Abstract
Context:
Prior studies showed that Axl /Tyro3 null mice have delayed first estrus and abnormal cyclicity due to developmental defects in GnRH neuron migration and survival.
Objective:
The objective of the study was to test whether the absence of Axl would alter reproductive function in mice and that mutations in AXL are present in patients with Kallmann syndrome (KS) or normosmic idiopathic hypogonadotropic hypogonadism (nIHH).
Design and Setting:
The sexual maturation of Axl null mice was examined. The coding region of AXL was sequenced in 104 unrelated, carefully phenotyped KS or nIHH subjects. Frequency of mutations was compared with other causes of GnRH deficiency. Functional assays were performed on the detected mutations.
Results:
Axl null mice demonstrated delay in first estrus and the interval between vaginal opening and first estrus. Three missense AXL mutations (p.L50F, p.S202C, and p.Q361P) and one intronic variant 6 bp upstream from the start of exon 5 (c.586-6 C>T) were identified in two KS and 2 two nIHH subjects. Comparison of the frequencies of AXL mutations with other putative causes of idiopathic hypogonadotropic hypogonadism confirmed they are rare variants. Testing of the c.586-6 C>T mutation revealed no abnormal splicing. Surface plasmon resonance analysis of the p.L50F, p.S202C, and p.Q361P mutations showed no altered Gas6 ligand binding. In contrast, GT1-7 GnRH neuronal cells expressing p.S202C or p.Q361P demonstrated defective ligand dependent receptor processing and importantly aberrant neuronal migration. In addition, the p.Q361P showed defective ligand independent chemotaxis.
Conclusions:
Functional consequences of AXL sequence variants in patients with idiopathic hypogonadotropic hypogonadism support the importance of AXL and the Tyro3, Axl, Mer (TAM) family in reproductive development.
GnRH neurons originate near the olfactory placode and migrate along olfactory nerves to the cribriform plate in which they turn caudally to target the hypothalamus. Projections extend to the median eminence to activate pituitary gonadotropin synthesis and ultimately control reproductive competence (1–4). Defects in migration and/or survival result in failure of normal sexual maturation in rodents and humans (5).
We initially identified the Tyro3, Axl, and Mer (TAM) family of receptor tyrosine kinases as differentially expressed in GnRH neuronal cells. Migratory NLT cells contain Axl and Tyro3, whereas Tyro3 and Mer are expressed in nonmigratory GT1-7 cells (6). Growth arrest specific gene 6 (Gas6) activation of Axl and Tyro3 mediated GnRH neuronal cell migration, survival, and gene expression (7–9). Classically, TAMs form homodimers or hetrodimers with each other or other receptor tyrosine kinases like mesenchymal epithelial transition factor (10). They have an intracellular carboxy-terminus containing a tyrosine kinase domain and an N-terminal extracellular domain containing two Ig-like domains and fibronectin type III (FNIII) repeats that are important for binding of the ligand Gas6 and protein processing, respectively. TAM receptors can also function independent of ligand via receptor/receptor interaction.
Analysis of mice null for both Axl and Tyro3 showed delayed first estrus, persistent abnormal estrus cyclicity (9), selective (38%) loss of GnRH neurons in the preoptic area during embryogenesis, and increased apoptosis along the migratory route, confirming a biological role in GnRH neuronal development. Based on the phenotype of these mice, we hypothesized that humans with Kallmann syndrome (KS) or normosmic idiopathic hypogonadotropic hypogonadism (nIHH) may harbor TAM mutations. In this report, we asked whether Axl null mice have reproductive defects and whether AXL mutations occur in human probands with KS or nIHH. We compared the frequency of mutations in AXL to prior putative causes of GnRH deficiency. Multiple assays were then performed to detect the functional importance of the human variants mutations in GnRH neuronal cells.
Patients and Methods
Phenotyping and ethnicity
KS or nIHH patients had absent or incomplete puberty by age 18 years and low sex steroids (serum T < 100 ng/dL in men or serum estradiol < 20 pg/mL in women) in the presence of low or normal gonadotropins; KS patients also had anosmia. No hypothalamic or pituitary abnormalities were detected with magnetic resonance imaging/computed tomography in an institutional review board-approved (Partners Healthcare System) study. Blood samples, health questionnaires, smell evaluation, and family history were gathered from participants and family members, when possible. Sense of smell was evaluated using the University of Pennsylvania Smell Identification Test (UPSIT). Subjects were given either a 12-item (when reporting congenital anosmia) or a 40-item test (when reporting at least partial sense of smell). Participants scoring in the fifth percentile or greater (based on gender and age) on the 40-item test were classified as normosmic and participants scoring less than the fifth percentiles were classified as anosmic. A normal sense of smell was confirmed if they answered nine or more items correctly (11). All probands and most of the control population from the 1000-genome database cohort have mainly European and African ancestry, and the National Heart, Lung, and Blood Institute (NHLBI) cohort are Caucasians from northern European descent.
Genotyping
The AXL coding region on chromosome 19q13.1–13.29 was sequenced in 104 unrelated carefully phenotyped subjects with KS or nIHH from the Massachusetts General Hospital database by PCR-based methodologies (Polymorphic DNA Technologies). Subject DNA was also sequenced in the 14 genes implicated in KS or nIHH (CHD7, FGF8, FGFR1, GNRH1, GNRHR, HS6ST1, KISS1, KISS1R, KAL1, NELF, PROK2, PROKR2, TAC3, and TACR3). The four detected mutations were compared with the 150 reproductively normal phenotyped and genotyped controls. In addition, the Axl mutants along with all published mutations in the 14 genes were examined in the Exome Variant Server, NHLBI Gene Ontology Exome Sequencing Project (ESP; Seattle, Washington; http://evs.gs.washington.edu/EVS/) and the 1000-genome project database (http://browser.1000genomes.org). Variants with published functional data supporting a biological effect of the mutation were then annotated to determine the frequency ranges for KS or nIHH variants.
Animals
Axl null mice were obtained from Greg Lemke (Salk Institute for Biological Studies, La Jolla, California) and established in a C57BL/6 × 129sv genetic background (12). Animal care and experimental procedures were performed as described in the Supplemental Methods, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org as per the Denver Veterans Affairs Institutional Animal Care and Use Committee guidelines. Female pups were checked daily for vaginal opening, within 1 week of weaning (d 21–23). Vaginal smears were collected daily until estrus and stained with Toluidine Blue.
Plasmids
Human AXL full-length plasmid pCMV6-XL5-human AXL was purchased from OriGene. Wild-type (WT) and mutant-human AXL constructs were made as described in the Supplemental Methods.
Cell culture and transfection
NLT from Sally Radovick (John Hopkins University, Baltimore, MD) and GT1-7 GnRH neuronal cells from Pamela Mellon (University of California San Diego, San Diego, California) were cultured as described in Supplemental Methods. GT1-7 cells that do not express endogenous AXL were transfected using Lipofectamine 2000 reagent (Life Technologies).
AXL protein processing
GT1-7 cells were serum starved for 12 hours after 24 hours of transfection. Cells were treated with the AXL ligand, Gas6 (400 ng/mL; Amgen), for 10 minutes, and the media were removed and concentrated using Centriplus (Millipore). Cell lysates and media were immunoblotted to detect AXL (Cell Signaling Technology) and cleaved AXL (R&D Systems). Densitometric analyses on three samples per group were performed for three separate experiments in duplicate.
Transwell migration assay
Transfected GT1-7 cells after 24 hours were serum starved for 5 hours. Cells were used in a migration assay in the absence and presence of Gas6 (13). Duplicate membranes (n = 6) in three separate experiments were evaluated.
Aggregation assay
GT1-7 neuronal cells were cultured to 80% confluence in 60-mm dishes, transfected, and stained during induction at 37°C and assayed for aggregation. After 24 hours, cells were trypsinized, resuspended in complete media, and passed through an 18-gauge syringe three times, diluted to 2 × 106/mL, and allowed to aggregate at 37°C on a rotary shaker at 100 rpm for 1 hour. Quantitation of aggregate formation was monitored under an inverted microscope. An aliquot of cell suspension was stained with Trypan Blue and cell aggregates were assessed using a hemocytometer. Aggregates having more than two cells were counted in duplicates and the total number of clumps per square from three separate experiments was plotted.
Statistical analysis
All data are expressed as mean ± SEM for each group and analyzed using GraphPad. Normality of data was determined using SAS (SAS Institute); data not found to be normal were natural log transformed. Statistical differences between two groups were analyzed using a Student's t test and data from receptor processing experiment were analyzed using nonparametric a Mann-Whitney U test. Multiple groups were analyzed using a two-way ANOVA with Tukey's multiple comparisions post hoc test. Values of P < .05 were considered statistically significant.
Results
Phenotype of AXL null mice
Deletion of both Axl and Tyro3 in mice results in a selective loss of GnRH neurons during embryogenesis and an adult reproductive phenotype of delayed sexual maturation and persistent proestrus (9). We therefore analyzed the phenotype of the Axl null mice, realizing that in many systems there is often redundancy and compensation with selective deletion of one family member. Axl−/− mice had no significant delay in vaginal opening compared with WT mice [34.8 ± 0.5 d (n = 30) in Axl−/− to 34.1 ± 0.6 d in WT]. However, they had a delay in first estrus to 38.4 ± 0.9 days (n = 28) compared with 35.5 ± 0.7 days (n = 12) in WT pups (P = .05) that was intermediate to that observed in Axl/Tyro3 null mice, which were delayed to 39.7 ± 1.3 days (n = 17) (P < .03). The time interval between vaginal opening and first estrus was also prolonged, averaging 3.6 days in Axl−/− mice compared with 1.4 days in WT and 5.6 days in Axl/Tyro3 null (P < .05). However, estrus cyclicity was restored in adulthood [4.5 d ± 0.2 in WT (n = 4) compared with 4.5 ± 0.26 d in Axl−/− (n = 6)], suggesting compensation. Together these data suggested an intermediate defect in reproductive function in mice with a single deleted TAM member and supported the evaluation of AXL mutations in humans.
AXL mutations are present in KS and nIHH patients
In a screen of 104 probands with KS or nIHH, four heterozygous novel AXL mutations were identified in two KS and two nIHH unrelated subjects (two males and two females. These included one intronic change 6 bp upstream from the start of exon 5 (c.586-6 C>T) and three missense mutants p.L50F, p.S202C, and p.Q361P (Figure 1). All probands were negative for 14 known genes implicated in KS or nIHH. One proband had whole-exome sequencing and was negative for newer candidates including WDR11, SEMA3A, SOX10, FGF17, IL17RD, DUSP6, SPRY4, and FLRT3.
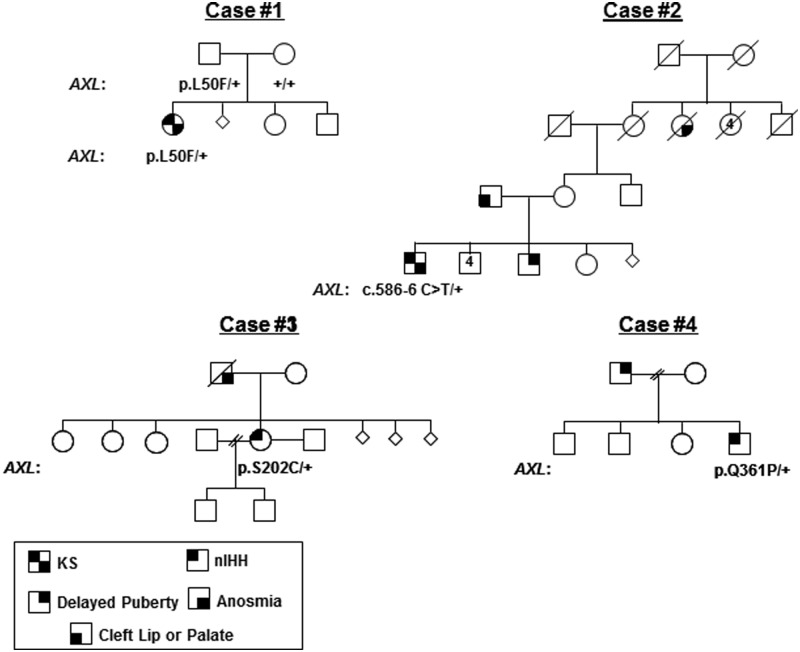
Figure 1.
Family pedigrees of probands identified with AXL mutations. Squares denote males and circles denote females. AXL mutation details are shown below each proband and phenotype details are presented in the text.
Clinical history and pedigree for probands with AXL
The pedigrees for the subjects are outlined in Figure 1.
Case 1
p.L50F was detected in a female proband with KS. Sense of smell was abnormal (only four of 12 correct using UPSIT scale). Frequent blood sampling (every 10 min) at age 24 years showed apulsatile LH (mean 0.42 IU/L). A second study at age 39 years confirmed the apulsatile pattern (mean 0.85 IU/L). Additional symptoms included bilateral clinodactyly, ostopenia, reduced elbow extension, and a flattened nasal bridge. The proband's mother required hormonal treatment to become pregnant. She screened negative for an AXL mutation. Interestingly, the p.L50F AXL substitution was detected in the proband's father, who had no history of delayed puberty.
Case 2
c.586-6C>T was detected in a male with KS and cryptorchidism. No formal smell testing was conducted. His father had a cleft palate and missing bicuspids, a brother reported constitutional delay of puberty (defined by completed growth at 18 y of age), and a maternal great aunt had anosmia.
Case 3
p.S202C was detected in a female proband with nIHH. No formal olfactory evaluations were conducted. At age 22 years, she had primary amenorrhea and was treated with estrogen and pulsatile GnRH. After her second cycle of pulsatile GnRH (75 ng/kg), she conceived and gave birth to a healthy son. Family history revealed an anosmic father, but none of her eight siblings had reproductive problems.
Case 4
p.Q361P was detected in a male subject with congenital nIHH and reversal after therapy (14). His sense of smell was normal and he scored in the 43rd percentile for men in his age on UPSIT scale (11). At age 19 years, testicular volumes were 7 mL (right) and 8 mL (left). Blood sampling every 10 minutes for 12 hours revealed a mean LH of 2.26 IU/L, pooled LH of 1.4 IU/L, LH mean amplitude of 1.6 IU/L, and 0.75 LH pulses using published criteria (15). At age 22 years, he showed increased LH activity: 7.5 IU/L mean LH, 5 IU/L pooled LH, 3.94 IU/L LH mean amplitude, and 9.5 LH pulses. The patient received no hormone therapy. Other phenotypic abnormalities included unilateral hearing loss, peripheral neuropathy, and learning disability. His father had constitutional delay of puberty. His three siblings were normal.
Analysis of human AXL mutations
Computer analysis to predict rarity of AXL variants
The intronic AXL variant c.586-6C>T and the missense AXL mutant p.S202C were not detected in the NHLBI-ESP (n = 6503) and 1000-genome databases and were considered to be novel variants. The p.L50F and p.Q361P mutants were detected in both databases at a frequency of 0.007% and 0.16%, respectively. To determine whether the low frequencies of p.L50F and p.Q361P were typical for KS or nIHH gene mutations, we determined the frequency of 227 published KS or nIHH variants. Forty-nine variants were found in the NHLBI-ESP database and 54 in the 1000-genome database (Supplemental Table 1). The minor allelic frequency for these established causes of human pubertal disorders ranged from 0.01% to 1.46%. Thus, the mutation frequencies of p.L50F and p.Q361P were well within the range of the other genes implicated in KS or nIHH and thus studied for their functional consequences.
In silico functional analysis
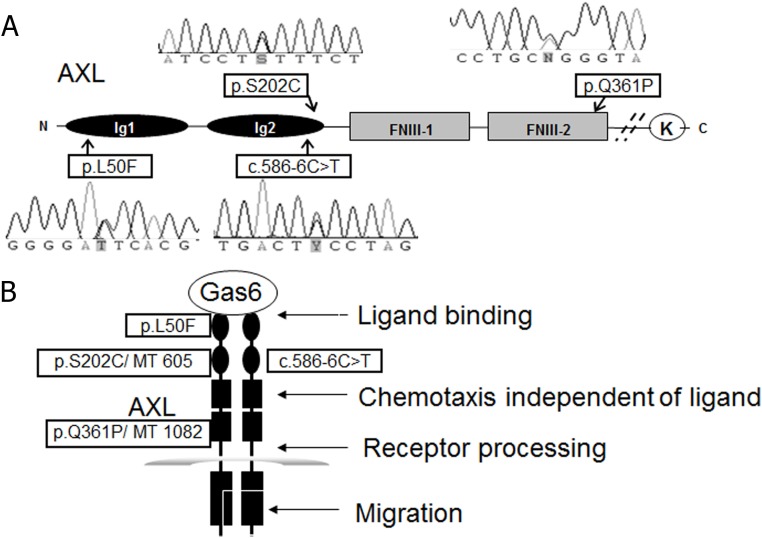
Three of the detected mutated residues were in highly conserved regions of the AXL extracellular domain (Figure 2-A). Table 1 summarizes mutations, locations, prevalence, predicted effect on protein function, and conservation. The intronic mutation c.585–6 C>T encodes the IG2 region and might be expected to alter splicing and thus stability of the protein (16). Leu50 in exon2 and Ser202 in exon5 encode for a change in the β-sheet B and F of the Ig1 and Ig2 domain, respectively, which have been shown to be important for the binding of the ligand, Gas6 (16). The p.Q361P mutation in exon8 encodes for a change in the second FNIII domain, which may serve a role in processing of AXL and is proximal to the site of cleavage of the N terminus, which occurs in many cells after ligand binding (17, 18). Predicted changes in protein domain structure by in silico protein prediction programs suggested the p.L50F mutation was not tolerated according to Sorting Tolerant From Intolerant (SIFT) (19) and p.Q361P is probably damaging according to Polyphen (20). Use of the Functional Analysis of Novel SNPs and Mutations Database (FANS) (21) predicted that the p.L50F mutation posed a medium risk and p.S202C mutation had a higher risk of perturbing AXL structure (20), whereas FANS predicted that p.Q361P is a nonconservative change that would alter protein function. The Mutation Taster tool (http://www.mutationtaster.org/) identified p.S202C as disease causing and predicted aberrant splicing resulting from the c.586-6 C>T mutation. Based on these data, we tested the mutants in the multiple functional assays as outlined in Figure 2B.
Figure 2.
A, Location of mutations within schema of AXL receptor molecule. B, The strategies to assess the effects of the mutations on Gas6 ligand binding, chemotaxis independent of ligand, receptor processing, and migration. K, tyrosine kinase; N, N terminus; C, C terminus.
Table 1.
In Silico Predictions of AXL Mutations: Nucleotide and Amino Acid Locations, Mutation Frequency, Conservation of Altered Residues, and in Silico Predicted Changes
| Case | Amino Acid Change | Nucleotide Change/Zygosity | Genomic Location |
In Silico Predicted Effect |
Conservation | |||
|---|---|---|---|---|---|---|---|---|
| Polyphen | SIFT | FANS | Mutation Taster | |||||
| 1 | L50F | c.148 C>T/Het | Exon 2 | Benign | Not tolerated | Medium risk | Polymorphism | M, R, D, C |
| 2 | — | c.586 − 6 C>T/Het | Intron 4 | — | — | — | Aberrant splicing of exon 5 & 6 | M, R, D, C |
| 3 | S202C | c.605 C>G/Het | Exon 5 | Benign | Tolerated | High risk | Disease causing | M, R, D, C, Z |
| 4 | Q361P | c.1082 A>C/Het | Exon 8 | Probably damaging | Not tolerated | High risk | Polymorphism | M, R, D, C |
Abbreviations: C, cow; D, dog; Het, heterozygous; M, mouse; R, rat; SIFT, Sorting Tolerant From Intolerant; Z, zebrafish.
Intronic AXL mutation did not alter in vitro splicing
To evaluate the significance of the intronic mutation 6 bp proximal to the start of exon 5, minigene constructs were created containing WT AXL or the c. 586-6C>T mutation, which is 6 bp upstream of the putative splice site and tested (Supplemental Figure 1A). No abnormal spliced products were detected and no difference in splicing efficiency was observed after RT-PCR (Supplemental Figure 1B). Thus, this single mutation alone was not sufficient to disrupt exon splicing.
Effects of AXL mutations on Gas6 binding determined by surface plasmon resonance (SPR)
The kinetics of interaction between the ligand, Gas6, with purified WT or mutant AXL extracellular domains were assessed using SPR (Supplemental Figure 2, A–C). The p.L50F and p.S202C mutations occur in regions reported to be important in binding of AXL to Gas6, and p.Q361P, a mutation in the FNIII domain whose function is not clear, were expected to have some effect on the binding of Gas6 (17). To our surprise, SPR revealed no significant differences in the binding kinetics of Gas6 ligand to mutant AXL proteins compared with WT AXL proteins. Thus, these mutations alone do not disrupt Gas6 ligand binding. We next examined their effects downstream on the signal transduction process.
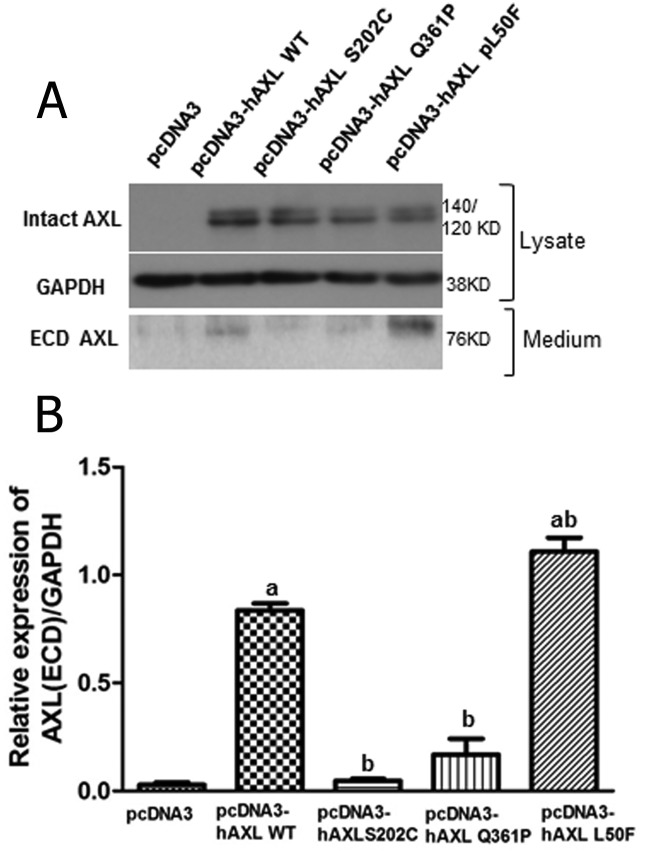
Effects of AXL mutations on Gas6-induced AXL processing
After Gas6 activation, AXL is phosphorylated and the amino-terminal portion of the molecule subsequent to the FNIII domain is cleaved to allow free N-terminal fragment and processing of the intracellular C-terminal kinase domain (17, 22, 23). Overexpression of both WT and mutant proteins in GT1-7 cells showed cleaved 76-kDa AXL extracellular domain (ECD) in the media (Figure 3 A). Compared with vector, WT AXL media contained increases in the AXL ECD (P < .05). Both the p.S202C and p.Q361P mutants demonstrated decreased receptor processing (30% and 28%, respectively, P < .05, Figure 3B) compared with WT-AXL, similar to vector controls. The p.L50F mutant augmented rather than decreased receptor processing. These data suggest that the signal from the ligand is not transmitted effectively through two of the AXL mutants, p.S202C and p.Q361P, resulting in inefficient receptor processing.
Figure 3.
Functional characterization of mutant AXL residues roles on Gas6-dependent AXL processing. A, AXL cleavage was determined in GT1-7 GnRH neuronal cells expressing WT or mutant AXL-Fc protein. For the cleavage assay, whole-cell protein lysates or concentrated media supernatant were immunoblotted to detect intact and extracellular AXL (ECD) levels. B, Bar graphs depicting effect of Gas6 on the mean relative expression of cleaved AXL (ECD) to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in vector, WT AXL, and mutant AXL overexpressing GT1-7 GnRH neuronal cells. Letters represent significant difference from each other (a, difference from vector control; b, difference from WT AXL overexpressing cells) (P < .05, n = 3).
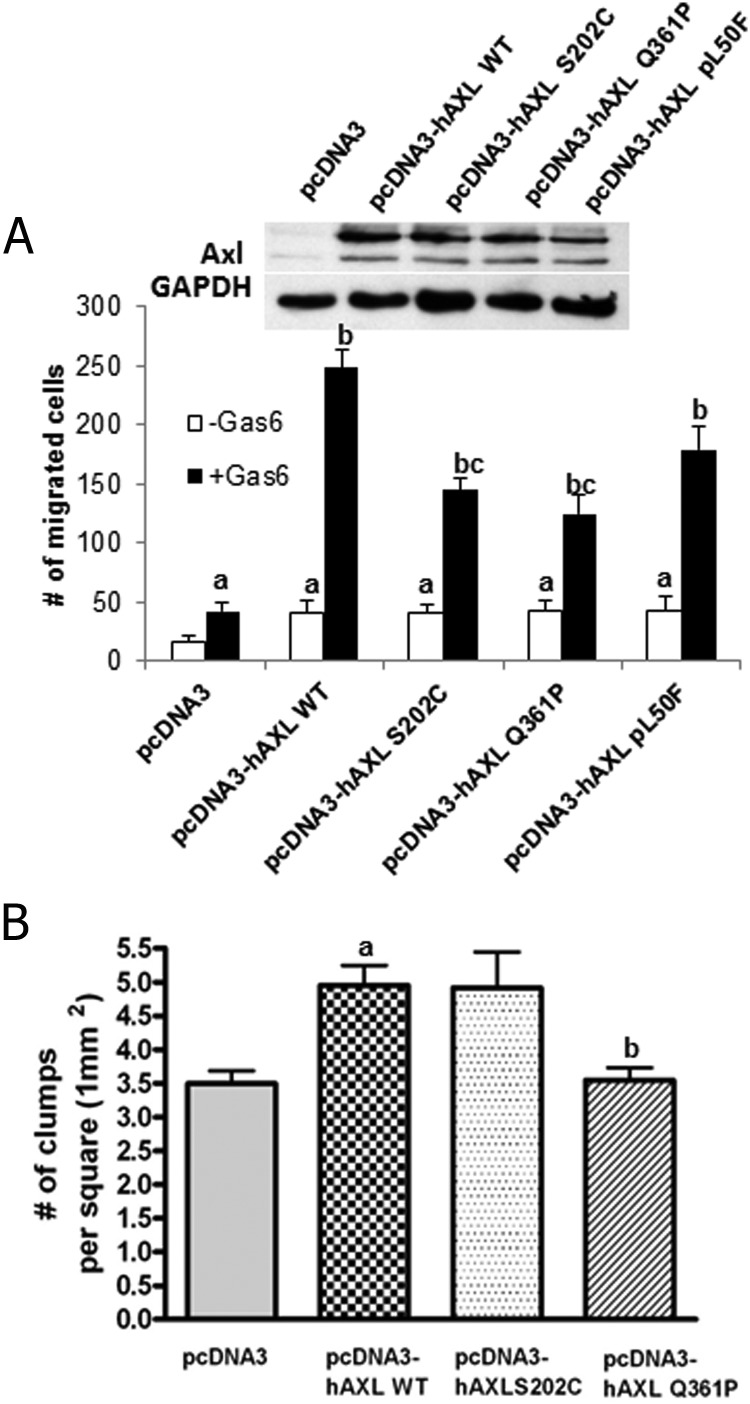
Effects of AXL mutations on Gas6-induced AXL mediated neuronal migration
To assess the effects of the mutants on GnRH neuronal cell migration, a transwell assay was performed. Overexpression of WT or mutant AXL was confirmed using immunoblot (Figure 4A, inset). Gas6 induced basal migration in GT1-7 neuronal cells transfected with vector control as compared with the PBS-treated controls (42 ± 7.7 vs 17 ± 1.5, P < .05) (Figure 4A). Cells expressing each of the mutant-AXL constructs (p.S202C, p.Q361P, and p.L50F) showed similar basal migration compared with WT AXL (41 ± 2.9, 42 ± 3.7, and 42 ± 5.4 vs 40 ± 5.8, P = NS). Gas6 increased the rate of migration 5.8-fold in WT AXL cells (247 ± 16.4 vs 40 ± 4.4 in WT AXL, P < .05). Cells expressing the p.S202C and p.Q361P mutants showed a loss of Gas6-induced migration to 3.4- and 2.9-fold, respectively, and 124 ± 16, P < .05, respectively). This reflects a defect in ligand induced neuronal migration of 41% and 49%, respectively (P < .05) compared with WT. Cells expressing the p.L50F mutant showed a nonsignificant impairment of migration compared with WT (27%, P = NS). These data suggest that both the AXL Ig2 domain and FNIII domain are required for optimal Gas6-induced GnRH neuronal cell migration.
Figure 4.
AXL mutation alters Gas6-induced GnRH neuronal migration and alters aggregation/chemotaxis in GT1-7 cells. A, GT1-7 cells overexpressing WT or mutant AXL were tested for functional effects on migration. Bar graph depicts effect of WT and mutant AXL overexpression (as shown in inset) on migration of GT1-7 neurons in the presence Gas6. The average number of cells that have migrated is shown and expressed as mean ± SEM, and letters represent significant difference from each other (a, difference from vector control and PBS treated group; b, difference from vector control and Gas6 treated group; c, difference from WT AXL overexpressing cells treated with Gas6) (P ≤ .05, two way ANOVA with post hoc Tukeys multiple comparisons test, n = 3). B, GT1-7 cells overexpressing WT or mutant AXL were tested for their aggregation ability. The number of clumps formed for each condition is shown. Letters represent significant difference from each other (a, difference from vector control; b, difference from WT AXL overexpressing cells) (n = 3). *, P ≤ .05.
Altered aggregation/chemotaxis with mutant AXL proteins
TAM expression on the cell surface is important for adhesive and chemotactic effects independent of Gas6 (18, 24), which may be important in GnRH neuron migration in vivo. To test the effects AXL mutations on adhesive interactions, WT and mutant AXL (p.S202C and p.Q361P) were overexpressed in GT1-7 GnRH neuronal cells (Figure 4B). Because mutant p.L50F did not show any significant defects in the previous functional assays, it was not tested. Overexpression of p.S202C mutation resulted in an approximately 30% increase in aggregation of the neuronal cells (P < .05), which was similar to the effects of WT AXL. Expression of the p.Q361P mutation, however, resulted in complete loss of aggregation, similar to cells expressing the empty vector and significantly different from cells expressing WT AXL or the p.S202C mutation (P < .05). Thus, the extracellular FNIII region is critical in ligand-independent cross talk of cell surface AXL molecules to promote adhesion.
Discussion
Based on the functional consequences of Axl/Tyro3 deletion on reproductive function and GnRH neuron development in mice, we hypothesized that selective mutation in TAM family members might have a reproductive phenotype in mice and may play a role in humans with disorders of pubertal onset or progression. More subtle reproductive defects were detected in mice null for Axl alone compared with our prior studies in Axl/Tyro3 null mice, suggesting the importance of cross talk between and the potential compensation by other TAM members, Tyro3 and Mer, in vivo. These animal studies justified a screen of human probands with GnRH deficiency.
In a screen of 104 probands, we detected no homozygous mutations but a 1.9% incidence of heterozygous mutations, which is higher than many other candidates implicated in KS or niHH (reviewed in Supplemental Table 1 and reference 25). Mutations were detected in both sexes and across a spectrum of phenotypes, including one patient with reversal of his reproductive defect after hormonal priming. Our screen of two large databases for the frequency of detected AXL mutations compared with 14 known genes revealed that of 227 published KS or nIHH variants, 49 were found in the NHLBI-ESP database and 54 were found in the 1000-genome database. When we assessed the frequency of the 24 mutations in eight KS or nIHH genes in which functional studies actually confirmed a defect (Supplemental Table 1), the minor allelic frequency ranged from 0.01% to 1.46%. Thus, the frequencies of the detected AXL mutants were well within this range. Prior pathogenic mutations from earlier studies were described as novel due to them being absent in control populations. The availability of larger genomic databases now reveals that many novel mutations are reclassifiable as rare variants and the strategies to evaluate rare variants for pathogencity require testing for functional effects using multiple approaches (26). The low penetrance and lower allelic frequency can help in identifying only the real mutations along with additional focus required to study the importance of oligogenicity (27) in this syndrome for preserving reproductive competence.
Based on multiple computer algorithms, the mutations were predicted to have deleterious functional effects; however, the p.L50F mutation, which was not detected in the databases but present in the proband's asymptomatic father, was not different from WT AXL in multiple functional assays. In contrast, the S202C and p.Q361P mutants showed defective receptor processing and diminished GnRH neuronal cell migration demonstrating the role of both the Ig2 and FNIII domains to mediate ligand-dependent effects. The defect in the adhesive properties of the p.Q361P mutation supports the importance of the FNIII region in ligand-independent actions of AXL. Together these human AXL mutations give novel insights into the structural domains that mediate ligand-dependent and -independent effects of the TAM proteins (10, 18, 28).
TAM family members contain a fibronectin domain that has been shown to bind heparan sulfate proteoglycans and alter ligand/receptor activation in other systems (reviewed in reference 29). This adaptor feature has been demonstrated for anosmin (30), fibroblast growth factor 8/fibroblast growth factor receptor 1 (31–33), and prokineticin 2/prokineticin receptor 2 (34, 35) all reported to be mutated in subjects with KS or niHH. Mutations in HS6ST1 in individuals with nIHH may contribute to defects in GnRH signaling through these ligand-receptor systems (36). In addition, Gas6/TAMs may cross talk with other transmembrane receptors like mesenchymal epithelial transition factor as we have recently shown (10) or G-protein coupled receptors via heparan sulfate proteoglycans (37). Mutations in these adaptor molecules may be implicated in future analyses of reproductive disorders.
Taken together, our data suggest that single mutations in AXL impair but do not destroy TAM function and raise the possibility that concomitant mutations in the ligand Gas6, or other TAM family members, or heterodimeric receptor partners may underlie the functional defects in these probands. In addition, because our prior studies showed that AXL may form heterodimers with Tyro3, single mutations in AXL may be compensated for by endogenous Tyro3 (28). Mutations in other components of this pathway may also represent targets for digenicity as has been recently suggested for the fibroblast growth factor/fibroblast growth factor receptor 1 interactome. The existence of an olfactory phenotype in a proband would also suggest a second mutation in addition to those in AXL. Because 60%–70% of subjects with KS or niHH do not have a known mutation (38), further characterization of novel candidates is important to understand the mechanism of the human disorder as well as shed light into the complexities of normal reproductive axis development. Because the Axl/Tyro3 null mouse show delayed sexual maturation with persistent estrus abnormalities (9) and an impaired LH surge mechanism (39), TAM family mutations should also be sought in probands with hypothalamic amenorrhea and oligoovulatory disorders. The wide spectrum of phenotypes with known genetic mutations (40) should direct us to broaden our perspective on the potential genetic defects in patients with acquired reproductive disorders.
Acknowledgments
We thank Angela Pierce and Brian Bliesner for their efforts to characterize the reproductive phenotype of AXL null mice. Surface Plasmon Resonance experiments were conducted in the Biophysics Core Facility at the University of Colorado School of Medicine. We thank Margaret Au (Massachusetts General Hospital) for her help in the initial mutant screening and Nelly Pitteloud (Centre Hospitalier Universitaire Vaudois) for her valuable inputs during the preparation of this manuscript. We acknowledge the NHLBI Gene Ontology Exome Sequencing Project, the Lung Gene Ontology Sequencing Project (HL-102923), the Women's Health Initiative Sequencing Project (HL-102924), the Broad Gene Ontology Sequencing Project (HL-102925), the Seattle Gene Ontology Sequencing Project (HL-102926), and the Heart Gene Ontology Sequencing Project (HL-103010).
This work was supported by National Institute of Health Grant HD31191 (to M.E.W.) and Grant U54 HD28138 (to W.F.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ECD
- extracellular domain
- ESP
- Exome Sequencing Project
- FANS
- Functional Analysis of Novel SNPs and Mutations Database
- FNIII
- fibronectin type III
- Gas6
- growth arrest-specific gene 6
- KS
- Kallmann syndrome
- NHLBI
- National Heart, Lung, and Blood Institute
- nIHH
- normosmic idiopathic hypogonadotropic hypogonadism
- SPR
- surface plasmon resonance
- TAM
- Tyro3, Axl, and Mer
- UPSIT
- University of Pennsylvania Smell Identification Test
- WT
- wild type.
References
- 1. Tobet SA, Schwarting GA. Minireview: recent progress in gonadotropin-releasing hormone neuronal migration. Endocrinology. 2006;147:1159–1165 [DOI] [PubMed] [Google Scholar]
- 2. Wierman ME, Pawlowski JE, Allen MP, Xu M, Linseman DA, Nielsen-Preiss S. Molecular mechanisms of gonadotropin-releasing hormone neuronal migration. Trends Endocrinol Metab. 2004;15:96–102 [DOI] [PubMed] [Google Scholar]
- 3. Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA. 1989;86:8132–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–164 [DOI] [PubMed] [Google Scholar]
- 5. Sykiotis GP, Pitteloud N, Seminara SB, Kaiser UB, Crowley WF., Jr Deciphering genetic disease in the genomic era: the model of GnRH deficiency. Sci Transl Med. 2010;2:32rv32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fang Z, Xiong X, James A, Gordon DF, Wierman ME. Identification of novel factors that regulate GnRH gene expression and neuronal migration. Endocrinology. 1998;139:3654–3657 [DOI] [PubMed] [Google Scholar]
- 7. Allen MP, Linseman DA, Udo H, et al. Novel mechanism for gonadotropin-releasing hormone neuronal migration involving Gas6/Ark signaling to p38 mitogen-activated protein kinase. Mol Cell Biol. 2002;22:599–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allen MP, Zeng C, Schneider K, et al. Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt. Mol Endocrinol. 1999;13:191–201 [DOI] [PubMed] [Google Scholar]
- 9. Pierce A, Bliesner B, Xu M, et al. Axl and Tyro3 modulate female reproduction by influencing gonadotropin-releasing hormone neuron survival and migration. Mol Endocrinol. 2008;22:2481–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salian-Mehta S, X M, Wierman ME. AXL and MET crosstalk to promote gonadotropin releasing hormone (GnRH) neuronal cell migration and survival. Mol Cell Endocrinol. 2013;374:92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178 [DOI] [PubMed] [Google Scholar]
- 12. Lu Q, Gore M, Zhang Q, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728 [DOI] [PubMed] [Google Scholar]
- 13. Allen MP, Xu M, Linseman DA, et al. Adhesion-related kinase repression of gonadotropin-releasing hormone gene expression requires Rac activation of the extracellular signal-regulated kinase pathway. J Biol Chem. 2002;277:38133–38140 [DOI] [PubMed] [Google Scholar]
- 14. Nachtigall LB, Boepple P, Pralong FP, Crowley WF., Jr Adult-onset idiopathic hypogonadotropic hypogonadism—a treatable form of male infertility. N Engl J Med. 1997;336:410–415 [DOI] [PubMed] [Google Scholar]
- 15. Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE. Free α-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab. 1999;84:1028–1036 [DOI] [PubMed] [Google Scholar]
- 16. Sasaki T, Knyazev PG, Clout NJ, et al. Structural basis for Gas6-Axl signalling. EMBO J. 2006;25:80–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellosta P, Costa M, Lin DA, Basilico C. The receptor tyrosine kinase ARK mediates cell aggregation by homophilic binding. Mol Cell Biol. 1995;15:614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Costa M, Bellosta P, Basilico C. Cleavage and release of a soluble form of the receptor tyrosine kinase ARK in vitro and in vivo. J Cell Physiol. 1996;168:737–744 [DOI] [PubMed] [Google Scholar]
- 19. Nq P, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu CK, Chen YH, Tang CY, et al. A Functional analysis of novel SNPs and mutations in human and mouse genomes. BMC Bioinformatics. 2008;9(suppl 12):S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fridell YW, Jin Y, Quilliam LA, et al. Differential activation of the Ras/extracellular-signal-regulated protein kinase pathway is responsible for the biological consequences induced by the Axl receptor tyrosine kinase. Mol Cell Biol. 1996;16:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Bryan JP, Frye RA, Cogswell PC, et al. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295–304 [DOI] [PubMed] [Google Scholar]
- 25. Brioude F, Bouligand J, Trabado S, et al. Non-syndromic congenital hypogonadotropic hypogonadism: clinical presentation and genotype-phenotype relationships. Eur J Endocrinol. 162:835–851 [DOI] [PubMed] [Google Scholar]
- 26. Norton N, Rieder MJ, Züchner S, et al. National Heart, Lung and Blood Institute GO Exome Sequencing Project. Evaluating pathogenicity of rare variants from dilated cardiomyopathy in the exome era. Circ Cardiovasc Genet. 2012;5:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dodé C, Rondard P. PROK2/PROKR2 signaling and Kallmann syndrome. Front Endocrinol (Lausanne). 2013;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pierce ABB, Xu M, Tobet S, Lemke G, Wierman ME. Axl and Tyro3 regulate GnRH neuron migration and modulate female reproductive function. Paper presented at: 90th Annual Meeting of The Endocrine Society; 2008; San Francisco, CA [Google Scholar]
- 29. Wierman ME, Kiseljak-Vassiliades K, Tobet S. Gonadotropin-releasing hormone (GnRH) neuron migration: initiation, maintenance and cessation as critical steps to ensure normal reproductive function. Front Neuroendocrinol. 2011;32(1):43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bulow HE, Berry KL, Topper LH, Peles E, Hobert O. Heparan sulfate proteoglycan-dependent induction of axon branching and axon misrouting by the Kallmann syndrome gene kal-1. Proc Natl Acad Sci USA. 2002;99:6346–6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dode C, Hardelin JP. Kallmann syndrome: fibroblast growth factor signaling insufficiency? J Mol Med. 2004;82:725–734 [DOI] [PubMed] [Google Scholar]
- 32. Gonzalez-Martinez D, Kim SH, Hu Y, et al. Anosmin-1 modulates fibroblast growth factor receptor 1 signaling in human gonadotropin-releasing hormone olfactory neuroblasts through a heparan sulfate-dependent mechanism. J Neurosci. 2004;24:10384–10392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim SH, Hu Y, Cadman S, Bouloux P. Diversity in fibroblast growth factor receptor 1 regulation: learning from the investigation of Kallmann syndrome. J Neuroendocrinol. 2008;20:141–163 [DOI] [PubMed] [Google Scholar]
- 34. Pitteloud N, Zhang C, Pignatelli D, et al. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 2007;104:17447–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monnier C, Dode C, Fabre L, et al. PROKR2 missense mutations associated with Kallmann syndrome impair receptor signalling activity. Hum Mol Genet. 2009;18:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tornberg J, Keefe K, Plummer L, et al. Heparan sulfate 6-O-sulfotransferase 1, a gene involved in extracellular sugar modifications, is mutated in patients with idiopathic hypogonadotrophic hypogonadism. Proc Natl Acad Sci USA. 2011;108:11524–11529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Melaragno MG, Wuthrich DA, Poppa V, et al. Increased expression of Axl tyrosine kinase after vascular injury and regulation by G protein-coupled receptor agonists in rats. Circ Res. 1998;83:697–704 [DOI] [PubMed] [Google Scholar]
- 38. Dodé C, Hardelin JP. Kallmann syndrome. Eur J Hum Genet. 2009;17:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pierce A, Bliesner B, Liu Z, et al. Hypothalamic but not pituitary or ovarian defects underlie the reproductive abnormalities in Axl/Tyro3 null mice. Mol Cell Endocrinol. 2011;339:151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caronia LM, Welt CK, Sykiotis GP, et al. A genetic basis for functional hypothalamic amenorrhea. N Engl J Med. 2011;364:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]