Abstract
Objective:
The objective of the study was to examine the association between prehospital serum 25-hydroxyvitamin D [25(OH)D]and the risk of mortality after hospital admission.
Design:
We performed a retrospective cohort study of adults hospitalized for acute care between 1993 and 2011.
Setting:
The study was conducted at two Boston teaching hospitals.
Patients:
A total of 24 094 adult inpatients participated in the study.
Intervention:
There was no intervention.
Measurements:
All patients had serum 25(OH)D measured before hospitalization. The exposure of interest was 25(OH)D categorized as less than 10 ng/mL, 10–19.9 ng/mL, 20–29.9 ng/mL, 30–49.9 ng/mL, 50–59.9 ng/mL, 60–69.9 ng/mL, and 70 ng/mL or greater. The main outcome measure was 90-day mortality. Adjusted odds ratios (ORs) were estimated by multivariable logistic regression with inclusion of potential confounders.
Results:
After adjustment for age, gender, race (white vs nonwhite), patient type (surgical vs medical), season of 25(OH)D draw, and the Deyo-Charlson index, patients with 25(OH)D levels less than 30 ng/mL or 60 ng/mL or greater had higher odds of 90-day mortality compared with patients with levels of 30–49.9 ng/mL [adjusted OR (95% confidence interval) for 25(OH)D <10 ng/mL, 10–19.9 ng/mL, 20–29.9 ng/mL, 50–59.9 ng/mL, 60–69.9 ng/mL, and ≥70 ng/mL was 2.01 (1.68–2.40), 1.89 (1.64–2.18), 1.34 (1.16–1.56), 0.94 (0.69–1.26), 1.52 (1.03–2.25), and 1.69 (1.09–2.61), respectively, compared with patients with 25(OH)D levels 30–49.9 ng/mL].
Limitations:
A causal relationship between either low or high 25(OH)D levels and increased mortality can not necessarily be inferred from this observational study.
Conclusions:
Analysis of 24 094 adult patients showed that 25(OH)D levels less than 20 ng/mL and 60 ng/mL or greater before hospitalization were associated with an increased odds of 90-day mortality. Although previous reports have suggested an association between low vitamin D status and mortality, these data raise the issue of potential harm from high serum 25(OH)D levels, provide a rationale for an upper limit to supplementation, and emphasize the need for caution in the use of extremely high doses of vitamin D among patients.
Low vitamin D status is associated with increased all-cause mortality in the general population (1, 2). Reviews of data from randomized controlled trials have demonstrated a 3%–7% reduction in all-cause mortality in nonhospitalized adults who receive vitamin D supplementation, compared with adults who receive a placebo (3, 4). Evidence supports the concept that vitamin D insufficiency is associated with major chronic diseases such as cardiovascular disease and osteoporosis as well as colorectal and breast cancer (5). Recently we reported that suboptimal levels of 25-hydroxyvitamin D [25(OH)D] prior to hospital admission is also associated with an increased risk of in-hospital mortality (6). These observational findings raise the potential for vitamin D supplementation as a therapeutic intervention to decrease mortality in hospitalized patients.
Serum 25(OH)D is the major circulating metabolite of vitamin D and the standard measure of vitamin D status (7); it also is used to assess therapeutic response to supplementation (8, 9). Vitamin D is considered to have an excellent safety profile with a broad therapeutic window (1, 8, 10). There is, however, significant controversy in the literature regarding the acceptable lower limit of 25(OH)D levels in adults (11); most notably, although The Endocrine Society recommendations advocate for levels of 30 ng/mL or greater (12), an analysis by the Institute of Medicine suggests that 25(OH)D levels of 20 ng/mL or greater are adequate (13). Similarly, guidelines related to the acceptable upper limit of 25(OH)D levels in adults are unclear. Serum 25(OH)D levels greater than 150 ng/mL are associated with toxicity (1, 8, 10), but levels up to 80 ng/mL had been reported as optimal (14). On the other hand, the 2011 Institute of Medicine guidelines suggest 50 ng/mL as the upper threshold for desirable 25(OH)D levels (13).
Although a number of existing studies have investigated the relationship between low 25(OH)D levels and undesirable health outcomes, only a few have reported mortality rates for 25(OH)D levels of 50 ng/mL or greater (15–18). These studies, which are limited to subjects in community-based settings, demonstrate a nonlinear association between 25(OH)D levels of 50 ng/mL or greater and an increased risk of mortality. Few individuals in the United States have 25(OH)D levels of 30 ng/mL or greater (19). Pastoral Hadzabe and Maasai tribe members living near the equator in north central Tanzania and Kenya have 25(OH)D levels around 43 ng/mL (20). 25(OH)D levels of 50 ng/mL or greater are generally expected only in individuals with extreme UVB exposure or high levels of vitamin D supplementation (20, 22).
Given the paucity of data regarding health outcomes in hospitalized patients with 25(OH)D levels of 50 ng/mL or greater, the dramatic increase in vitamin D testing during routine medical care (23), burgeoning sales of vitamin D supplements (24), and the current trend of using increasingly large doses of vitamin D to treat low 25(OH)D levels and to maintain general health (25, 26), we performed a two-center observational study of a large cohort of hospitalized adults among whom 25(OH)D had been measured within 1 year before hospitalization. The objective of this study was to test our hypothesis that 25(OH)D levels before hospital admission have a U-shaped association with all-cause mortality.
Materials and Methods
Source population
We abstracted administrative and laboratory data from individuals admitted to two teaching hospitals in Boston, Massachusetts: Brigham and Women's Hospital (BWH), with 793 beds, and Massachusetts General Hospital (MGH), with 902 beds. The two hospitals provide primary as well as tertiary care to an urban and suburban population in addition to a diverse population within eastern Massachusetts and the surrounding region. BWH and MGH are both level 1 trauma centers, and both have 45 000–47 000 hospital admissions per year. BWH and MGH are members of Partners HealthCare, which is the largest health care provider in Massachusetts.
Data sources
Data on all patients admitted to BWH or MGH between August 3, 1993, and January 5, 2011, were obtained through the Research Patient Data Registry (RPDR), a computerized registry that serves as a central data warehouse for all inpatient and outpatient records at Partners HealthCare sites. The RPDR has been used for other clinical research studies (27). Approval for the study was granted by the Partners Human Research Committee Institutional Review Board. Requirement for consent was waived because the data were analyzed anonymously.
Study population
During the study period, there were 24 915 individual patients who were aged 18 years or older on the day of hospital admission, had serum 25(OH)D level measured between 7 and 365 days before admission, and were assigned a Diagnostic-Related Group (DRG), which is a system to classify hospital cases used by the Centers for Medicare and Medicaid Services (28). Exclusions included 23 foreign patients without Social Security numbers because vital status in this study was determined by the Social Security Administration Death Master File; 618 patients with missing laboratory data [calcium, creatinine, hematocrit, or white blood cell (WBC) count within the first 48 hours of hospital admission]; and 216 patients who received high-dose vitamin D supplementation (oral Ergocalciferol ≥50 000 IU) between the 25(OH)D blood draw and the index hospital admission. Thus, 24 094 patients constituted the total study cohort.
Exposure of interest and comorbidities
The exposure of interest was prehospital serum 25(OH)D level obtained 7–365 days prior to the date of hospital admission and categorized a priori as 25(OH)D less than 10 ng/mL or less than 25 nmol/L;10–19.9 ng/mL or 25–49.9 nmol/L; 20–29.9 ng/mL or 50.0–74.9 nmol/L; 30–49.9 ng/mL or 75–124.9 nmol/L; 50–59.9 ng/mL or 125–149.9 nmol/L; 60–69.9 ng/mL or 150–174.9 nmol/L; and 70 ng/mL or greater or 175 nmol/L or greater. The cut points for 25(OH)D less than 10 ng/mL, 10–19.9 ng/mL, and 20–29.9 ng/mL were taken from existing national clinical guidelines (12). The conversion factor to SI units is: 1 ng/mL = 2.496 nmol/L. In cases in which a patient had serum their 25(OH)D levels measured more than once in the year prior to hospitalization, the serum 25(OH)D measured closest to the date of hospital admission was used.
We used the Deyo-Charlson index to assess the burden of chronic illness, with higher scores indicating more comorbidity (29), using the International Classification of Diseases, ninth edition coding algorithms, which are well studied and validated (30, 31). Patient admission type was defined as medical or surgical and incorporates the DRG methodology, devised by the Centers for Medicare and Medicaid Services (28). Intensive care unit admission was determined by current procedural terminology code 99291 (critical care, first 30–74 min) assignment during hospital admission and has been validated in the RPDR database (27). Race was either self-determined or designated by a patient representative/health care proxy.
25(OH)D assays
Between 1993 and 2011, different assays were used at the two hospitals: chemiluminescence assay, RIA, or liquid chromatography-mass spectroscopy (LC-MS). Dates, times, and type of 25(OH)D assay were recorded. The clinical laboratories in which the assays were performed are Clinical Laboratory Improvement Amendments certified. The 25(OH)D assays were tested for imprecision by the clinical laboratories at the two hospitals. Imprecision testing with human serum specimens showed within-run coefficients of variation of 4.5% or less for the chemiluminescence assay, 10.8% or less for the RIA, and 8.6% or less for LC-MS. The method corrections were not used when institutions changed assays, and because this was a retrospective, observational study, the determination of the between-method assay differences was not possible.
End points
The primary end point was all-cause 90-day mortality. Ninety-day mortality was chosen based on previous studies in patients at BWH and MGH to provide a sufficient event rate (32, 33). The secondary outcomes included 30-day, 365-day, and in-hospital mortality.
Assessment of mortality
Information on vital status for the study cohort was obtained from the Social Security Administration Death Master File, which has high sensitivity and specificity for mortality (34–37). We have validated the accuracy of the Social Security Administration Death Master File for in-hospital and out-of-hospital mortality in our administrative database (27). One hundred percent of the cohort had at least 365-day follow-up. The censoring date was January 5, 2012.
Power calculations and statistical analysis
By extrapolating data from other studies (6, 15–18), we assumed that 90-day mortality would be 4% higher among patients with prehospital 25(OH)D levels less than 20 ng/mL or 60 ng/mL or greater compared with those with prehospital 25(OH)D 30–49.9 ng/mL. With an alpha error level of 5% and a power of 80%, the sample size thus required for our primary end point (90 d mortality) was 350 patients with prehospital 25(OH)D less than 20 ng/mL, 350 patients with prehospital 25(OH)D 60 ng/mL or greater, and 4830 patients with prehospital 25(OH)D 30–49.9 ng/mL.
Categorical variables were described by frequency distribution and compared across 25(OH)D groups using contingency tables and χ2 testing. Continuous variables were examined graphically (eg, histogram, box plot) and in terms of summary statistics (mean, SD, median, interquartile range) and then compared across exposure groups using one-way ANOVA. The primary outcome was 90-day mortality. Unadjusted associations between 25(OH)D groups and 90-day mortality were estimated using logistic regression. Adjusted odd ratios (ORs) were estimated using multivariable logistic regression with inclusion of potential confounders [ie, variables thought to plausibly associate with both 25(OH)D levels and 90 d mortality]. For the primary model, the specification of each continuous covariate (as a linear vs categorical term) was adjudicated by the empirical association with the primary outcome using Akaike's Information Criterion; overall model fit was assessed using the Hosmer Lemeshow test. Models for secondary analyses were specified identically to the primary model. Locally weighted scatter plot smoothing (LOWESS) (38, 39) was used to graphically represent the relationship between prehospital 25(OH)D level and the 90-day mortality rate. We tested the significance of the interaction using the likelihood ratio test. For the time to mortality, we estimated the survival curves according to group with the use of the Kaplan-Meier method (40) and compared the results by means of the log-rank test. All P values were two tailed, with P < .05 considered statistically significant. All analyses were performed using STATA 12.0MP statistical software.
Results
Patient characteristics of the study cohort were stratified according to prehospital 25(OH)D levels (Table 1). The mean age at hospital admission was 61 years. Most patients were female, white, and had a medically related DRG. Factors that significantly differed between stratified groups included age, gender, race (white vs nonwhite), patient type (surgical vs medical), Deyo-Charlson index, and admission creatinine level. The mean prehospital level of 25(OH)D was 27.9 ng/mL (SD 15.1) with the median 25(OH)D of 26 ng/mL. Nearly half of the 25(OH)D measurements (48%) occurred in the 3 months before hospital admission. Thirteen percent of the patients received critical care services. In-hospital mortality rate was 2%, whereas 30-, 90-, and 365-day mortality rates were 4%, 7%, and 13%, respectively. Table 2 indicates that age, gender, race, patient type, and the Deyo-Charlson index were significant predictors of 90-day mortality.
Table 1.
Patient Characteristics by Prehospital Vitamin D Status
| Prehospital 25(OH)D, ng/mL |
P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| <10.0 | 10–19.9 | 20–29.9 | 30–49.9 | 50–59.9 | 60–69.9 | ≥70.0 | Total | ||
| n | 2,309 | 5,622 | 6,216 | 8,163 | 1,095 | 390 | 299 | 24 094 | |
| Age, y, mean (SD) | 58.7 (18.1) | 58.9 (17.7) | 60.3 (17.7) | 63.3 (17.1) | 65.8 (16.5) | 65.1 (17.3) | 63.9 (17.6) | 61.2 (17.6) | <.0001a |
| Sex, n, % | <.0001 | ||||||||
| Female | 1,403 (61) | 3,314 (59) | 3,771 (61) | 5,300 (65) | 782 (71) | 287 (74) | 204 (68) | 15 061 (63) | |
| Male | 906 (39) | 2,308 (41) | 2,445 (39) | 2,863 (35) | 313 (29) | 103 (26) | 95 (32) | 9033 (37) | |
| Race, n, % | <.0001 | ||||||||
| White | 1,589 (69) | 4,151 (74) | 4,877 (78) | 6,903 (85) | 975 (89) | 336 (86) | 250 (84) | 19 081 (79) | |
| Nonwhite | 720 (31) | 1,471 (26) | 1,339 (22) | 1,260 (15) | 120 (11) | 54 (14) | 49 (16) | 5013 (21) | |
| Patient type, n, % | <.0001 | ||||||||
| Medical | 1,636 (71) | 3,570 (64) | 3,669 (59) | 4,592 (56) | 635 (58) | 248 (64) | 175 (59) | 14 525 (60) | |
| Surgical | 673 (29) | 2,052 (36) | 2,547 (41) | 3,571 (44) | 460 (42) | 142 (36) | 124 (41) | 9569 (40) | |
| Deyo-Charlson index, n, % | <.0001 | ||||||||
| 0–3 | 685 (30) | 1967 (35) | 2486 (40) | 3138 (38) | 391 (36) | 125 (32) | 92 (31) | 8884 (37) | |
| 4–6 | 569 (25) | 1461 (26) | 1551 (25) | 2093 (26) | 320 (29) | 111 (28) | 86 (29) | 6191 (26) | |
| ≥6 | 1,055 (46) | 2194 (39) | 2179 (35) | 2932 (36) | 384 (35) | 154 (39) | 121 (40) | 9019 (37) | |
| Season of 25(OH)D draw | <.0001 | ||||||||
| Spring | 758 (33) | 1630 (29) | 1691 (27) | 2054 (25) | 276 (25) | 110 (28) | 84 (28) | 6603 (27) | |
| Summer | 407 (18) | 1179 (21) | 1576 (25) | 2266 (28) | 307 (28) | 92 (24) | 83 (28) | 5910 (25) | |
| Winter | 650 (28) | 1504 (27) | 1345 (22) | 1614 (20) | 224 (20) | 84 (22) | 52 (17) | 5473 (23) | |
| Fall | 494 (21) | 1309 (23) | 1604 (26) | 2229 (27) | 288 (26) | 104 (27) | 80 (27) | 6108 (25) | |
| Creatinine, n, % | <.0001 | ||||||||
| ≤0.8 mg/dL | 537 (23) | 1488 (26) | 1745 (28) | 2223 (27) | 315 (29) | 103 (26) | 79 (26) | 6490 (27) | |
| 0.9–1.5 mg/dL | 967 (42) | 2594 (46) | 3064 (49) | 4236 (52) | 525 (48) | 201 (52) | 139 (46) | 11 726 (49) | |
| 1.6–3.0 mg/dL | 404 (18) | 790 (14) | 747 (12) | 1057 (13) | 162 (15) | 47 (12) | 47 (16) | 3254 (14) | |
| Calcium, n, % | .013 | ||||||||
| ≥10.5 mg/dL | 231 (10) | 653 (10) | 766 (12) | 867 (11) | 110 (11) | 46 (13) | 27 (10) | 2700 (11) | |
| Hematocrit < 30%, n, % | 523 (23) | 1081 (17) | 857 (14) | 1041 (14) | 147 (15) | 56 (16) | 52 (18) | 3757 (16) | <.0001 |
| > 90 d between 25(OH)D and admission, n, % | 979 (42) | 2970 (48) | 3315 (53) | 4370 (57) | 520 (54) | 191 (53) | 150 (53) | 12 495 (52) | <.0001 |
| 90-Day mortality, n, % | 228 (10) | 496 (9) | 389 (6) | 411 (5) | 53 (5) | 31 (8) | 25 (8) | 1633 (7) | <.0001 |
P values were determined by χ2 unless designated by superscript a. Columns may not add up to 100% due to rounding.
When P values were not determined by χ2, then the P value was determined by Kruskal Wallis.
Table 2.
Multivariable-Adjusted Associations Between Covariates and All-Cause 90-Day Mortality
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Age, y (per 1 y) | 1.02 | 1.02–1.03 | <.0001 |
| Sex | |||
| Male | 1.42 | 1.28–1.58 | <.0001 |
| Female | 1 | Referent | |
| Race | |||
| Nonwhite | 0.68 | 0.59–0.78 | <.0001 |
| White | 1 | Referent | |
| Patient type | |||
| Surgical | 0.43 | 0.38–0.49 | <.0001 |
| Medical | 1 | Referent | |
| Deyo-Charlson index | |||
| 0–3 | 1 | Referent | |
| 4–6 | 1.98 | 1.63–2.41 | <.0001 |
| ≥6 | 4.63 | 3.89–5.50 | <.0001 |
| Season of 25(OH)D draw | |||
| Spring | 1 | Referent | |
| Summer | 0.95 | 0.82–1.10 | .51 |
| Winter | 1.06 | 0.91–1.23 | .43 |
| Fall | 1.09 | 0.94–1.27 | .25 |
Estimates for each variable are adjusted for all other variables in the table.
Primary outcome
Prehospital vitamin D status was a strong predictor of 90-day mortality (Figure 1). The odds of 90-day mortality in the 25(OH)D less than 10 ng/mL, 25(OH)D 10–19.9 ng/mL, 25(OH)D 20–29.9 ng/mL, 25(OH)D 60–69.9 ng/mL, and 25(OH)D 70 ng/mL or greater groups was 2.1-, 1.8-, 1.3-, 1.6-, and 1.7-fold, respectively, that of the 30–49.9 ng/mL group (Table 3). Vitamin D status remained a significant predictor of the odds of 90-day mortality after adjustment for age, gender, race, patient type, the Deyo-Charlson index, and season of 25(OH)D draw. The adjusted odds of 90-day mortality in the 25(OH)D less than 10 ng/mL, 25(OH)D 10–19.9 ng/mL, 25(OH)D 20–29.9 ng/mL, 25(OH)D 60–69.9 ng/mL, and 25(OH)D 70 ng/mL or greater groups was 2.0-, 1.9-, 1.3-, 1.5-, and 1.7-fold, respectively, that of the 30–49.9 ng/mL group (Table 3).
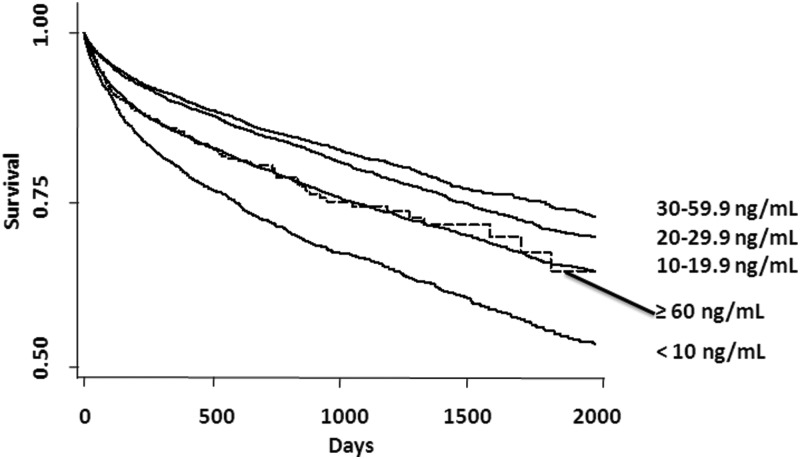
Figure 1.
Time-to-event curves for all-cause mortality. Unadjusted event rates were calculated with the use of the Kaplan-Meier methods and compared with the use of the log-rank test. Categorization of 25(OH)D is per the primary analyses with 25(OH)D 60–69.9 ng/mL and 25(OH)D ≥70.0 ng/mL groups shown as ≥60.0 ng/mL. The global comparison log rank P value is <.0001.
Table 3.
Unadjusted and Adjusted Associations Between Prehospital 25(OH)D Level and All-Cause 90-Day Mortality
| Unadjusted |
Adjusted |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| 90-Day mortality | ||||||
| <10 ng/mL | 2.07 | 1.75–2.45 | <.0001 | 2.01 | 1.68–2.40 | <.0001 |
| 10–19.9 ng/mL | 1.83 | 1.59–2.09 | <.0001 | 1.89 | 1.64–2.18 | <.0001 |
| 20–29.9 ng/mL | 1.26 | 1.09–1.45 | .002 | 1.34 | 1.16–1.56 | <.0001 |
| 30–49.9 ng/mL | 1 | Referent | 1 | Referent | ||
| 50–59.9 ng/mL | 0.96 | 0.72–1.29 | .78 | 0.94 | 0.69–1.26 | .67 |
| 60–69.9 ng/mL | 1.63 | 1.11–2.38 | .012 | 1.52 | 1.03–2.25 | .037 |
| ≥70 ng/mL | 1.72 | 1.13–2.62 | .012 | 1.69 | 1.09–2.61 | .018 |
| 365-Day mortality | ||||||
| <10 ng/mL | 2.48 | 2.19–2.81 | <.0001 | 2.58 | 2.26–2.96 | <.0001 |
| 10–19.9 ng/mL | 1.84 | 1.66–2.04 | <.0001 | 2.00 | 1.79–2.23 | <.0001 |
| 20–29.9 ng/mL | 1.27 | 1.14–1.42 | <.0001 | 1.39 | 1.24–1.56 | <.0001 |
| 30–49.9 ng/mL | 1 | Referent | 1 | Referent | ||
| 50–59.9 ng/mL | 1.11 | 0.90–1.37 | .32 | 1.09 | 0.88–1.35 | .45 |
| 60–69.9 ng/mL | 1.42 | 1.05–1.93 | .022 | 1.33 | 0.96–1.82 | .083 |
| ≥70 ng/mL | 1.68 | 1.21–2.32 | .002 | 1.66 | 1.18–2.34 | .004 |
Estimates adjusted for age, sex, race (white vs nonwhite), patient type (surgical vs medical), season of 25(OH)D draw, and Deyo-Charlson index.
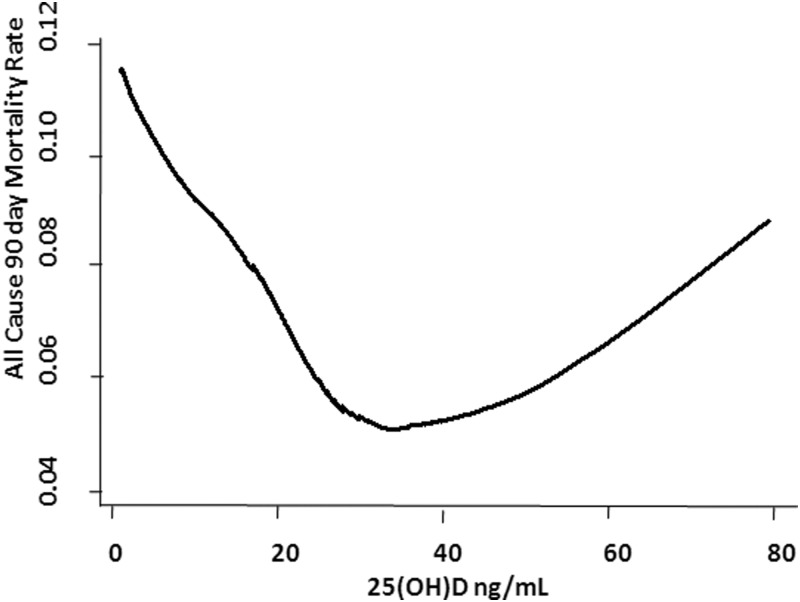
Additional adjustment for assay type (chemiluminescence, RIA, or LC-MS) did not materially alter the results: the fully adjusted OR of 90-mortality in the 25(OH)D less than 10 ng/mL, 25(OH)D 10–19.9 ng/mL, 25(OH)D 20–29.9 ng/mL, 25(OH)D 60–69.9 ng/mL, and 25(OH)D 70 ng/mL or greater groups was 1.75 [95% confidence interval (CI) 1.45–2.10], 1.68 (95% CI 1.45–1.94), 1.17 (95% CI 1.00–1.36), 1.56 (95% CI, 1.05–2.31), and 1.71 (95% CI 1.11–2.64), respectively, that of the 30–49.9 ng/mL group. Additional adjustment for year of 25(OH)D draw did not change these results (data not shown). Although limited by statistical power, the adjusted results did not materially differ by hospital site [χ2 (1, N = 24 094) = 1.61, P = .20]. LOWESS plot (Figure 2) shows a near-inverse linear association between 25(OH)D level and risk of 90-day mortality up to 25(OH)D levels near 25 ng/mL. Between 25(OH)D levels of 25 ng/mL and 50 ng/mL, there was flattening of the curve. Beyond the 25(OH)D levels of 50 ng/mL, the curve appears to show a linear association between the 25(OH)D level and an increased risk of 90-day mortality.
Figure 2.
Vitamin D status versus all-cause 90-day mortality. Locally weighted scatter plot smoothing (LOWESS) utilized to represent the nonlinear association between pre-hospital 25(OH)D level and 90-day mortality rate.
Secondary outcome
The odds of 365-day mortality in the 25(OH)D less than 10 ng/mL, 25(OH)D 10–19.9 ng/mL, 25(OH)D 20–29.9 ng/mL, 25(OH)D 60–69.9 ng/mL, and 25(OH)D 70 ng/mL or greater groups was 2.5-, 1.8-, 1.3-, 1.4-, and 1.7-fold, respectively, that of the 30–49.9 ng/mL group (Table 3). Vitamin D status remained a predictor of the odds of 90-day mortality after adjustment for age, gender, race, patient type, the Deyo-Charlson index, and season of 25(OH)D draw. The adjusted odds of 365-day mortality in the 25(OH)D less than 10 ng/mL, 25(OH)D 10–19.9 ng/mL, 25(OH)D 20–29.9 ng/mL, 25(OH)D 60–69.9 ng/mL, and 25(OH)D 70 ng/mL or greater groups was 2.6-, 2.0-, 1.4-, 1.3-, and 1.7-fold, respectively, that of the 30–49.9 ng/mL group (Table 3).
Subanalyses
Although statistical power was compromised, when patients with 25(OH)D measured more than 90 days before hospital admission were excluded (n = 11 599), the odds of 90-day mortality in patients with 25(OH)D less than 10 ng/mL or 25(OH)D of 70 ng/mL or greater vitamin D was 2.16 (95% CI 1.74–2.68) and 2.11(95% CI 1.23–3.62), respectively, relative to that of the 30–49.9 ng/mL group. In this subanalysis, the multivariable adjusted odds of 90-day mortality in patients with 25(OH)D less than 10 ng/mL or 25(OH)D 70 ng/mL or greater vitamin D was 2.07 (95% CI 1.65–2.60) and 2.00 (95% CI 1.14–3.51), respectively, relative to that of the 30–49.9 ng/mL group.
Limiting the analysis to those with 25(OH)D measured by either chemiluminescence or RIA (n = 12 373) yielded a similar U-shaped association (data not shown). Limiting the analysis to only those with 25(OH)D measured by LC-MS (n = 11 271) also yielded a U-shaped association between prehospital 25(OH)D and mortality with an OR for 90-day mortality as follows: less than 10 ng/mL (1.89, 95% CI 1.36–2.62), 10–19.9 ng/mL (2.01, 95% CI 1.60–2.51), 20–29.9 ng/mL (1.32, 95% CI 1.07–1.64), 50–59.9 ng/mL (0.99, 95% CI 0.68–1.44), 60–69.9 ng/mL (2.02, 95% CI 1.27–3.23), and 70 ng/mL or greater (1.97, 95% CI 1.13–3.42), fully adjusted all relative to 25(OH)D 30–49.9 ng/mL. To assess discrimination of 25(OH)D for 90-day mortality, we used receiver-operating characteristic curve analysis and determined the area under the curve (AUC). Estimating the AUC shows that 25(OH)D has similar discriminative power for 90-day mortality, regardless of assay used (total cohort AUC = 0.57; chemiluminescence assay AUC = 0.58; RIA AUC = 0.55; and LC-MS AUC = 0.56).
Effect modification
Analyses based on fully adjusted models were performed to evaluate the 25(OH)D 90-day mortality association, and P value for interaction was determined to explore for any evidence of effect modification. We individually tested for effect modification by gender, patient type (surgical vs medical), creatinine, calcium, hematocrit, assay, hospital of admission, and the time between 25(OH)D draw and hospitalization by adding an interaction term to the multivariate models. Quantitative effect modification existed with gender, patient type (surgical vs medical), creatinine, calcium, hematocrit, assay, hospital of admission, and the time between 25(OH)D draw and hospitalization in which the direction of the association did not change but the strength differed across strata of the effect modifier (Supplemental Table1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Season of 25(OH)D draw did not emerge as an effect modifier of the association between 25(OH)D and 90-day mortality (P interaction = .36).
Furthermore, individually running the adjusted model with and without terms for the time between 25(OH)D draw and hospital admission, calcium, creatinine, and WBC count, the 90-day mortality estimates in each case are similar. The odds of 90-day mortality in the 25(OH)D 70 ng/mL or greater group was 1.66 (95% CI 1.07–2.56) with additional adjustment for time between 25(OH)D draw and admission; OR 1.70 (95% CI 1.10–2.62) with calcium, OR 1.67 (95% CI 1.08–2.58) with creatinine, OR 1.70 (95% CI 1.10–2.63) with WBC count, all relative to 25(OH)D 30–49.9 ng/mL. This indicates that the 25(OH)D 90-day mortality relationship is not materially confounded by the time between 25(OH)D draw and hospital admission, calcium, creatinine, or WBC count.
Discussion
In this study, we investigated whether prehospital 25(OH)D was associated with all-cause mortality after hospital admission. Our data suggest that in hospitalized adults, prehospital 25(OH)D levels less than 30 ng/mL and 60 ng/mL or greater are associated with a significantly increased odds of 90-day mortality compared with prehospital 25(OH)D levels of 30–49.9 ng/mL. This finding was not materially altered after a multivariable analysis, suggesting that the 25(OH)D levels at either end of the spectrum prior to hospitalization may be an independent marker of poor outcome. However, because our study is observational and not interventional, the inference of a causal relationship between 25(OH)D levels and outcomes is limited.
Although the association between low vitamin D status and the risk of mortality in hospitalized patients has been previously reported (6), mortality risk in hospitalized patients with 25(OH)D levels of 60 ng/mL or greater is a novel observation. Patients in this study with 25(OH)D levels of 60 ng/mL are likely a heterogenous group. A transient 25(OH)D level of 60 ng/mL or greater may have resulted from vitamin D supplementation of patients who had low serum 25(OH)D, which itself may be a marker for poor outcome (6). Alternatively, a more chronic 25(OH)D level of 60 ng/mL or greater may itself have deleterious consequences (1, 8, 10, 41). Indeed, a recent 15-year follow-up report on participants from the third National Health and Nutrition Evaluation Survey (NHANES III) suggested that outpatients with 25(OH)D of 48 ng/mL or greater appear to have increased mortality compared with individuals with levels between 30 and 39.9 ng/mL (42). Furthermore, in community-dwelling men, 25(OH)D greater than 39 ng/mL are associated with increased cancer risk but not cardiovascular mortality (16). It is unclear whether our observation of worse outcomes in 25(OH)D 60–69.9 ng/mL and 25(OH)D 70 ng/mL or greater groups is due to vitamin D concentrations or a reflection of reverse causation. In support of supplementation being important for the observed differences in our study is that a seasonal pattern is not particularly strong and patients with 25(OH)D of 60 ng/mL or greater are more likely to be older, white, and female with a higher Deyo-Charlson index score (Table 1).
Prolonged daily intakes of 10 000 IU have been reported to be safe (10), and the tolerable upper daily limit recommended by The Endocrine Society is 10 000 IU (12), whereas the Institute of Medicine suggests a maximum daily intake of 4000 IU (13). Serum 25(OH)D levels greater than 150 ng/mL are associated with hypercalcemia, hypercalciuria, and pathological calcifications in the kidney and other organs (1, 8, 10). However, vitamin D toxicity in the form of hypercalcemia is usually observed only when 25(OH)D concentrations are consistently above 160–200 ng/mL (43). Vitamin D intoxication may be life threatening but is rare because most cases are attributable to prolonged and accidental intakes of greater than 40 000 IU per day (1, 41). Documented adverse effects of high-dose vitamin D supplementation with doses up to 600 000 IU include the increased risk of falls and fractures (44, 45) as well as rare cases of mild hypercalcemia (45, 46).
A biological explanation for the observed results in this study is not readily apparent, but it may be related to recent evidence suggesting threshold-mediated anti- and proinflammatory properties associated with 25(OH)D levels. In a secondary analysis of a large cohort of community-dwelling adults (47), serum C-reactive protein (CRP) levels were found to be inversely related to increasing 25(OH)D levels up to approximately 20 ng/mL, after which, each 10-ng/mL increment in the 25(OH)D level was associated with a 0.6-mg/L increase in CRP levels. On average, a 25(OH)D level of 20 ng/mL was associated with a CRP level of 2 mg/L in this cohort and a 25(OH)D level of 60 ng/mL would therefore be associated with a CRP level of greater than 4 mg/L. It is unclear whether these baseline elevations in CRP are associated with an exaggerated inflammatory state after an acute hospitalization. Another potential explanation for our findings may be related to the role of vitamin D in promoting the intestinal absorption of phosphorus. Phosphate levels have been shown to be directly associated with mortality (48, 49).
The present study has all the inherent limitations of a retrospective study. Because our study is observational, causality is limited. Selection bias may exist because the patient cohort under study had vitamin D status investigated for a particular reason that may be absent in other patients. Ascertainment bias may be present because not every patient had 25(OH)D measured prior to hospitalization and thus were not included in our study. These issues may decrease the generalizability of our results to all hospitalized patients. We are unable to adjust for PTH levels drawn at the time of the serum 25(OH)D determination in the study cohort. Additionally, we do not have albumin measurements at the time of 25(OH)D determination because low albumin has been noted to correlate with low 25(OH)D levels (50, 51). Despite adjustment for multiple potential confounders, there may be residual confounding variables leading to the observed differences in outcomes.
All cohort patients had 25(OH)D measured prior to hospitalization. A prior study in an outpatient population noted the intraperson Pearson correlation coefficient for 25(OH)D at 3 years between blood draws was 0.70 after adjustments for age, race, and season (52). Our subanalysis demonstrates the preservation of the observed vitamin D status-mortality association with less than 90 days between the 25(OH)D level and hospital admission. Despite this observation, 25(OH)D levels at the time of hospitalization are not available in this cohort and may have changed since the preadmission values were determined (53–55). Additionally, we do not have data available on over-the-counter supplementation or vitamin D prescribed by providers outside clinics related to the hospitals under study. We are therefore unable to determine whether 25(OH)D levels of 60 ng/mL or greater are a result of aggressive supplementation.
The present study has several strengths. For example, our study has sufficient numbers of patients to ensure the adequate reliability of our estimates (n = 24 094, 90 d mortality rate = 7%). We have sufficient statistical power to detect a clinically relevant difference in the 90-day mortality if one exists. We used all-cause mortality as a primary end point, which is an unbiased and clinically relevant outcome. The Deyo-Charlson index accounts for chronic conditions that may alter hospital mortality (56). Preservation of the vitamin D status-mortality association after the exclusion of patients with 25(OH)D levels drawn more than 90 days prior to hospitalization and the absence of confounding relative to the time between 25(OH)D draw and hospital admission suggests that the likelihood of reverse causation related to the timing of 25(OH)D draw is low.
In summary, these data demonstrate that both patients with preadmission 25(OH)D levels less than 30 ng/mL and those with 25(OH)D levels of 60 ng/mL or greater may be at increased risk of 90-day mortality after hospital admission and that this risk is independent of other factors. Although our study cannot determine causation or be considered evidence in favor of adjusting current vitamin D supplementation dose recommendations, our clinical data related to the risk of mortality with 25(OH)D levels of 60 ng/mL or greater raise a number of questions that merit further investigation and should encourage the careful monitoring of vitamin D status in hospitalized patients. Our illustration of potential harm at 25(OH)D levels of 60 ng/mL, a level lower than the hypercalcemic toxicity threshold [25(OH)D > 150 ng/mL], highlights the potential importance of monitoring 25(OH)D concentrations during vitamin D therapy (14). This is especially important, given an emerging trend in the use of higher supplementation doses (25, 26) and recent policy statements calling for the elimination of routine 25(OH)D testing based on the previously assumed therapeutic index of vitamin D therapy (21, 57).
Acknowledgments
Author contributions include the following: K.A. and K.B.C. jointly conceived the study and designed and implemented the analysis with assistance from A.A.L., E.G., S.A.Q., and C.A.C.; K.B.C. assembled the input data, wrote the code, ran the model, and analyzed the output data; K.A., S.A.Q., C.A.C., and K.B.C. wrote the manuscript; and K.A., S.A.Q., A.A.L, F.K.G., T.R.P., C.A.C., E.G., and K.B.C. edited the manuscript and provided conceptual advice.
No conflicts of interest, including relevant financial interests, activities, relationships, and affiliations exist for any authors.
This manuscript is dedicated to the memory of our dear friend and colleague, Nathan Edward Hellman, MD, PhD.
Disclosure Summary: No conflicts of interest, including relevant financial interests, activities, relationships, and affiliations exists for any authors.
S.A.Q. received support from the National Institutes of Health Grants 5T32GM007592-33 and UL1 RR025758. C.A.C. received support from National Institutes of Health Grants R01 AI093723 and U01 AI087881. K.B.C. received support from National Institutes of Health Grant K08AI060881.
For editorial see page 1164
- AUC
- area under the curve
- BWH
- Brigham and Women's Hospital
- CI
- confidence interval
- CRP
- C-reactive protein
- DRG
- Diagnostic-Related Group
- LC-MS
- liquid chromatography-mass spectroscopy
- MGH
- Massachusetts General Hospital
- 25(OH)D
- 25-hydroxyvitamin D
- OR
- odds ratio
- RPDR
- Research Patient Data Registry
- WBC
- white blood cell.
References
- 1. Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95(1):91–100 [DOI] [PubMed] [Google Scholar]
- 2. Ginde AA, Scragg R, Schwartz RS, Camargo CA., Jr Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older US adults. J Am Geriatr Soc. 2009;57(9):1595–1603 [DOI] [PubMed] [Google Scholar]
- 3. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(16):1730–1737 [DOI] [PubMed] [Google Scholar]
- 4. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2011(7):CD007470. [DOI] [PubMed] [Google Scholar]
- 5. Peterlik M. Vitamin D insufficiency and chronic diseases: hype and reality. Food Funct. 2012;3(8):784–794 [DOI] [PubMed] [Google Scholar]
- 6. Lange N, Litonjua AA, Gibbons FK, Giovannucci E, Christopher KB. Pre-hospital vitamin D concentration, mortality, and bloodstream infection in a hospitalized patient population. Am J Med. 2013;126(7):640.e619–e627 [DOI] [PubMed] [Google Scholar]
- 7. Lai JK, Lucas RM, Clements MS, Harrison SL, Banks E. Assessing vitamin D status: pitfalls for the unwary. Mol Nutr Food Res. 2010;54(8):1062–1071 [DOI] [PubMed] [Google Scholar]
- 8. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281 [DOI] [PubMed] [Google Scholar]
- 9. Heaney RP. Assessing vitamin D status. Curr Opin Clin Nutr Metab Care. 2011;14(5):440–444 [DOI] [PubMed] [Google Scholar]
- 10. Vieth R. Vitamin D toxicity, policy, and science. J Bone Miner Res. 2007;22(suppl 2):V64–V68 [DOI] [PubMed] [Google Scholar]
- 11. Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26(3):455–457 [DOI] [PubMed] [Google Scholar]
- 12. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930 [DOI] [PubMed] [Google Scholar]
- 13. Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011 [PubMed] [Google Scholar]
- 14. Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc. 2010;85(8):752–757; quiz 757–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michaelsson K, Baron JA, Snellman G, et al. Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr. 2010;92(4):841–848 [DOI] [PubMed] [Google Scholar]
- 17. Jia X, Aucott LS, McNeill G. Nutritional status and subsequent all-cause mortality in men and women aged 75 years or over living in the community. Br J Nutr. 2007;98(3):593–599 [DOI] [PubMed] [Google Scholar]
- 18. Visser M, Deeg DJ, Puts MT, Seidell JC, Lips P. Low serum concentrations of 25-hydroxyvitamin D in older persons and the risk of nursing home admission. Am J Clin Nutr. 2006;84(3):616–622; quiz 671–672 [DOI] [PubMed] [Google Scholar]
- 19. Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luxwolda MF, Kuipers RS, Kema IP, van der Veer E, Dijck-Brouwer DA, Muskiet FA. Vitamin D status indicators in indigenous populations in East Africa. Eur J Nutr. 2013;52(3):1115–1125 [DOI] [PubMed] [Google Scholar]
- 21. Bolland MJ, Grey A, Davidson JS, Cundy T, Reid IR. Should measurement of vitamin D and treatment of vitamin D insufficiency be routine in New Zealand? NZ Med J. 2012;125(1349):83–91 [PubMed] [Google Scholar]
- 22. Binkley N, Novotny R, Krueger D, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92(6):2130–2135 [DOI] [PubMed] [Google Scholar]
- 23. Bilinski K, Boyages S. The rise and rise of vitamin D testing. BMJ. 2012;345:e4743. [DOI] [PubMed] [Google Scholar]
- 24. Nutrition Business Journal. 2012 Global supplement, nutrition industry report, 2012. http://newhope360.com/nutrition-business-journal Accessed March 2, 2013
- 25. Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85(1):6–18 [DOI] [PubMed] [Google Scholar]
- 26. Haines ST, Park SK. Vitamin D supplementation: what's known, what to do, and what's needed. Pharmacotherapy. 2012;32(4):354–382 [DOI] [PubMed] [Google Scholar]
- 27. Zager S, Mendu ML, Chang D, et al. Neighborhood poverty rate and mortality in patients receiving critical care in the academic medical center setting. Chest. 2011;139(6):1368–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rapoport J, Gehlbach S, Lemeshow S, Teres D. Resource utilization among intensive care patients. Managed care vs traditional insurance. Arch Intern Med. 1992;152(11):2207–2212 [PubMed] [Google Scholar]
- 29. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383 [DOI] [PubMed] [Google Scholar]
- 30. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139 [DOI] [PubMed] [Google Scholar]
- 31. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682 [DOI] [PubMed] [Google Scholar]
- 32. Braun A, Chang D, Mahadevappa K, et al. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39(4):671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Braun AB, Gibbons FK, Litonjua AA, Giovannucci E, Christopher KB. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit Care Med. 2012;40(1):63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12(7):462–468 [DOI] [PubMed] [Google Scholar]
- 35. Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schisterman EF, Whitcomb BW. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul Health Metr. 2004;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Newman TB, Brown AN. Use of commercial record linkage software and vital statistics to identify patient deaths. J Am Med Inform Assoc. 1997;4(3):233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cleveland W. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836 [Google Scholar]
- 39. Cleveland W, Devlin S. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83(403):596–610 [Google Scholar]
- 40. Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481 [Google Scholar]
- 41. Lowe H, Cusano NE, Binkley N, Blaner WS, Bilezikian JP. Vitamin D toxicity due to a commonly available “over the counter” remedy from the Dominican Republic. J Clin Endocrinol Metab. 2011;96(2):291–295 [DOI] [PubMed] [Google Scholar]
- 42. Sempos CT, Durazo-Arvizu RA, Dawson-Hughes B, et al. Is there a reverse J-shaped association between 25-hydroxyvitamin D and all-cause mortality? Results from the US Nationally Representative NHANES. J Clin Endocrinol Metab. 2013;98(7):3001–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vieth R. The mechanisms of vitamin D toxicity. Bone Miner. 1990;11(3):267–272 [DOI] [PubMed] [Google Scholar]
- 44. Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women—a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford). 2007;46(12):1852–1857 [DOI] [PubMed] [Google Scholar]
- 45. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815–1822 [DOI] [PubMed] [Google Scholar]
- 46. Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87(3):688–691 [DOI] [PubMed] [Google Scholar]
- 47. Amer M, Qayyum R. Relation between serum 25-hydroxyvitamin D and C-reactive protein in asymptomatic adults (from the continuous National Health and Nutrition Examination Survey 2001 to 2006). Am J Cardiol. 2012;109(2):226–230 [DOI] [PubMed] [Google Scholar]
- 48. Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112(17):2627–2633 [DOI] [PubMed] [Google Scholar]
- 49. Larsson TE, Olauson H, Hagstrom E, et al. Conjoint effects of serum calcium and phosphate on risk of total, cardiovascular, and noncardiovascular mortality in the community. Arterioscler Thromb Vasc Biol. 2010;30(2):333–339 [DOI] [PubMed] [Google Scholar]
- 50. Premaor MO, Alves GV, Crossetti LB, Furlanetto TW. Hyperparathyroidism secondary to hypovitaminosis D in hypoalbuminemic is less intense than in normoalbuminemic patients: a prevalence study in medical inpatients in southern Brazil. Endocrine. 2004;24(1):47–53 [DOI] [PubMed] [Google Scholar]
- 51. Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954–959 [DOI] [PubMed] [Google Scholar]
- 52. Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15(3):255–265 [DOI] [PubMed] [Google Scholar]
- 53. Krishnan A, Ochola J, Mundy J, et al. Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit Care. 2010;14(6):R216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mata-Granados JM, Vargas-Vasserot J, Ferreiro-Vera C, Luque de Castro MD, Pavon RG, Quesada Gomez JM. Evaluation of vitamin D endocrine system (VDES) status and response to treatment of patients in intensive care units (ICUs) using an on-line SPE-LC-MS/MS method. J Steroid Biochem Mol Biol. 2010;121(1–2):452–455 [DOI] [PubMed] [Google Scholar]
- 56. Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;34(10):2576–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clinical utility of vitamin d testing: an evidence-based analysis. Ont Health Technol Assess Ser. 2010;10(2):1–93 [PMC free article] [PubMed] [Google Scholar]