Abstract
Context:
Mineralocorticoid synthesis by the nonhuman primate periovulatory follicle enhances luteinization. Whether a similar event occurs in women undergoing in vitro fertilization (IVF) is unknown.
Objective:
The objective of the study was to determine whether human luteinized granulosa cells (LGCs) produce mineralocorticoids derived from 21-hydroxylase activity and also express mRNA for 21-hydroxylase and the mineralocorticoid receptor.
Design:
This was a prospective cohort study.
Setting:
The study was conducted at an academic center.
Patients:
LGC lipid content and follicle fluid (FF) hormone analysis was performed on 27 nonobese IVF women. LGCs from six additional nonobese IVF women were used for gene expression studies.
Intervention:
At oocyte retrieval, FF was aspirated from the first follicle (≥16 mm in size) of each ovary and pooled LGCs were collected.
Main Outcome Measures:
FF steroid analysis was performed by liquid chromatography-tandem mass spectrometry. LGCs were stained with lipid fluorescent dye BODIPY FL C16 to estimate lipid content by confocal microscopy as a cholesterol source for steroidogenesis in vivo. Quantitative real-time PCR was performed using LGCs to detect 21-hydroxylase and mineralocorticoid receptor mRNA expression. Pearson correlation coefficients determined associations between FF steroid levels and LGC lipid content.
Results:
FF levels of the 21-hydroxylase-derived steroids, 11-deoxycorticosterone [DOC, 39.97, median (13.94–63.02) ng/mL] and 11-deoxycortisol [11DOC, 2.07 (0.69–5.01) ng/mL], along with the 21-hydroxylase precursor 17-hydroxyprogesterone [1268.21 (493.26–3558.39) ng/mL], positively correlated with LGC lipid content (84 ± 43 fluorescent units/sample) (P ≤ .05, all steroids). 21-Hydroxylase and mineralocorticoid receptor mRNA expression was detected in LGCs.
Conclusions:
Human LGCs likely synthesize 21-hydroxylase-derived mineralocorticoids from cholesterol-containing lipid in vivo to promote postovulatory luteinization via mineralocorticoid receptor-mediated events.
In mammals, steroid synthesis by luteinized granulosa cells (LGCs) begins with cholesterol as substrate stored in intracellular lipid droplets after uptake from circulating lipoproteins (1–4). During steroidogenesis, cholesterol is transported as a rate-limiting step into mitochondria via steroidogenic acute regulatory protein and translocator protein, in which side-chain cleavage cytochrome P450 (P450scc) converts cholesterol to pregnenolone (P5) as a precursor for further steroidogenesis (5). Metabolism of P5 then occurs between two competing enzymes, with 17α-hydroxylase/17–20 lyase catalyzing the synthesis of 17-hydroxypregnenolone (17OHP5) and dehydroepiandrosterone (DHEA) from P5 (δ5 pathway), which otherwise is predominantly metabolized to progesterone (P4) by 3β-hydroxysteroid dehydrogenase (δ4 pathway) (6). Additionally, in macaque LGCs, induction of 21-hydroxylase by human chorionic gonadotropin (hCG) catalyzes the synthesis of 11-deoxycorticosterone (DOC) and 11-deoxycortisol (11 DOC) from P4 and 17-hydroxyprogesterone (17OHP4), respectively, with DOC acting through the mineralocorticoid receptor to promote luteinization (7).
It remains unclear, however, whether LGCs from women undergoing ovarian stimulation for in vitro fertilization (IVF) exhibit sufficient 21-hydroxylase for mineralocorticoid production in vivo, although IVF patients have higher levels of mineralocorticoids in their follicles than blood (8). If so, human LGCs may produce mineralocorticoids within periovulatory follicles from intracellular lipid as a source of cholesterol for steroidogenesis. Using confocal microscopy for LGC lipid quantification (4), combined with molecular studies for 21-hydroxylase and mineralocorticoid receptor mRNA expression, the present study investigates in nonobese women undergoing ovarian stimulation for IVF whether LCGs produce 21-hydroxylase-derived mineralocorticoids in proportion to their intracellular lipid content and whether LGCs also express mRNAs for 21-hydroxylase and the mineralocorticoid receptor.
Our IVF data demonstrate that follicle fluid (FF) levels of DOC and 11DOC, as 21-hydroxylase-derived steroids, positively correlate with the lipid content of LGCs, which also express mRNA for 21-hydroxylase and mineralocorticoid receptor. Therefore, FSH-primed human LGCs exposed to hCG have the capacity to synthesize 21-hydroxylase-derived mineralocorticoids from cholesterol-containing lipid in vivo, which may promote postovulatory luteinization via mineralocorticoid receptor-mediated events.
Materials and Methods
Study participants
After the approval by the University of California, Los Angeles, Institutional Review Board, nonobese women undergoing gonadotropin therapy for IVF were recruited; all women signed informed consent before study participation. All study participants were between the ages of 25 and 44 years and had normal serum prolactin levels and thyroid function studies. Exclusion criteria included women with galactorrhea, endometriomas, or ovarian cysts greater than 18 mm in diameter as possible modifiers of ovarian responsiveness to gonadotropin therapy (9–11). Patients undergoing IVF who were obese [body mass index (BMI) ≥ 30 kg/m2] were also excluded to eliminate possible effects of obesity on ovarian cell lipid content and intrafollicular steroidogenesis (4, 12, 13).
Gonadotropin stimulation for IVF and oocyte retrieval
Women undergoing ovarian stimulation for IVF received a GnRH antagonist (Ganirelix; Merck & Co Inc), a luteal phase leuprolide acetate (Lupron; TAP Pharmaceuticals), or a microdose leuprolide acetate ovarian stimulation protocol (14–16). Recombinant human (rh) FSH or urinary gonadotropins were initially started at a dose of 225–450 IU sc daily for the first 3 days and then increased or decreased thereafter as clinically indicated. Serial estradiol (E2) levels and transvaginal sonographic measurements of ovarian follicles were performed until at least two dominant follicles reached 17 mm or greater in diameter and serum E2 levels reached approximately 300 pg/mL per dominant follicle. hCG (10 000 IU, im), choriogonadotropin-α (500 μg sc, Ovidrel; EMD Serono, Inc), or leuprolide acetate (4 mg sc every 12 h for two doses) was then administered, followed by transvaginal oocyte retrieval 35.5 hours later.
Preparation of follicular fluid samples
At oocyte retrieval, FF uncontaminated by blood was aspirated separately from the first follicle of each ovary, as previously described (12, 15, 17, 18). Each follicle was selected for study by accessibility and size of at least 16 mm in mean diameter of three perpendicular planes to eliminate variability in FF steroid production by follicle size (19). Follicular fluid was then transported on ice to the laboratory, where it was centrifuged for 10 minutes at 1500 rpm at 4°C. All FF samples were stored in 0.5-mL aliquots at −80°C for later hormone determinations.
FF hormone assays
Follicular fluid levels of P5, P4, DOC, corticosterone (C), 18-hydroxycorticosterone (18OHC), 17OHP5, 17OHP4, 11DOC, cortisol (F), cortisone (CN), DHEA, androstenedione (A4), T, and E2 were measured by liquid chromatography-tandem mass spectrometry (Quest Diagnostics Nichols Institute), as previously described (20). Briefly, FF samples were prepared by acidifying 100 μL of FF with 20% formic acid containing three internal standards. After mixing, samples were incubated at room temperature for 15–20 minutes before being placed in the refrigerated autosampler for injection. The Aria TLX System (Thermo Electron) injected samples onto an extraction column at a high flow rate, allowing steroids to bind to large particles of the extraction column, whereas protein and debris flowed through and were discarded. Flow was reversed and the sample was eluted off the extraction column and transferred to a reverse-phase C8 analytical column. A binary HPLC gradient was applied, separating 14 steroids and three internal standards from each other and their metabolites. The steroids then were quantitated using a TSQ Quantum Ultra (Thermo Fisher) triple quadrupole tandem mass spectrometer, permitting isolation of the parent compound within ±0.5 mass to charge ratio within the first quadrupole. In the second quadrupole, the parent ions collided with argon to generate daughter ions that were selected in the third quadrupole. Inclusion of deuterated steroids as internal standards enabled absolute quantitation of steroids by correcting for procedural losses.
To estimate the accuracy for each FF steroid measurement, reproducibility of known standards was determined by the calculated mean target concentration of individual standards from 10 independent runs. All steroid results were within the acceptable range of 80%–120%, with a coefficient of variation (CV) of 15% or less. In addition, three quality control pools were tested over a 5-day interval, with 10 replicates of each pooled sample assayed daily to quantify spike recovery of standards. The difference of the mean value for each level of quality control material from target was less than 15%.
Intraassay CVs were as follows: P5, 9.8%; P4, 3.4%; DOC, 4.6%; C, 7.4%; 18OHC, 12.6%; 17OHP5, 7.7%; 17OHP4, 3.4%; 11DOC, 3.3%; F, 8.3%; CN, 4.9%; DHEA, 9.9%; A4, 4.4%; T, 5.5%; and E2, 10%. Interassay CVs were as follows: P5, 13.1%; P4, 8.6%; DOC, 7.3%; C, 9.1%; 18OHC, 9.3%; 17OHP5, 11.7%; 17OHP4, 6.3%; 11DOC, 7.7%; F, 11.5%; CN, 7.7%; DHEA, 11.3%; A4, 6.5%; T, 10.6%; and E2, 13%. Lower limits of quantification (nanomoles) for each hormone were as follows: P5, 1.11; P4, 0.32; DOC, 0.48; C, 0.49; 18OHC, 0.72; 17OHP5, 0.99; 17OHP4, 0.51; 11DOC, 0.43; F, 0.72; CN, 0.42; DHEA, 0.8; A4, 0.31; T, 0.35; and E2, 0.01.
Preparation of LGCs
LGCs obtained from pooled FF at oocyte retrieval were transferred to culture dishes (21, 22). Cells were washed several times in 5 mL of 4-morpholinepropanesulfonic acid (MOPS)-buffered medium (G-MOPS; VitroLife), containing 10% serum substitute supplement (Irvine Scientific), resuspended in 100 μL of rh-hyaluronidase (40–120 U/mL) (ICSI Cumulase; Malov), and then pipetted up and down for 1 minute before being placed in the MOPS-buffered medium. Pooled LGCs were initially centrifuged at 1600 rpm for 5 minutes at 24°C in the IVF laboratory. Cell samples were then transported on ice to the research laboratory, where they were resuspended in PBS and centrifuged for 5 minutes at 800 rpm at 20°C within 1–2 hours.
Isolated LGCs were immediately fixed with 4% paraformaldehyde in cell suspension for 20 minutes. Endogenous lipid content of LGCs was determined using the fluorescence probe BODIPY FL C16 (0.8 μg/mL) (1 hour in the dark at room temperature) (Invitrogen). Cell staining included a 5-minute incubation with 4′,6-diamidino-2-phenylindole (DAPI; 0.5 μg/mL; Invitrogen) to identify the nuclei. Cells were resuspended in 30 μL of PBS and 3 μL of 10% polyvinylpyrrolidone solution with human serum albumin (Irvine Scientific) before being placed on glass slides for imaging.
Confocal microscopy and analysis
Images were captured using the Leica TCS-SP2-AOBS confocal microscope with ×63 oil objective, using identical magnification and gain settings. The 488-line argon laser was used at 667 V to capture the BODIPY FL C16 lipid stain (Invitrogen); the diode 405 nm laser was used at 436 V to capture DAPI nuclear stain. Image acquisition was performed using Leica confocal software version 2.61 Build 1537. Fluorescent images of LGC lipid content were quantified using ImageJ (http://rsbweb.nih.gov/ij/) to determine mean fluorescence (fluorescence/unit area) of 20 cells per patient. All images were quantified by one investigator.
Images taken as single channel images were converted to overlay images, and all images were saved in tag image file format (TIFF) format. Single-channel images of BODIPY FL C16 (Invitrogen) and DAPI were used to create an overlay image, with single-channel BODIPY FL C16 used for lipid quantification in ImageJ (National Institutes of Health). Background staining was accounted for by using five negatively stained regions per cell, which were subtracted from the total mean fluorescence.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total cellular RNA was isolated from LGCs of six additional nonobese IVF patients, using an RNeasy kit (QIAGEN) and the manufacturer's protocol. First-strand cDNA was synthesized using a first-strand RT2 kit (QIAGEN); mRNA was quantified by qRT-PCR using RT2 quantitative PCR master mix according to manufacturer's protocol (QIAGEN). qRT-PCR was performed on an ABI 7300 (Applied Biosystems) using standard temperature cycling conditions. Human primers for 21-hydroxylase (CYP21A2; chromosome 6: exons 1–2, length 105 bp) and mineralocorticoid receptor (NR3C2; chromosome 4: exons 7–8, length 68 bp; Life Technologies Corp) were used to detect mRNAs for CYP21A2and NR3C2, respectively, in LGCs. To verify primer sets for CYP21A2 and NR3C2, cDNA obtained from mRNAs of 295R adrenocortical cells (American Type Culture Collection) and human kidney tissue, respectively, were used as positive controls. To detect mRNA markers of LGC luteinization, human primers for LH/choriogonadotropin receptor (CGR; chromosome 2: exons 1–2, length 105 bp); P450scc side chain cleavage cytochrome P450 (CYP11A1; chromosome 15: exons 2–3, length 77 bp); and 11β-hydroxysteroid dehydrogenase (11βHSD), type 1 (chromosome 1: exons 3–4, length 67 bp) and type 2 (chromosome 16: exons 1–2, length 50 bp) also were used (Life Technologies Corp). The human primer for glyceraldehyde-3-phosphate dehydrogenase (GAPDH: chromosome 12: exons 6–7, length 93 bp) was used to detect GAPDH mRNA as an internal control housekeeping gene (Life Technologies Corp). As a negative control, first-strand DNA synthesis was performed without mRNA followed by qRT-PCR. Each measurement was performed in triplicate with at least three independent experiments conducted for each sample.
The relative expression of target genes was measured using the comparative critical threshold (Ct) method. The results were expressed as mRNA expression relative to GADPH using the formula 2-δCt (δCt is the difference between the mean Ct values of targeted genes and the mean Ct value GAPDH).
Statistical analysis
Pearson correlation coefficients based on steroid pathway analysis were used to examine the associations between FF steroid levels and LGC lipid content (23), followed by a regression analysis (24) to estimate the least squares regression lines for significant variables. Pearson correlation coefficients also were used to examine the relationships of patient age, BMI, and amount of gonadotropins administered with LCG lipid content. Statistical analyses were performed in SPSS 21 (IBM Corp).
A value of P ≤ .05 was considered statistically significant. IVF cycle and patient characteristics are reported as mean ± SD. Due to skewed steroid data, FF levels data are reported as median (range). The mRNA levels of CYP21A2, NR3C2 LH/CGR, CYP11A1, and type 1/type 2 11βHSD in cells are expressed as mean ± SD.
Results
Twenty-seven nonobese IVF patients (aged 36.2 ± 4.5 y; BMI, 22.6 ± 3.0 kg/m2: mean ± SD) were recruited for study with the primary diagnoses of endometriosis (n = 2), advanced maternal age (n = 6), unexplained infertility (n = 3), exclusive male factor infertility (n = 5), hypogonadotropic hypogonadism (n = 2), oocyte donation (n = 3), polycystic ovary syndrome (n = 2), recurrent pregnancy loss (n = 2), tubal factor (n = 1), and fertility preservation (n = 1). Characteristics of the IVF cycles were as follows: cycle day 3 serum FSH and E2 levels, 7.2 ± 3.4 mIU/mL and 34.7 ± 15.3 pg/mL, respectively; total amounts of rhFSH and hCG administered, 3180 ± 945 IU and 208 ± 148 IU, respectively; maximal serum E2 levels, 3274 ± 1617 pg/mL; and total numbers of oocytes retrieved, 15.9 ± 8.7. Within the age and BMI ranges of these nonobese IVF patients, the total amounts of rhFSH or hCG administered were unrelated to LGC lipid content (patient age, R2 = 0.07, P = .2; BMI, R2 = 0.07, P = .2; total amounts of rhFSH, R2 < 0.01, P = .9; and hCG, R2 < 0.01, P = .9 administered).
FF contained measurable amounts of mineralocorticoids, glucocorticoids, and sex steroids. Within FF, P4 [13 245 median (4405–20 485 range) ng/mL; (international unit conversion to nanomoles, 3.18)] and 17OHP4 [1268.21 (493.26–3558.39) ng/mL; (international unit conversion, 3.03)] were the two steroids in greatest concentration, representing 83% and 9% of all steroids combined (Figure 1). Intrafollicular P4 and 17OHP4 levels were followed in decreasing order of concentration by E2 [527.015 (219.899–977.928) ng/mL (international unit conversion, 3.67)]; P5 [275.07 (105.40–1724.12) ng/mL (international unit conversion, 3.16)]; F [59.3 (21.7–100.9) ng/mL (international unit conversion, 2.76)]; DOC [39.97 (13.94–63.02) ng/mL (international unit conversion, 3.03)]; and CN [13.8 (6.2–22.5) ng/mL (international unit conversion, 2.77)], with lower levels of 17OHP5 [2.29 (range 0.52–56.22) ng/mL (international unit conversion, 3.01)]; 11DOC [2.07 (0.69–5.01) ng/mL (international unit conversion, 2.89)]; C [1.50 (0.45–4.57) ng/mL (international unit conversion, 2.89)]; DHEA [0.89 (0.31–5.51) ng/mL (international unit conversion, 3.47)]; A4 [0.87 (0.08–19.59) ng/mL (international unit conversion, 3.49)]; T [0.43 (0.16–1.48) ng/mL (international unit conversion, 3.47); and 18OHC [0.31 (0.25–0.49) ng/mL (international unit conversion, 2.76)].
Figure 1.
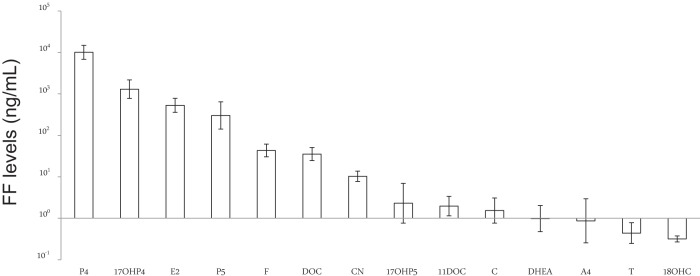
Mean intrafollicular steroid levels in nonobese women undergoing ovarian stimulation for IVF. Follicular fluid was collected at oocyte retrieval, and steroid levels were measured by liquid chromatography-tandem mass spectrometry. Intraassay CVs for all steroid measurements varied between 4% and 13%. All values are expressed in nanograms per milliliter and log transformed before statistical analysis. Error bars represent 1 SD.
The amounts of P5, 17OHP5, and P4 in FF as precursors for further steroid synthesis were unrelated to LGC lipid content (P5, R2 = 0.02, P = .5; 17OHP5, R2 = 0.01, P = .6; P4, R2 = 0.08, P = .2), which appeared as a homogenous pattern of intracytoplasmic fluorescence (mean 83.97 ± 42.93; range 24–194 fluorescent units) (Figure 2). In contrast, FF concentrations of the steroids directly derived from 21-hydroxylase activity, namely DOC and 11DOC, were positively correlated with LGC lipid content (DOC, R2 = 0.20, P ≤ .025; 11DOC, R2 = 0.15, P ≤ .05), as were FF levels of the steroid substrate for 21-hydroxylase activity, 17OHP4 (R2 = 0.19, P ≤ .025) (Figure 3). Intrafollicular levels of downstream steroid products of 11-hydroxylase action, namely C, 18OHC, and F, were unrelated to LGC lipid content (C, R2 = 0.05, P = .27; 18OHC, R2 = 0.01, P = .89; F, R2 = 0.10, P = .11), consistent with the presence of 21-hydroxylase, but not 11β-hydroxylase, activity in nonhuman primate LGCs (7). The amounts of CN and sex steroids in follicles also were unrelated to LGC lipid content (CN, R2 = 0.04, P = .31; DHEA, R2 = 0.02, P = .5; A4, R2 = 0.01, P = .2; T, R2 = 0.06, P = .2; E2, R2 = 0.08, P = .2).
Figure 2.
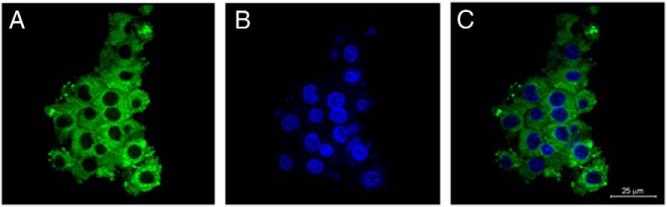
Lipid content of human LGCs. Cells were collected from pooled follicle fluid at oocyte retrieval. After immediate cell fixation, immunofluorescence studies were performed (see Materials and Methods). A, LGC lipid content detected by BODIPY FL C16 staining (green; Invitrogen). B, LGC nuclei stained with DAPI (blue). C, LGC overlap of lipid content and cell nuclei. Single-channel and overlap images were taken with a confocal microscope with a ×63 oil objective. Background staining was accounted for by using five negatively stained regions per cell, which were subtracted from the total mean fluorescence.
Figure 3.
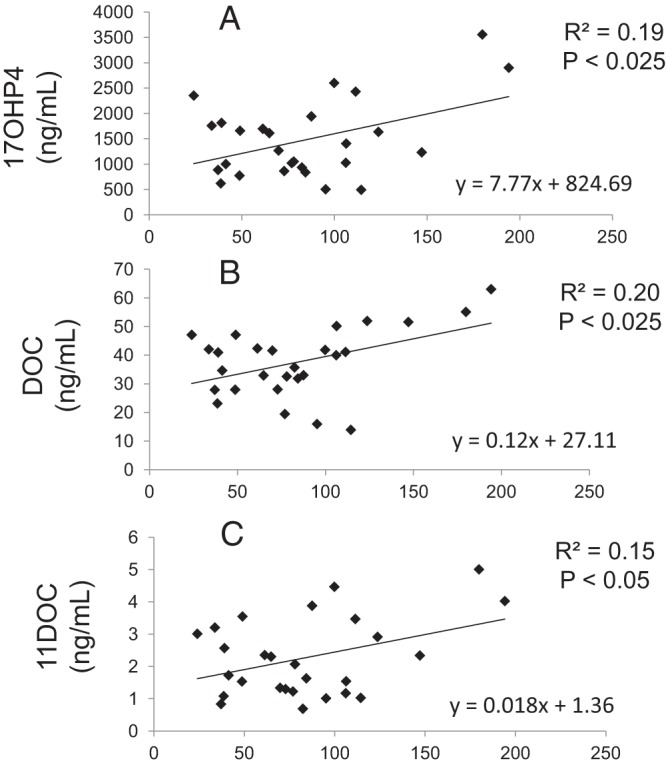
Regression of human LGC lipid content with folllicular fluid steroid levels. LGC lipid content was determined with BODIPY FL C16 staining (Invitrogen) by confocal microscopy, using ImageJ (National Institutes of Health) to determine mean fluorescence (immunofluorescence units per area) of at least 20 cells per patient. FF steroid levels were determined by liquid chromatography-tandem mass spectrometry. All values were expressed in nanograms per milliliter. The LGC lipid content positively correlated with intrafollicular levels of 17OHP4 (P ≤ .025), DOC (P ≤ .025), and 11DOC (P ≤ .05). Regression analysis was used to estimate least squares regressions lines for significant variables.
Gene expression analysis
In LGCs, mRNA expressions of CYP21A2 and NR3C2 were 0.0000889 ± 0.000006 (δCt = 13.475 935 ± 0.533 421) and 0.001 58 ± 0.000 078 (δCt = 9.350 499 ± 0.220 215) relative to GADPH (mean ± SD), respectively (Figure 4A). mRNA levels of CYP21A2 and NR3C2 were 0.019 95 ± 0.000 98 (δCt = 5.647 314 ± 0.245 596) in H295R adrenocortical cells and 0.211 517 ± 0.017 24 (δCt = 2.253 76 ± 0.183 74) in human kidney tissue as positive controls, respectively, relative to GADPH. Therefore, CYP21A2 and NR3C2 mRNA levels in LGCs were lower than those in H295R adrenocortical and human renal cells, respectively, but still detectable. mRNA expression of CYP21A2 or NR3C2 was undetected in the absence of mRNA during first-strand DNA synthesis (data not shown).
Figure 4.
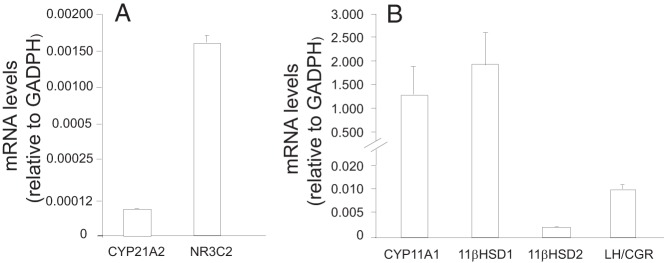
Target gene expression in human LGCs. Total RNA of LGCs from pooled FF at oocyte retrieval was isolated as described in Materials and Methods. CYP21A2 and NR3C2 (A) and CYP11A1, type 1 11βHSD, type 2 11βHSD, and LH/CGR (B) mRNA levels relative to GADPH were determined by qRT-PCR and calculated using the formula 2-δCt. Each measurement was performed in triplicate with at least three independent experiments conducted for each sample (six total samples). H295R adrenocortical cells and human kidney tissue were used as positive controls for CYP21A2 and NRC3, respectively. Error bars represent 1 SD.
Molecular studies confirmed mRNA expression of markers for luteinization as follows: CYP11A1, 1.332 551 ± 0.599 535 (δCt = −0.404 956 ± 0.182 196); type 1 11βHSD, 1.921 028 ± 0.714 864 (δCt = −0.909 716 ± 0.338 529); type 2 11βHSD, 0.001 830 ± 0.000 149 (δCt = 9.267 299 ± 0.752 112); and LH/CGR, 0.009 742 ± 0.000 894 (δCt = 6.798 525 ± 0.623 893) relative to GADPH (mean ± SD), respectively (Figure 4B).
Discussion
Luteinization of the periovulatory follicle requires steroid synthesis from nonesterified cholesterol (25, 26). Cholesterol stored as lipid in LGCs is converted to P5 by the mitochondrial cytochrome P450 enzyme, P450scc, producing P5 as substrate for P4 synthesis by the microsomal enzyme, 3β-hydroxysteroid dehydrogenase (25, 26). In macaques, P4 produced by LGCs is further converted via 21-hydroxylase activity to DOC, which acts as a mineralocorticoid to enhance hCG-stimulated steroidogenesis (7). Similar mineralocorticoid production by human follicles, however, remains controversial, although IVF patients have higher levels of mineralocorticoids in their follicles than blood (8).
Our study examined whether human LGCs of IVF patients express sufficient 21-hydroxylase activity for mineralocorticoid production in vivo. To do so, FF steroid levels by liquid chromatography-tandem mass spectrometry were correlated with LGC lipid content as a source of cholesterol substrate derived from circulating lipoproteins (1, 2, 4) to determine whether 21-hydroxylase-derived mineralocorticoids were present in follicles in proportion to LGC lipid content. Specifically, confocal microscopy was used to measure the lipid content of LCGs after their immediate fixation at oocyte retrieval while in cell suspension, thereby avoiding cell culture techniques that alter human LGC lipid accumulation in vitro (4). Using these combined techniques, FF concentrations of DOC and 11DOC, as 21-hydroxylase-derived steroids, were positively correlated with LGC lipid content. This relationship was further supported by our molecular studies that demonstrated CYP21A2 mRNA expression in LGCs, suggesting that human LGCs may be capable of 21-hydroxylase-derived mineralocorticoid production in vivo.
In macaque LGCs, steroids derived from 21-hydroxylase do not undergo further metabolism due to lack of 11-hydroxylase (7). Consequently, these 21-hydroxylase-derived steroids can accumulate in the periovulatory follicle and act via mineralocorticoid receptors present on LGCs to promote luteinization (7). Several lines of evidence support the possibility of a similar phenomenon in periovulatory follicles of our IVF patients. First, FF levels of DOC were present in sufficient amounts (∼113 nM) to interact with the mineralocorticoid receptor (dissociation constant value, ∼1 nM) (27). Second, FF levels of C and 18OHC, as 11-hydroxylase-derived steroids, were low in amounts and unrelated to LCG lipid content, agreeing with absence of 11β-hydroxylase in primate LGCs (7). Third, measurable amounts of NR3C2 mRNA were present in LGCs, implying possible expression of mineralocorticoid receptors by these cells. Because 21-hydroxylase-derived DOC is produced by FSH-primed macaque granulosa cells exposed to hCG (7), a similar phenomenon in IVF patients likely allows sufficient DOC to saturate LGC mineralocorticoid receptors for maximal luteinization in the presence of other competing ligands, such as glucocorticoids (28).
The mineralocorticoid receptor, however, does not differentiate between steroids with different configurations at positions 11β, β17, and β18 (28). Consequently, 11DOC production from 17OHP4 by human LGCs, although lower than that of DOC due to the preferential metabolism of P5 via βHSD vs 17α-hydroxylase/17–20 lyase (6, 26), may interfere with mineralocorticoid receptor binding activity (6, 29). Cortisol production may also interfere with mineralocorticoid receptor binding activity via interconversion of cortisone with cortisol by two granulosa cell-derived 11βHSD isoforms: nicotinamide adenine dinucleotide phosphate-dependent type 1 11βHSD (with predominant reductase activity for cortisol synthesis) and nicotinamide adenine dinucleotide-dependent type 2 11βHSD (with dehydrogenase activity for cortisone synthesis) (30–32). Human LGCs in our study expressed greater amounts of type 1 than type 2 11βHSD mRNA, agreeing with human granulosa cells expressing type 2, 11βHSD during early folliculogenesis and switching to type 1, 11βHSD expression in response to hCG (30–32).
In our study, FF DOC levels were unrelated to follicle size (R2 = 0.02; P = .5), implying a limited role for local mineralocorticoids on transfer of small circulating molecules with plasma across a follicular membrane into a follicle containing hydrolyzed glycosaminoglycans (33). Moreover, administration of spironolactone, a mineralocorticoid receptor antagonist, does not impair follicle growth and can induce ovulation in some hyperandrogenic anovulatory women (34).
Nevertheless, mineralocorticoids produced by mature human follicles likely enter the circulation because a luteal phase rise in circulating DOC levels is only partially suppressed by dexamethasone (35, 36). Whether such an event is physiologically relevant in fluid homeostasis, however, is controversial because adult human carriers of mineralocorticoid receptor mutations maintain normal sodium balance, blood pressure, and extracellular water from secondary activation of the renin-angiotensin-aldosterone system (37). Similarly, although FF and serum mineralocorticoid levels increase during gonadotropin therapy for IVF (8), any ovarian mineralocorticoid action accompanying ovarian hyperstimulation syndrome is likely overshadowed by activation of the renin-angiotensin-aldosterone system secondary to hypovolemia from ascites due to ovarian vascular endothelial growth factor production (38, 39).
Several factors may have led us to underestimate the relationship between 21-hydroxylase-derived steroid production and LGC lipid in vivo. Variability in clinical characteristics may have been one factor, but patient and IVF cycle characteristics were unrelated to LGC lipid content, given the age and BMI ranges studied. More likely, FF steroid measurements from a first aspirated ovarian follicle did not account for the hormone variability between follicles, as shown in 10 subjects with size-matched FF samples uncontaminated by blood (CVs: 17OHP4, 24%; DOC, 14%; and 11DOC, 30%). Similarly, LGC lipid measurements between different images of the same patient determined by one investigator showed a CV of 41.3 across varying cellular lipid means and different patients, confirming variation between images. Additionally, because cholesterol as a source for steroid production is acquired from the circulation, synthesized de novo and/or stored in lipid droplets, the LGC lipid pool is not an obligatory step in ovarian steroidogenesis, nor does it identify dynamic changes in lipid turnover as cholesterol forms into esters and undergoes hydrolysis (40).
We conclude that FSH-primed human LGCs exposed to hCG have the capacity to synthesize 21-hydroxylase-derived mineralocorticoids from cholesterol-containing lipid in vivo, which may promote postovulatory luteinization via mineralocorticoid receptor-mediated events. This positive correlation between FF levels of 21-hydroxylase-derived steroids and LGC lipid content in vivo will require additional studies to explore the physiological role of this relationship in human ovarian folliculogenesis.
Acknowledgments
We acknowledge Erica Keller and Paul Aguilera for their contributions. Confocal laser-scanning microscopy was performed at the California NanoSystems Institute Advanced Light Microscopy/Spectroscopy Shared Resource Facility at the University of California, Los Angeles, supported with funding from National Institutes of Health-National Center for Research Resources shared resources (Grant CJX1–44385-WS-29646) and National Science Foundation Major Research Instrumentation Grant CHE-0722519. Statistical analyses were funded by the National Institutes of Health/National Center for Advancing Translational Sciences/ University of California, Los Angeles, Clinical and Translational Science Institute Grant UL1TR000124.
This work was supported in part by the Department of Obstetrics and Gynecology, University of California, Los Angeles, and by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, through Cooperative Agreement U54 HD071836.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- A4
- androstenedione
- BMI
- body mass index
- C
- corticosterone
- CGR
- choriogonadotropin receptor
- CN
- cortisone
- Ct
- critical threshold
- CV
- coefficient of variation
- CYP11A1
- P450scc side chain cleavage cytochrome P450
- CYP21A2
- 21-hydroxylase
- DAPI
- 4′,6-diamidino-2-phenylindole
- DHEA
- dehydroepiandrosterone
- DOC
- 11-deoxycorticosterone
- 11DOC
- 11-deoxycortisol
- E2
- estradiol
- F
- cortisol
- FF
- follicle fluid
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- hCG
- human chorionic gonadotropin
- 11βHSD
- 11β-hydroxysteroid dehydrogenase
- IVF
- in vitro fertilization
- LGC
- luteinized granulosa cell
- MOPS
- 4-morpholinepropanesulfonic acid
- NR3C2
- mineralocorticoid receptor
- 18OHC
- 18-hydroxycorticosterone
- 17OHP4
- 17-hydroxyprogesterone
- 17OHP5
- 17-hydroxypregnenolone
- P4
- progesterone
- P5
- pregnenolone
- P450scc
- side-chain cleavage cytochrome P450
- qRT-PCR
- quantitative real-time PCR
- rh
- recombinant human.
References
- 1. Azhar S, Tsai L, Medicherla S, Chandrasekher Y, Giudice L, Reaven E. Human granulosa cells use high density lipoprotein cholesterol for steroidogenesis. J Clin Endocrinol Metab. 1998;83:983–991 [DOI] [PubMed] [Google Scholar]
- 2. Parinaud J, Perret B, Ribbes H, Chap H, Pontonnier G, Douste-Blazy L. High density lipoprotein and low density lipoprotein utilization by human granulosa cells for progesterone synthesis in serum-free culture: respective contributions of free and esterified cholesterol. J Clin Endocrinol Metab. 1987;64:409–417 [DOI] [PubMed] [Google Scholar]
- 3. Rajan VP, Menon KM. Cholesterol flux between high density lipoproteins and cultured rat luteal cells. Endocrinology. 1989;124:1857–1862 [DOI] [PubMed] [Google Scholar]
- 4. Singh P, Amin M, Keller E, et al. A novel approach to quantifying ovarian cell lipid content and lipid accumulation in vitro by confocal microscopy in lean women undergoing ovarian stimulation for in vitro fertilization (IVF). J Assist Reprod Genet. 2013;30:733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salway JG. Metabolism at a Glance. 3rd ed Malden, MA: Blackwell Publishing; 2010:72 [Google Scholar]
- 6. Conley AJ, Bird IM. The role of cytochrome P450 17α-hydroxylase and 3β-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the δ5- and δ4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56:789–7997 [DOI] [PubMed] [Google Scholar]
- 7. Fru KN, VandeVoort CA, Chaffin CL. Mineralocorticoid synthesis during the periovulatory interval in macaques. Biol Reprod. 2006;75:568–574 [DOI] [PubMed] [Google Scholar]
- 8. Sneeringer R, Penzias AS, Barrett B, Usheva A. High levels of mineralocorticoids in preovulatory follicular fluid could contribute to oocyte development. Fertil Steril. 2011;95:182–187 [DOI] [PubMed] [Google Scholar]
- 9. Nakamura E, Otsuka F, Inagaki K, et al. A novel antagonistic effect of the bone morphogenetic protein system on prolactin actions in regulating steroidogenesis by granulosa cells. Endocrinology. 2010;151:5506–5518 [DOI] [PubMed] [Google Scholar]
- 10. Coccia ME, Rizzello F, Mariani G, Bulletti C, Palagiano A, Scarselli G. Impact of endometriosis on in vitro fertilization and embryo transfer cycles in young women: a stage-dependent interference. Acta Obstet Gynecol Scand. 2011;90:1232–1238 [DOI] [PubMed] [Google Scholar]
- 11. Qublan HS, Amarin Z, Tahat YA, Smadi AZ, Kilani M. Ovarian cyst formation following GnRH agonist administration in IVF cycles: incidence and impact. Hum Reprod. 2006;21:640–644 [DOI] [PubMed] [Google Scholar]
- 12. Dumesic DA, Lesnick TG, Abbott DH. Increased adiposity enhances intrafollicular estradiol levels in normoandrogenic ovulatory women receiving gonadotropin-releasing hormone analog/recombinant human follicle-stimulating hormone therapy for in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1438–1441 [DOI] [PubMed] [Google Scholar]
- 13. Robker RL, Wu LL, Yang X. Inflammatory pathways linking obesity and ovarian dysfunction. J Reprod Immunol. 2011;88:142–148 [DOI] [PubMed] [Google Scholar]
- 14. Shamonki MI, Spandorfer SD, Rosenwaks Z. Ultrasound-guided embryo transfer and the accuracy of trial embryo transfer. Hum Reprod. 2005;20:709–716 [DOI] [PubMed] [Google Scholar]
- 15. Dumesic DA, Lesnick TG, Stassart JP, Ball GD, Wong A, Abbott DH. Intrafollicular antimullerian hormone levels predict follicle responsiveness to follicle-stimulating hormone (FSH) in normoandrogenic ovulatory women undergoing gonadotropin releasing-hormone analog/recombinant human FSH therapy for in vitro fertilization and embryo transfer. Fertil Steril. 2009;92:217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shastri SM, Barbieri E, Kligman I, Schoyer KD, Davis OK, Rosenwaks Z. Stimulation of the young poor responder: comparison of the luteal estradiol/gonadotropin-releasing hormone antagonist priming protocol versus oral contraceptive microdose leuprolide. Fertil Steril. 2011;95:592–595 [DOI] [PubMed] [Google Scholar]
- 17. Phy JL, Conover CA, Abbott DH, et al. Insulin and messenger ribonucleic acid expression of insulin receptor isoforms in ovarian follicles from nonhirsute ovulatory women and polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2004;89:3561–3566 [DOI] [PubMed] [Google Scholar]
- 18. Foong SC, Abbott DH, Zschunke MA, Lesnick TG, Phy JL, Dumesic DA. Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome patients undergoing gonadotropin therapy for in vitro fertilization. J Clin Endocrinol Metab. 2006;91:2327–2333 [DOI] [PubMed] [Google Scholar]
- 19. Desforges-Bullet V, Gallo C, Lefebvre C, Pigny P, Dewailly D, Catteau-Jonard S. Increased anti-Mullerian hormone and decreased FSH levels in follicular fluid obtained in women with polycystic ovaries at the time of follicle puncture for in vitro fertilization. Fertil Steril. 2010;94:198–204 [DOI] [PubMed] [Google Scholar]
- 20. Legro RS, Schlaff WD, Diamond MP, et al. Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab. 2010;95:5305–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang SS, Carrillo AJ, Darling DS. Expression of multiple thyroid hormone receptor mRNAs in human oocytes, cumulus cells, and granulosa cells. Mol Hum Reprod. 1997;3:555–562 [DOI] [PubMed] [Google Scholar]
- 22. Ferrero H, Delgado-Rosas F, Garcia-Pascual CM, et al. Efficiency and purity provided by the existing methods for the isolation of luteinized granulosa cells: a comparative study. Hum Reprod. 2012;27:1781–1789 [DOI] [PubMed] [Google Scholar]
- 23. Petrie A, Sabin C. Medical Statistics at a Glance. 1st ed Oxford, UK: John Wiley and Sons Ltd; 2000:67–69 [Google Scholar]
- 24. Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993;14:43–68 [DOI] [PubMed] [Google Scholar]
- 25. Doody KJ, Lorence MC, Mason JI, Simpson ER. Expression of messenger ribonucleic acid species encoding steroidogenic enzymes in human follicles and corpora lutea throughout the menstrual cycle. J Clin Endocrinol Metab. 1990;70:1041–1045 [DOI] [PubMed] [Google Scholar]
- 26. Sano Y, Suzuki K, Arai K, Okinaga S, Tamaoki BI. Changes in enzyme activities related to steroidogenesis in human ovaries during the menstrual cycle. J Clin Endocrinol Metab. 1981;52;994–1001 [DOI] [PubMed] [Google Scholar]
- 27. Lan NC, Graham B, Bartter FC, Baxter JD. Binding of steroids to mineralocorticoid receptors: implications for in vivo occupancy by glucocorticoids. J Clin Endocrinol Metab. 1982;54:332–342 [DOI] [PubMed] [Google Scholar]
- 28. Bureik M, Brück N, Hübel K, Bernhardt R. The human mineralocorticoid receptor only partially differentiates between different ligands after expression in fission yeast. FEMS Yeast Res. 2005;5:627–633 [DOI] [PubMed] [Google Scholar]
- 29. Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970 [DOI] [PubMed] [Google Scholar]
- 30. Tetsuka M, Thomas FJ, Thomas MJ, Anderson RA, Mason JI, Hillier SG. Differential expression of messenger ribonucleic acids encoding 11β-hydroxysteroid dehydrogenase types 1 and 2 in human granulosa cells. J Clin Endocrinol Metab. 1997;82:2006–2009 [PubMed] [Google Scholar]
- 31. Michael AE, Evagelatou M, Norgate DP, et al. Isoforms of 11beta-hydroxysteroid dehydrogenase in human granulosa-lutein cells. Mol Cell Endocrinol. 1997;132:43–52 [DOI] [PubMed] [Google Scholar]
- 32. Thurston LM, Chin E, Jonas KC, et al. Expression of 11β-hydroxysteroid dehydrogenase (11βHSD) proteins in luteinizing human granulosa-lutein cells. J Endocrinol. 2003;178:127–135 [DOI] [PubMed] [Google Scholar]
- 33. Gosden RG, Hunter RH, Telfer E, Torrance C, Brown N. Physiological factors underlying the formation of ovarian follicular fluid. J Reprod Fertil. 1988;82:813–825 [DOI] [PubMed] [Google Scholar]
- 34. Evron S, Shapiro G, Diamant YZ. Induction of ovulation with spironolactone (Aldactone) in anovulatory oligomenorrheic and hyperandrogenic women. Fertil Steril. 1981;36:468–471 [DOI] [PubMed] [Google Scholar]
- 35. Wenner MM, Stachenfeld NS. Blood pressure and water regulation: understanding sex hormone effects within and between men and women. J Physiol (Lond). 2012;590:5949–6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parker CR, Rush AJ, MacDonald PC. Serum concentrations of deoxycorticosterone in women during the luteal phase of the ovarian cycle are not suppressed by dexamethasone treatment. J Steroid Biochem. 1983;19:1313–1317 [DOI] [PubMed] [Google Scholar]
- 37. Escoubet B, Couffignal C, Laisy JP, et al. Cardiovascular effects of aldosterone: insight from adult carriers of mineralocorticoid receptor mutations. Circ Cardiovasc Genet. 2013;6:381–390 [DOI] [PubMed] [Google Scholar]
- 38. Navot D, Margalioth EJ, Laufer N, et al. Direct correlation between plasma renin activity and severity of the ovarian hyperstimulation syndrome. Fertil Steril. 1987;48:57–61 [DOI] [PubMed] [Google Scholar]
- 39. Manno M, Tomei F. Renin-angiotensin system activation during severe OHSS: cause or effect? Fertil Steril. 2008;89:488. [DOI] [PubMed] [Google Scholar]
- 40. Strauss JF., III The synthesis and metabolism of steroid hormones. In Strauss JF, III, Barbieri RL, eds. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 6th ed Philadelphia: Saunders Elsevier; 2009:80–83 [Google Scholar]


