser response to injectable medications, given the larger volume of distribution. Although it has been demonstrated that obese women require higher doses of gonadotropins to achieve a similar response to their normal-weight counterparts (15), this is unlikely to be the sole mechanism, given that studies controlling for total gonadotropin dose have still demonstrated poorer IVF outcomes in the obese population (16). Accurate study of the bioavailability of gonadotropins is difficult because most women retain low levels of endogenous gonadotropins despite suppression of the GnRH axis. By contrast, nonpregnant women have undetectable levels of hCG, making accurate assessment of its bioavailability more feasible.
Bioavailability of hCG in obese vs normal-weight women has been examined by a single group to date (12). In the 2003 study by Chan et al (12), obese women had a mean BMI of 29 kg/m2; although Asians are considered obese at a BMI of 25 kg/m2 or greater, the generalizability of the results to other ethnic populations in whom obesity is defined as a BMI of 30 kg/m2 or greater is limited. Moreover, the study compared im vs sc injections of u-hCG; although sc injection of u-hCG is frequently used in clinical practice, the formulation is not approved by the FDA for this route of administration. The investigators found that obese women had significantly lower bioavailability of hCG as compared with their normal-weight counterparts, regardless of the route of administration, and that both obese and normal-weight women had higher bioavailability after an im injection of u-hCG relative to sc. This is in contrast to the results of the present study that reveal similar bioavailability between normal-weight and obese women after an im injection of u-hCG but diminished bioavailability after an sc injection of r-hCG in the obese population.
Results from the ovarian steroid testing did not substantially change the conclusions of the present study, perhaps not surprisingly, because the study was not powered to assess each of these secondary outcomes. The only consistent findings were an increase in the levels of 17-OHP and T after both the im injection of u-hCG and after the sc injection of r-hCG. The magnitude of the increase in 17-OHP was noted to be more pronounced in the obese subjects but still present in the normal-weight women, whereas the increase in T was present only among the obese women. A rise in serum 17-OHP and T after LH or hCG stimulation has been previously demonstrated to be more pronounced in women with PCOS and is thought to be due to an exaggerated response of the delta-4 steroidogenic pathway (17–19). Although PCOS was an exclusion criterion for the present study, it is possible that the more pronounced elevations of these steroids in the obese subjects reflect a component of undiagnosed ovarian dysfunction in this population.
A key strength of the current study is that all im injections were performed under sonographic guidance by a single investigator, thereby ensuring accurate differentiation between sc and im delivery of hCG. It is notable that a standard-size needle would not have penetrated the gluteal muscle in 36% of the obese participants. These results appear to provide a conservative estimate as compared with those presented by the few studies on the topic, which have demonstrated an sc layer thickness beyond the reach of a standard needle in 50%–98% of women at the site of a dorsogluteal injection (20, 21). Some of this variation may be attributable to differences in sonographic measurements. Even the thinnest obese participant in the present study had a distance of 2.8 cm between the skin and gluteal muscle and may therefore fail to self-administer a true im injection if the needle was not inserted to its base. As mentioned previously, the FDA has approved only the im injection of u-hCG and the sc injection of r-hCG. The findings of the present study indicate that many obese women undergoing IVF who are instructed to self-administer im u-hCG at the dorsogluteal site may be inadvertently administering the medication in a sc fashion. This inaccurate administration may impact drug delivery and efficacy, although the precise clinical implications are not known.
Some limitations should be considered in the evaluation of this pilot study. Only two formulations of hCG were compared in the present study: 250 μg r-hCG and 10 000 IU u-hCG. These doses were selected for comparison because they were the two most commonly used in our clinical infertility practice. The treatment equivalence between these doses has been demonstrated with respect to the number of oocytes retrieved, the number of normally fertilized embryos at fertilization check, and the implantation rate (14). This is further supported by a randomized controlled trial of 250 μg and 500 μg r-hCG in obese women with a mean BMI of 29 kg/m2 that found no difference in oocyte/embryo parameters between the groups (22). Early studies on u-hCG have also demonstrated equivalence between the administration of 5000 and 10 000 IU of u-hCG with respect to oocyte recovery, although patient BMI was not specified (23).
Because participants in this study were healthy volunteers rather than infertile women, it remains unknown whether the observed pharmacokinetic differences will translate into clinical differences in terms of oocyte yield or maturity, clinical pregnancy rate, and live birth rates. Indeed, the aforementioned randomized trial comparing 250 μg and 500 μg r-hCG in obese women identified significantly lower serum hCG levels (73.9 ± 18.8 vs 146 ± 48.1 mIU/mL) but comparable clinical pregnancy rates (50.0% vs 47.1%) after the 250-μg dose. Trials of the early hCG formulation Profasi revealed that a mean serum hCG level of 129 mIU/mL or greater 1 day after injection was sufficient to induce follicular maturation and adequate luteinization (9). It is notable that the Cmax in the present study after sc injection of r-hCG in the obese population was only 89 mIU/mL, suggesting that a sc formulation may be inadequate in the obese population.
Baseline levels of estradiol, T, SHBG, DHEA, and 17-OHP in our study population are reflective of follicular-phase levels demonstrated by other investigators (17, 24). Although the interassay CV of DHEA was unexpectedly elevated at 29.7%, there were no significant differences in DHEA levels in the participant samples, either between normal and obese women or between im and sc injections, suggesting a potential error with the QC sample used to calculate the CV. Additionally, although most progesterone levels were within the standard ranges for the follicular phase of the menstrual cycle, our results reveal that four women (two normal weight, two obese) may have prematurely ovulated prior to their study visit and were therefore inadvertently assessed in their luteal phase. It is worth noting that the results of the study remained unchanged when these women were removed from the analysis.
When compared with normal-weight women, obese women had significantly lower serum hCG levels after sc injection but comparable levels after im injection. Significant increases in 17-OHP and T levels were noted 36 hours after hCG injection; baseline concentrations of other ovarian steroids did not appear to significantly differ between normal and obese women nor change in response to hCG injection. Notably, many obese women would not have been able to self-administer an im injection using a standard 1.5-in. needle. It will be important to determine whether these pharmacokinetic differences translate into clinical differences in an infertile population. Until that time, practitioners may want to consider alternate routes of hCG administration to ensure im delivery in the obese population.
Acknowledgments
We acknowledge Dr Robert Barbieri for his guidance and consultation in the design of this study.
This work was conducted with support from the Department of Obstetrics and Gynecology, Brigham and Women's Hospital, Expanding the Boundaries Grant; Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources); and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170–05; and financial contributions from Harvard University and its affiliated academic health care centers.
Disclosure Summary: D.K.S., S.A.M., and K.F.B.C. have nothing to declare. E.S.G. receives royalties from Up to Date.
Footnotes
- AUC
- area under the curve
- BMI
- body mass index
- Cmax
- peak serum concentration of hCG
- CV
- coefficient of variation
- DHEA
- dehydroepiandrosterone
- ECLIA
- electrochemiluminescence immunoassay
- FDA
- US Food and Drug Administration
- HCCRC
- Harvard Catalyst Clinical Research Center
- hCG
- human chorionic gonadotropin
- IVF
- in vitro fertilization
- 17-OHP
- 17-hydroxyprogesterone
- PCOS
- polycystic ovary syndrome
- QC
- quality control
- r-hCG
- recombinant form of hCG
- Tmax
- time to reach Cmax
- u-hCG
- urinary formulation of hCG.
References
- 1. Elkind-Hirsch KE, Bello S, Esparcia L, Phillips K, Sheiko A, McNichol M. Serum human chorionic gonadotropin levels are correlated with body mass index rather than route of administration in women undergoing in vitro fertilization—embryo transfer using human menopausal gonadotropin and intracytoplasmic sperm injection. Fertil Steril. 2001;75(4):700–704 [DOI] [PubMed] [Google Scholar]
- 2. Sills ES, Drews CD, Perloe M, Kaplan CR, Tucker MJ. Periovulatory serum human chorionic gonadotropin (hCG) concentrations following subcutaneous and intramuscular nonrecombinant hCG use during ovulation induction: a prospective, randomized trial. Fertil Steril. 2001;76(2):397–399 [DOI] [PubMed] [Google Scholar]
- 3. Mannaerts BM, Geurts TB, Odink J. A randomized three-way cross-over study in healthy pituitary-suppressed women to compare the bioavailability of human chorionic gonadotrophin (Pregnyl) after intramuscular and subcutaneous administration. Hum Reprod. 1998;13(6):1461–1464 [DOI] [PubMed] [Google Scholar]
- 4. Carrell DT, Jones KP, Peterson CM, Aoki V, Emery BR, Campbell BR. Body mass index is inversely related to intrafollicular HCG concentrations, embryo quality and IVF outcome. Reprod Biomed Online. 2001;3(2):109–111 [DOI] [PubMed] [Google Scholar]
- 5. Stelling JR, Chapman ET, Frankfurter D, Harris DH, Oskowitz SP, Reindollar RH. Subcutaneous versus intramuscular administration of human chorionic gonadotropin during an in vitro fertilization cycle. Fertil Steril. 2003;79(4):881–885 [DOI] [PubMed] [Google Scholar]
- 6. Farrag A, Costantini A, Manna C, Grimaldi G. Recombinant HCG for triggering ovulation increases the rate of mature oocytes in women treated for ICSI. J Assist Reprod Genet. 2008;25(9–10):461–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The European Recombinant Human Chorionic Gonadotrophin Study Group. Induction of final follicular maturation and early luteinization in women undergoing ovulation induction for assisted reproduction treatment—recombinant HCG versus urinary HCG. Hum Reprod. 2000;15(7):1446–1451 [PubMed] [Google Scholar]
- 8. Al-Inany HG, Aboulghar M, Mansour R, Proctor M. Recombinant versus urinary human chorionic gonadotrophin for ovulation induction in assisted conception. Cochrane Database Syst Rev. 2005(2):CD003719. [DOI] [PubMed] [Google Scholar]
- 9. Driscoll GL, Tyler JP, Hangan JT, Fisher PR, Birdsall MA, Knight DC. A prospective, randomized, controlled, double-blind, double-dummy comparison of recombinant and urinary HCG for inducing oocyte maturation and follicular luteinization in ovarian stimulation. Hum Reprod. 2000;15(6):1305–1310 [DOI] [PubMed] [Google Scholar]
- 10. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 49(2):71–87 [DOI] [PubMed] [Google Scholar]
- 11. Gray DS, Fujioka K. Use of relative weight and body mass index for the determination of adiposity. J Clin Epidemiol. 1991;44(6):545–550 [DOI] [PubMed] [Google Scholar]
- 12. Chan CC, Ng EH, Chan MM, et al. Bioavailability of hCG after intramuscular or subcutaneous injection in obese and non-obese women. Hum Reprod. 2003;18(11):2294–2297 [DOI] [PubMed] [Google Scholar]
- 13. Harris ID, Python J, Roth L, Alvero R, Murray S, Schlaff WD. Physicians' perspectives and practices regarding the fertility management of obese patients. Fertil Steril. 2011;96(4):991–992 [DOI] [PubMed] [Google Scholar]
- 14. Chang P, Kenley S, Burns T, et al. Recombinant human chorionic gonadotropin (rhCG) in assisted reproductive technology: results of a clinical trial comparing two doses of rhCG (Ovidrel) to urinary hCG (Profasi) for induction of final follicular maturation in in vitro fertilization-embryo transfer. Fertil Steril. 2001;76(1):67–74 [DOI] [PubMed] [Google Scholar]
- 15. Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology—a systematic review. Hum Reprod Update. 2007;13(5):433–444 [DOI] [PubMed] [Google Scholar]
- 16. Shah DK, Missmer SA, Berry KF, Racowsky C, Ginsburg ES. Effect of obesity on oocyte and embryo quality in women undergoing in vitro fertilization. Obstet Gynecol. 2011;118(1):63–70 [DOI] [PubMed] [Google Scholar]
- 17. Rosencrantz MA, Coffler MS, Haggan A, et al. Clinical evidence for predominance of δ-5 steroid production in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96(4):1106–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnes RB, Rosenfield RL, Burstein S, Ehrmann DA. Pituitary-ovarian responses to nafarelin testing in the polycystic ovary syndrome. N Engl J Med. 1989;320(9):559–565 [DOI] [PubMed] [Google Scholar]
- 19. Rosenfield RL, Barnes RB, Ehrmann DA. Studies of the nature of 17-hydroxyprogesterone hyperresonsiveness to gonadotropin-releasing hormone agonist challenge in functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1994;79(6):1686–1692 [DOI] [PubMed] [Google Scholar]
- 20. Nisbet AC. Intramuscular gluteal injections in the increasingly obese population: retrospective study. BMJ. 2006;332(7542):637–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zaybak A, Gunes UY, Tamsel S, Khorshid L, Eser I. Does obesity prevent the needle from reaching muscle in intramuscular injections? J Adv Nurs. 2007;58(6):552–556 [DOI] [PubMed] [Google Scholar]
- 22. Kahraman S, Karlikaya G, Kavrut M, Karagozoglu H. A prospective, randomized, controlled study to compare two doses of recombinant human chorionic gonadotropin in serum and follicular fluid in woman with high body mass index. Fertil Steril. 2010;93(6):2084–2087 [DOI] [PubMed] [Google Scholar]
- 23. Abdalla HI, Ah-Moye M, Brinsden P, Howe DL, Okonofua F, Craft I. The effect of the dose of human chorionic gonadotropin and the type of gonadotropin stimulation on oocyte recovery rates in an in vitro fertilization program. Fertil Steril. 1987;48(6):958–963 [DOI] [PubMed] [Google Scholar]
- 24. Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98(19):1406–1415 [DOI] [PubMed] [Google Scholar]