Abstract
Background:
Subclinical hypothyroidism is common in the elderly, yet its relationship with weight and body composition is unclear.
Objective:
We examined the relationship between subclinical hypothyroidism and weight change and body composition in older adults.
Methods:
A total of 427 subclinically hypothyroid and 2864 euthyroid U.S. individuals ≥65 years old enrolled in the Cardiovascular Health Study and not taking thyroid preparations were included. Analyses of 6-year weight change were performed, compared by thyroid status. A cross-sectional analysis of thyroid status and body composition was performed in a subset of 1276 participants who had dual-energy x-ray absorptiometry scans. Models were risk factor-adjusted and stratified by sex.
Results:
Overall, participants lost weight during follow-up (−0.38 kg/y in men, −0.37 kg/y in women). Subclinical hypothyroidism, when assessed at a single time point or persisting over 2 years, was not associated with a difference in weight change compared with euthyroidism. Subclinical hypothyroidism was also not associated with differences in lean mass, fat mass, or percent fat compared with euthyroidism. A TSH level 1 mU/L higher within the euthyroid or subclinical hypothyroid range was associated with a 0.51-kg higher baseline weight in women only (P < .001) but not with weight change in either sex. A 1 ng/dL higher free T4 level was associated with lower baseline weight and 0.32 kg/y greater weight loss in women only (P = .003). Baseline weight and weight change did not differ by T3 levels.
Conclusions:
Our data do not support a clinically significant impact of subclinical hypothyroidism on weight status in the elderly.
Subclinical hypothyroidism in the elderly is of unclear clinical significance, with inconsistent findings across observational studies and a lack of consensus regarding treatment (1). Frequently, patients with overt hypothyroidism complain of weight gain, and treatment of overt hypothyroidism may result in modest weight loss (2). However, the association between subclinical hypothyroidism and weight is less well characterized, especially in the elderly. Obesity is an increasingly concerning global health problem associated with increased morbidity and a shortened life span (3–5). The strength of association between higher weight and mortality is weaker in the elderly (6, 7), and other measures of adiposity such as lean and fat mass may be better predictors of mortality risk in this population.
It has been suggested in studies of middle-aged adults that subclinical hypothyroidism and higher TSH within the euthyroid range are related to higher weight and body mass index (BMI) (8–12) or higher fat mass (13, 14). However, several studies in middle-aged adults have found that higher T3 levels in euthyroid or subclinically hypothyroid patients are also related to higher weight and BMI (8, 12, 14, 15), suggesting that thyroid hormone-induced changes in metabolic rate may not explain the relationship between subclinical hypothyroidism and weight. This calls into question the direction of causality between subclinical thyroid disease and body composition. Few studies have evaluated subclinical hypothyroidism and weight in the elderly, and no studies of older participants were longitudinal or included assessments of T3 and free T4 (FT4) levels.
The Cardiovascular Health Study (CHS) is a large cohort study of community-dwelling individuals aged 65 years and older that included both subclinically hypothyroid and euthyroid participants. Using data from the CHS, we sought to assess the relationship between thyroid function and weight change and body composition in an elderly population.
Subjects and Methods
Study population
The CHS is a population-based, longitudinal study of 5888 adults aged 65 and older (16). Enrollment of the original cohort of 5201 adults occurred between May 1989 and June 1990, and an additional cohort of 687 African Americans was enrolled in 1992 and 1993. Eligible individuals were identified from an age- and sex-stratified random sample of the Medicare eligibility rosters in four U.S. communities: Washington County, Maryland; Allegheny County, Pennsylvania; Sacramento County, California; and Forsyth County, North Carolina. To be eligible, individuals had to be noninstitutionalized, expecting to remain in the area for the following 3 years, not in active treatment for cancer, not wheelchair-bound at home, not requiring a proxy respondent at entry, and capable of providing medical consent. Household members of the sampled individuals were recruited, if eligible. The institutional review boards of all 4 sites and the coordinating center at the University of Washington in Seattle approved the study. All participants gave informed consent.
Annual study visits through 1999 included a detailed medical history, physical examination, and assessment of health status. Blood was drawn after a 12-hour fast, and serum was processed and stored in −70°C freezers for future testing.
Thyroid function testing
Serum TSH was measured in 2009 and 2010 from banked samples from both cohorts obtained at the 1992/1993 (n = 3996) and 1994/1995 (n = 4005) visits. FT4 and total T3 were measured in all participants at the 1992/1993 visit and in those with abnormal TSH levels at the 1994/1995 visit. Thyroid function assays were performed at the CHS Central Blood Analysis Laboratory at the University of Vermont using chemiluminescent immunoassays on the Elecsys 2010 analyzer (Roche Diagnostics). TSH concentrations were measured with a third-generation assay with a functional sensitivity of 0.005 mU/L and 2.1% intra-assay and 3.1% interassay coefficients of variation. The FT4 assay had a functional sensitivity of 0.23 ng/dL (2.96 pmol/L) and 1.7% intra-assay and 3.3% interassay coefficients of variation. A reference range of 0.7 to 1.7 ng/dL (9–22 pmol/L) was used. The total T3 assay had a functional sensitivity of 19.5 ng/dL (0.3 nmol/L), reference range of 84.4 to 201.3 ng/dL (1.3–3.1 nmol/L), and 4.2% intra-assay and 4.7% interassay coefficients of variation.
Assessment of outcome variables
Anthropometric measures were performed by trained personnel using standardized protocols. Standing height was measured at the 1992/1993 visit using a stadiometer calibrated in centimeters. Weight was measured annually through 1999 using a balance-beam scale calibrated in kilograms. BMI was calculated as weight in kilograms divided by height in meters squared. Dual-energy x-ray absorptiometry (DXA) was performed in 1994 and 1995 at the Pennsylvania and California sites only. Whole-body DXA scans were obtained using Hologic QDR-2000 densitometers (Hologic, Inc). Blinded radiologists read the scans at the University of California, San Francisco, Reading Center using Hologic software version 7.10.
Assessment of covariates
Thyroid medication use was assessed annually via examination of medication bottles. Covariates assessed at the 1992/1993 visit included age, race, smoking status (never, former, or current), kilocalories per week of self-reported physical activity, and self-reported health.
Statistical analysis
Characteristics of participants who were not taking thyroid hormone preparations and were either subclinically hypothyroid (TSH 4.51–19.99 mU/L with normal FT4) or euthyroid (TSH 0.45–4.50 mU/L) in 1992/1993 were compared using χ2 or t tests. Generalized estimating equations (GEEs) with an exchangeable correlation structure were used to examine the relationship between thyroid status in those who were subclinically hypothyroid or euthyroid at a single time point (the 1992/1993 visit) and annual weight change through the 1998/1999 visit, in participants who had at least 1 additional weight measurement during follow-up. In additional analyses, GEEs were used to analyze the relationship between continuous measures of TSH, FT4, and total T3 and annual weight change in the combined group of subclinically hypothyroid and euthyroid individuals at the 1992/1993 visit. Weight change through 1998 and 1999 was also analyzed using GEEs in those who were euthyroid or subclinically hypothyroid at both 1992/1993 and 1994/1995 visits (persistently euthyroid or persistently or transiently subclinically hypothyroid). Linear regression was used to assess the relationship between the change in TSH and change in weight between the 1992/1993 and 1994/1995 visits, excluding subjects with changes in TSH of more than 10 mU/L. Scatterplots were used to display the association. All analyses were adjusted for age, race, smoking, activity level, self-reported health, and thyroid hormone initiation and were sex-stratified unless otherwise noted. Because thyroid hormone initiation could be influenced by thyroid and weight status, analyses were repeated without adjustment for thyroid hormone initiation.
Participants who were not taking thyroid hormone preparations and were either subclinically hypothyroid (n = 118) or euthyroid (n = 1158) in 1994/1995 were included in analyses of body composition by DXA (lean body mass, absolute and percent total body fat, and arm and trunk fat). Cross-sectional associations were examined using linear regression to compare individuals with subclinical hypothyroidism with those with euthyroidism and to analyze continuous measures of TSH and associations with DXA outcomes. Analyses were adjusted for age, race, smoking, activity level, and self-reported health and were sex-stratified.
Results
Of the 3996 participants with TSH in 1992 and 1993, 3789 were in the euthyroid or subclinical hypothyroid range and 3520 remained after excluding participants taking thyroid medications. An additional 229 participants did not have at least 2 measurements of weight at the 1992/1993 visit or beyond and were excluded, leaving 3291 in the analysis. Slightly fewer had T3 (n = 3248) or FT4 (n = 3268) measurements. There were 427 with subclinical hypothyroidism (268 women and 159 men) and 2864 who were euthyroid (1623 women and 1241 men) (Table 1). Compared with euthyroid women, women with subclinical hypothyroidism were more likely to be Caucasian but were otherwise similar. Subclinically hypothyroid men were slightly older, more likely to be Caucasian, and less likely to have ever smoked than euthyroid men. FT4 levels were lower in those with subclinical hypothyroidism, whereas total T3 levels were slightly higher in women with subclinical hypothyroidism compared with their euthyroid counterparts. Sixty-four (2.2%) of the euthyroid participants and 105 (24.6%) of the subclinically hypothyroid participants initiated thyroid medications during follow-up.
Table 1.
Characteristics of Study Sample at Baseline (1992/1993)a
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Subclinical Hypothyroid, n = 268 | Euthyroid, n = 1623 | P Value | Subclinical Hypothyroid, n = 159 | Euthyroid, n = 1241 | P Value | |
| TSH, mU/L | 6.7 (2.6) | 2.2 (1.0) | NA | 6.7 (2.6) | 2.1 (0.92) | NA |
| Age, y | 74.9 (5.3) | 74.4 (5.1) | .11 | 76.1 (5.7) | 74.7 (5.3) | .001 |
| Caucasian | 240 (89.6) | 1291 (79.5) | <.001 | 144 (90.6) | 1041 (83.9) | .028 |
| Activity level, kcal | 675 (226–1481) | 735 (245–1725) | .64b | 1072 (547–2102) | 1117 (430–2375) | .89b |
| Ever smoker | 106 (39.6) | 700 (43.1) | .27 | 102 (64.1) | 900 (72.5) | .028 |
| Good to excellent self-reported health | 219 (81.7) | 1288 (79.4) | .37 | 134 (84.3) | 1001 (80.7) | .27 |
| BMI, kg/m2 | 27.1 (5.3) | 26.8 (5.3) | .48 | 26.6 (3.8) | 26.7 (3.8) | .59 |
| T3, ng/dL | 1.86 (0.36) | 1.80 (0.34) | .009 | 1.74 (0.28) | 1.77 (0.30) | .24 |
| FT4, ng/dL | 1.07 (0.17) | 1.21 (0.17) | <.001 | 1.10 (0.17) | 1.23 (0.17) | <.001 |
Abbreviations: IQR, interquartile range; NA, not available.
Data are expressed as number (percentage), except for age, TSH, T3, and FT4, which are expressed as mean (SD) and activity level, which is expressed as median (IQR). NA indicates comparisons not performed due to selection of groups based on this measure.
According to t test of mean difference on the natural log scale.
Overall, women experienced an average annual weight loss of 0.37 kg/y and men experienced an average annual weight loss of 0.38 kg/y over 6 years of follow-up. Annual weight change did not differ between subclinically hypothyroid and euthyroid individuals in adjusted models (women, loss of 0.35 vs loss of 0.38 kg/y, P = .63; men, loss of 0.34 vs loss of 0.38 kg/y, P = .48; Table 2). A 1 mU/L higher TSH was associated with a 0.51-kg higher baseline weight in women (P < .001) with no difference in men and no association between TSH as a continuous measure and weight change in either sex (Table 2). Baseline total T3 had no association with baseline weight or weight change. A 1 ng/dL higher FT4 was associated with a 4.60-kg lower baseline weight in women (P = .005) and a 0.32-kg greater annual weight loss (P = .003) in women only. Results were unchanged in analyses without adjustment for thyroid hormone initiation (Supplemental Table 1, published on The Endocrine Society's Journals Online website at http://jcem.endojournals.org).
Table 2.
Association of Thyroid Function in 1992/1993 With Average Annual Change in Weight Through 1998/1999a
| Womena | P Value | Mena | P Value | |
|---|---|---|---|---|
| Average weight change/y | ||||
| Euthyroid | −0.38 (−0.42 to −0.34) | −0.38 (−0.43 to −0.34) | ||
| Subclinical hypothyroid | −0.35 (−0.45 to −0.26) | .63b | −0.34 (−0.46 to −0.21) | .48b |
| TSH, effect per mU/L | ||||
| Initial weight | 0.51 (0.24 to 0.79) | <.001 | 0.03 (−0.30 to 0.36) | .86 |
| Average annual weight changec | 0.01 (−0.01 to 0.03) | .23 | 0.01 (−0.01 to 0.04) | .18 |
| T3, effect per ng/dL | ||||
| Initial weight | 1.19 (−0.50 to 2.89) | .17 | 0.52 (−1.60 to 2.63) | .63 |
| Average annual weight changec | −0.03 (−0.13 to 0.07) | .58 | 0.10 (−0.03 to 0.24) | .13 |
| FT4, effect per ng/dL | ||||
| Initial weight | −4.60 (−7.79 to −1.40) | .005 | −2.91 (−6.40 to 0.58) | .10 |
| Average annual weight changec | −0.32 (−0.53 to −0.11) | .003 | −0.09 (−0.32 to 0.14) | .45 |
Adjusted for age, race, kilocalories of activity (natural log), smoking status, self-reported health, and initiation of thyroid medication use. Results are shown as kg (95% CI).
For whether slope differs significantly from that in the euthyroid group.
Change in slope per unit change in biomarker, given an average estimated slope of −0.37 in women and −0.38 in men.
Those participants who were stably euthyroid (n = 2165), stably subclinically hypothyroid (n = 191), or transiently subclinically hypothyroid (n = 123) over a 2-year period were included in an additional analysis. Average annual weight change did not differ between persistently or transiently subclinically hypothyroid and persistently euthyroid women or men in adjusted analyses (women, loss of 0.34 and 0.45 vs loss of 0.46 kg/y, P = .24; men, loss of 0.49 and 0.41 vs loss of 0.52 kg/y, P = .79) (Table 3). Twenty-eight (1.3%) of the participants who were persistently euthyroid in 1994 and 1995, 13 (10.6%) of those who were transiently subclinically hypothyroid, and 52 (27.2%) of those who were persistently subclinically hypothyroid started thyroid medication during follow-up. Results were unchanged in analyses without adjustment for thyroid hormone initiation (Supplemental Table 2).
Table 3.
Average Annual Weight Change from 1994/1995 to 1998/1999 by Persistence of Thyroid Groups Determined From Measurements in 1992/1993 and 1994/1995a
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| n | Estimated Annual Weight Change, kg (95% CI) | P Value | n | Estimated Annual Weight Change, kg (95% CI) | P Value | |
| Euthyroid | 1224 | −0.46 (−0.52 to −0.40) | 941 | −0.52 (−0.59 to −0.46) | ||
| Transient | 83 | −0.45 (−0.68 to −0.23) | .93b | 40 | −0.41 (−0.73 to −0.10) | .51b |
| Persistent subclinical hypothyroid | 118 | −0.34 (−0.54 to −0.14) | .24b | 73 | −0.49 (−0.74 to −0.23) | .77b |
Adjusted for age, race, kilocalories of activity (ln), smoking status, self-reported health, and initiation of thyroid medications.
For difference in rate of change from the euthyroid group.
Concurrent changes in TSH and weight from 1992/1993 to 1994/1995 could be assessed in 1169 men and 1579 women who were not taking thyroid preparations at either visit, after the removal of 9 participants with absolute changes of >10 mU/L. The average 2-year change in TSH was modest, with a mean (SD) of −0.08 (1.00) mU/L in men and −0.21 (1.19) mU/L in women. The unadjusted association of change in TSH with change in weight appeared linear (Figure 1). In an adjusted linear regression model, a 1 mU/L change in TSH was associated with a 0.27-kg (95% confidence interval [CI], 0.05–0.48 kg) increase in weight in men (P = .014) and a 0.18-kg (95% CI, 0.03–0.34 kg) increase in weight in women (P = .019).
Figure 1.
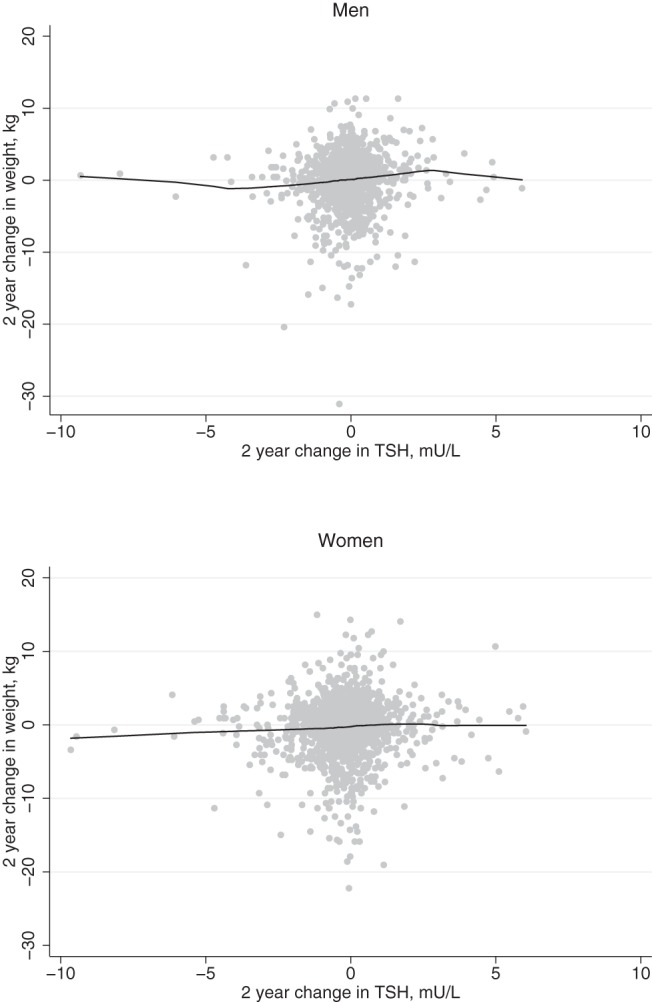
Scatterplots of change in weight vs change in TSH over a 2-year follow-up in men and women.
Characteristics of the subgroup of 1276 participants who had DXA scans performed at the 1994/1995 visit are described in Supplemental Table 3. Similar to the overall cohort, men and women with subclinical hypothyroidism were slightly older and more likely to be white compared with those with euthyroidism. Subclinical hypothyroidism was not associated with lean body mass, fat mass, or percent body fat in this subgroup (Table 4). In women, a 1 mU/L higher TSH level within the normal or subclinical hypothyroid range was associated with a 0.46-kg higher total fat mass (P = .02), 0.038-kg higher mean arm fat (P = .03), 0.28-kg higher trunk fat (P = .006), and 0.31% higher percent fat (P = .04) but not with trunk to arm fat ratio or lean body mass (Table 4). No associations were seen between TSH level and body composition in men.
Table 4.
Association of Thyroid Function With Body Composition (1994/1995) in Subset of Subjects With DXAa
| Women, n = 695 | P Value | Men, n = 581 | P Value | |
|---|---|---|---|---|
| Entries are the differences in the subclinical hypothyroid group compared with the euthyroid group | ||||
| Total fat mass, kg | 1.1 (−1.2 to 3.5) | .34 | 1.0 (−1.3 to 3.3) | .39 |
| Total lean mass, kg | 0.40 (−0.85 to 1.7) | .53 | −0.52 (−2.4 to 1.3) | .58 |
| Percent fat, % | 0.86 (−0.97 to 2.7) | .36 | 0.97 (−0.99 to 2.9) | .33 |
| TSH (mU/L) Entries are the regression estimates for 1 mU/L higher TSH | ||||
| Total fat mass, kg | 0.46 (0.08 to 0.84) | .02 | 0.19 (−0.13 to 0.51) | .24 |
| Total lean mass, kg | 0.19 (−0.01 to 0.39) | .07 | −0.01 (−0.26 to 0.24) | .94 |
| Percent fat, % | 0.31 (0.02 to 0.60) | .04 | 0.13 (−0.14 to 0.40) | .35 |
| Mean arm fat, kg | 0.038 (0.005 to 0.071) | .03 | 0.015 (−0.008 to 0.037) | .20 |
| Trunk fat, kg | 0.28 (0.08 to 0.48) | .006 | 0.084 (−0.12 to 0.29) | .42 |
| Trunk fat/mean arm fat | 0.056 (−0.01 to 0.12) | .095 | −0.022 (−0.12 to 0.08) | .65 |
Adjusted for age, race, kilocalories of activity (ln), smoking status, and self-reported health.
Discussion
In this large population-based study of adults aged 65 years or older, we report no difference in weight trajectory between those with subclinical hypothyroidism and euthyroid individuals, even in those with persistent subclinical hypothyroidism over 2 years. In addition, BMI, lean body mass, and fat mass were not significantly higher in men and women with subclinical hypothyroidism than in their euthyroid counterparts. These findings are particularly relevant in light of previous data from CHS demonstrating that thyroid hormone initiation was higher in individuals whose BMI was above 25 kg/m2 (17), a group in whom weight management may influence prescription of thyroid hormone medication.
To our knowledge, there are only 4 other studies that evaluated the association between thyroid status in the euthyroid and subclinically hypothyroid range and anthropometric measures in older participants. None of these studies were longitudinal in design or evaluated FT4 or T3 levels, but their cross-sectional associations with subclinical hypothyroidism are congruent with our null findings (18–21). A larger body of data exists in middle-aged adults, in whom TSH within the euthyroid range has been found to be positively related to weight and BMI in most (8–12, 22–29), but not all (15, 30) studies. Three of these studies examined weight longitudinally (9, 10, 31). Fox et al (10) failed to find a relationship between baseline TSH within the euthyroid range and weight change, but all 3 studies found a positive association between change in TSH and change in weight or BMI over time. One recent study found no difference in BMI between euthyroid and subclinically hypothyroid participants (32). In addition, 2 studies of middle-aged adults assessed the relationship between TSH and body composition. In a British study of euthyroid subjects, TSH was positively associated with percent body fat measured by electrical bioimpedance (13). In a Greek study of euthyroid men and women, sc fat by ultrasound was positively associated with TSH only in univariate analysis but not in the fully adjusted model (14).
Several studies have assessed serum T4 or T3 levels in relation to weight or BMI in middle-aged subjects, although a consistent pattern of associations has not emerged. FT4 has been shown to have no association (12, 14, 22–24, 30) or an inverse relationship (15) with BMI, and FT3 has been shown to have no association (22) or to be positively associated (12, 14) with BMI in euthyroid subjects. In a Danish study of euthyroid and subclinically hypothyroid subjects, FT4 but not FT3 was inversely related to BMI (8). In a longitudinal study, higher FT3 and FT4 were each associated with greater risk of becoming obese over a 6-year follow-up (33).
Overall, the differences in weight, BMI, and fat mass within the subclinically hypothyroid and euthyroid range found in our study and other published studies in elderly and middle-aged adults were small. Although many studies showed statistically significant relationships between thyroid status and anthropomorphic measures, apart from the results in women found in one study (10), the magnitudes of the differences were modest and of questionable clinical relevance. Furthermore, it is unclear whether treatment of thyroid status within the subclinical and euthyroid ranges would result in meaningful improvements in body composition in overweight patients.
The direction of causality in the relationship between thyroid status and weight and body composition is not known. An association between resting energy expenditure and FT4 has been demonstrated in 18 euthyroid male volunteers (34), and lower thyroid function within the subclinical and euthyroid range could lead to weight gain, possibly through a decreased metabolic rate (8). However, patients with subclinical hypothyroidism in randomized trials who are treated with levothyroxine do not show significant weight loss (35, 36). Even in overtly hypothyroid patients, treatment inconsistently affects weight (2, 13). It has been shown that the decrease in body weight seen in overtly hypothyroid patients who initiate levothyroxine is due to excretion of excess body water (37). Conversely, it has been suggested that adipose tissue may affect thyroid function, as evidenced by studies that demonstrate decreases in TSH with weight loss (38, 39). This association between weight loss and TSH is proposed to be mediated by leptin. In times of energy excess, rising levels of leptin are hypothesized to stimulate TSH and increase T4 to T3 conversion (40).
In individual analyses of TSH, FT4, and total T3, sex-specific differences were found, with stronger associations of thyroid function tests with weight and body composition in women than in men. This was not due to lower power secondary to fewer men with subclinical hypothyroidism in the cohort, because the effect sizes were smaller in men. Four previous studies found similar sex-specific differences in middle-aged participants, with stronger associations between TSH and weight or BMI in women than in men (9, 10, 24, 26), whereas 3 others showed no sex-specific differences (11, 12, 31). Between-sex differences in leptin and resting fat oxidation have been described in older people (41, 42), although the role that differential effects from thyroid hormone plays in these observations is unknown.
A major strength of our study is the use of a large population-based cohort of older men and women with a 6-year longitudinal follow-up of weight. FT4 and T3 were collected in addition to TSH, allowing accurate categorization of participants as subclinically hypothyroid and for use in separate analyses. All thyroid function testing was performed using banked samples, and results were not provided to study participants or their physicians. Assessment of thyroid function at 2 time points allowed analysis of weight change in those who were persistently subclinically hypothyroid. We further defined the type of body mass associated with TSH by using DXA, although this was only at a single time point. All of our analyses were adjusted for multiple covariates, including physical activity. We are unable to comment on the relationship between thyroid function and body composition in populations younger than age 65 years. In addition, measurements of thyroid autoantibodies were not performed, so that we were not able to discriminate between individuals with thyroid autoimmunity and those with elevated TSH levels due to other causes. Furthermore, CHS is an observational study, and thus we did not evaluate the effects of treating subclinical hypothyroidism on weight and body composition.
In conclusion, our data suggest that subclinical hypothyroidism does not represent an independent risk factor for weight gain in men and women aged 65 and older. There is a cross-sectional relationship between TSH, FT4, weight, and fat mass in older women, but the clinical relevance and directionality of the relationship remains unclear. Intentional weight loss in the elderly remains controversial, and our findings do not support the treatment of subclinical hypothyroidism to prevent weight gain or treat obesity in the elderly.
Acknowledgments
This work was supported by Grant R01AG032317 from the National Institute on Aging (NIA); T32DK007314 from the National Institute of Diabetes, Digestive, and Kidney Disorders; and Contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and Grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contributions from the National Institute of Neurological Disorders and Stroke. Additional support was provided by Grant AG023629 from the NIA. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CHS
- Cardiovascular Health Study
- CI
- confidence interval
- DXA
- dual-energy x-ray absorptiometry
- FT4
- free T4
- GEE
- generalized estimating equation.
References
- 1. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142–1154 [DOI] [PubMed] [Google Scholar]
- 2. Tzotzas T, Krassas GE, Konstantinidis T, Bougoulia M. Changes in lipoprotein(a) levels in overt and subclinical hypothyroidism before and during treatment. Thyroid. 2000;10:803–808 [DOI] [PubMed] [Google Scholar]
- 3. Ford ES, Mokdad AH. Epidemiology of obesity in the Western Hemisphere. J Clin Endocrinol Metab. 2008;93:S1–S8 [DOI] [PubMed] [Google Scholar]
- 4. Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589 [DOI] [PubMed] [Google Scholar]
- 5. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7 [DOI] [PubMed] [Google Scholar]
- 7. Cohen-Mansfield J, Perach R. Is there a reversal in the effect of obesity on mortality in old age? J Aging Res. 2011;2011:765071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–4024 [DOI] [PubMed] [Google Scholar]
- 9. Nyrnes A, Jorde R, Sundsfjord J. Serum TSH is positively associated with BMI. Int J Obes (Lond). 2006;30:100–105 [DOI] [PubMed] [Google Scholar]
- 10. Fox CS, Pencina MJ, D'Agostino RB, et al. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med. 2008;168:587–592 [DOI] [PubMed] [Google Scholar]
- 11. Asvold BO, Bjøro T, Vatten LJ. Association of serum TSH with high body mass differs between smokers and never-smokers. J Clin Endocrinol Metab. 2009;94:5023–5027 [DOI] [PubMed] [Google Scholar]
- 12. Kitahara CM, Platz EA, Ladenson PW, Mondul AM, Menke A, Berrington de Gonzalez A. Body fatness and markers of thyroid function among U.S. men and women. PLoS One. 2012;7:e34979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pinkney JH, Goodrick SJ, Katz J, et al. Leptin and the pituitary-thyroid axis: a comparative study in lean, obese, hypothyroid and hyperthyroid subjects. Clin Endocrinol (Oxf). 1998;49:583–588 [DOI] [PubMed] [Google Scholar]
- 14. Alevizaki M, Saltiki K, Voidonikola P, Mantzou E, Papamichael C, Stamatelopoulos K. Free thyroxine is an independent predictor of subcutaneous fat in euthyroid individuals. Eur J Endocrinol. 2009;161:459–465 [DOI] [PubMed] [Google Scholar]
- 15. Makepeace AE, Bremner AP, O'Leary P, et al. Significant inverse relationship between serum free T4 concentration and body mass index in euthyroid subjects: differences between smokers and nonsmokers. Clin Endocrinol (Oxf). 2008;69:648–652 [DOI] [PubMed] [Google Scholar]
- 16. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276 [DOI] [PubMed] [Google Scholar]
- 17. Somwaru LL, Arnold AM, Cappola AR. Predictors of thyroid hormone initiation in older adults: results from the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2011;66:809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132:270–278 [DOI] [PubMed] [Google Scholar]
- 19. Moon MK, Lee YJ, Choi SH, et al. Subclinical hypothyroidism has little influences on muscle mass or strength in elderly people. J Korean Med Sci. 2010;25:1176–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Westerink J, van der Graaf Y, Faber DR, Visseren FL. The relation between thyroid-stimulating hormone and measures of adiposity in patients with manifest vascular disease. Eur J Clin Invest. 2011;41:159–166 [DOI] [PubMed] [Google Scholar]
- 21. Yeap BB, Alfonso H, Chubb SA, et al. Higher free thyroxine levels are associated with frailty in older men: the Health In Men Study. Clin Endocrinol (Oxf). 2012;76:741–748 [DOI] [PubMed] [Google Scholar]
- 22. Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clin Endocrinol (Oxf). 2005;62:487–491 [DOI] [PubMed] [Google Scholar]
- 23. Ambrosi B, Masserini B, Iorio L, et al. Relationship of thyroid function with body mass index and insulin-resistance in euthyroid obese subjects. J Endocrinol Invest. 2010;33:640–643 [DOI] [PubMed] [Google Scholar]
- 24. Díez JJ, Iglesias P. Relationship between thyrotropin and body mass index in euthyroid subjects. Exp Clin Endocrinol Diabetes. 2011;119:144–150 [DOI] [PubMed] [Google Scholar]
- 25. Mehran L, Amouzegar A, Delshad H, Azizi F. Association between serum TSH concentration and body mass index in euthyroid subjects: the role of smoking. Arch Iran Med. 2012;15:400–403 [PubMed] [Google Scholar]
- 26. Zhang J, Sun H, Chen L, et al. Relationship between serum TSH level with obesity and NAFLD in euthyroid subjects. J Huazhong Univ Sci Technolog Med Sci. 2012;32:47–52 [DOI] [PubMed] [Google Scholar]
- 27. Souza Ade M, Sichieri R. Relationship between body mass index and thyrotropin in euthyroid women: differences by smoking, race and menopausal status. Obes Facts. 2011;4:175–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lai Y, Wang J, Jiang F, et al. The relationship between serum thyrotropin and components of metabolic syndrome. Endocr J. 2011;58:23–30 [DOI] [PubMed] [Google Scholar]
- 29. Ruhla S, Weickert MO, Arafat AM, et al. A high normal TSH is associated with the metabolic syndrome. Clin Endocrinol (Oxf). 2010;72:696–701 [DOI] [PubMed] [Google Scholar]
- 30. Manji N, Boelaert K, Sheppard MC, Holder RL, Gough SC, Franklyn JA. Lack of association between serum TSH or free T4 and body mass index in euthyroid subjects. Clin Endocrinol (Oxf). 2006;64:125–128 [DOI] [PubMed] [Google Scholar]
- 31. Svare A, Nilsen TI, Bjoro T, Asvold BO, Langhammer A. Serum TSH related to measures of body mass: longitudinal data from the HUNT Study, Norway. Clin Endocrinol (Oxf). 2011;74:769–775 [DOI] [PubMed] [Google Scholar]
- 32. Wang CY, Chang TC, Chen MF. Associations between subclinical thyroid disease and metabolic syndrome. Endocr J. 2012;59:911–917 [DOI] [PubMed] [Google Scholar]
- 33. Soriguer F, Valdes S, Morcillo S, et al. Thyroid hormone levels predict the change in body weight: a prospective study. Eur J Clin Invest. 2011;41:1202–1209 [DOI] [PubMed] [Google Scholar]
- 34. Boivin M, Camirand A, Carli F, Hoffer LJ, Silva JE. Uncoupling protein-2 and -3 messenger ribonucleic acids in adipose tissue and skeletal muscle of healthy males: variability, factors affecting expression, and relation to measures of metabolic rate. J Clin Endocrinol Metab. 2000;85:1975–1983 [DOI] [PubMed] [Google Scholar]
- 35. Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of l-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab. 2007;92:1715–1723 [DOI] [PubMed] [Google Scholar]
- 36. Kong WM, Sheikh MH, Lumb PJ, et al. A 6-month randomized trial of thyroxine treatment in women with mild subclinical hypothyroidism. Am J Med. 2002;112:348–354 [DOI] [PubMed] [Google Scholar]
- 37. Karmisholt J, Andersen S, Laurberg P. Weight loss after therapy of hypothyroidism is mainly caused by excretion of excess body water associated with myxoedema. J Clin Endocrinol Metab. 2011;96:E99–E103 [DOI] [PubMed] [Google Scholar]
- 38. Sari R, Balci MK, Altunbas H, Karayalcin U. The effect of body weight and weight loss on thyroid volume and function in obese women. Clin Endocrinol (Oxf). 2003;59:258–262 [DOI] [PubMed] [Google Scholar]
- 39. Chikunguwo S, Brethauer S, Nirujogi V, et al. Influence of obesity and surgical weight loss on thyroid hormone levels. Surg Obes Relat Dis. 2007;3:631–635; discussion 635–636 [DOI] [PubMed] [Google Scholar]
- 40. Zimmermann-Belsing T, Brabant G, Holst JJ, Feldt-Rasmussen U. Circulating leptin and thyroid dysfunction. Eur J Endocrinol. 2003;149:257–271 [DOI] [PubMed] [Google Scholar]
- 41. Toth MJ, Gardner AW, Arciero PJ, Calles-Escandon J, Poehlman ET. Gender differences in fat oxidation and sympathetic nervous system activity at rest and during submaximal exercise in older individuals. Clin Sci (Lond). 1998;95:59–66 [PubMed] [Google Scholar]
- 42. Isidori AM, Strollo F, Morè M, et al. Leptin and aging: correlation with endocrine changes in male and female healthy adult populations of different body weights. J Clin Endocrinol Metab. 2000;85:1954–1962 [DOI] [PubMed] [Google Scholar]


