Abstract
Context:
Severe deficiencies of testosterone (T) and GH are associated with low bone mineral density (BMD) and increased fracture risk. Replacement of T in hypogonadal men improves several bone parameters. Replacement of GH in GH-deficient men improves BMD.
Objective:
Our objective was to determine whether T and GH treatment together improves the structural and mechanical parameters of bone more than T alone in men with hypopituitarism.
Design and Subjects:
This randomized, prospective, 2-year study included 32 men with severe deficiencies of T and GH due to panhypopituitarism.
Intervention:
Subjects were randomized to receive T alone (n = 15) or T and GH (n = 17) for 2 years.
Main Outcome Measures:
We evaluated magnetic resonance microimaging-derived structural (bone volume fraction [BVF] and trabecular thickness) and mechanical (axial stiffness [AS], a measure of bone strength) properties of the distal tibia at baseline and after 1 and 2 years of treatment.
Results:
Treatment with T and GH did not affect BVF, thickness, or AS differently from T alone. T treatment in all subjects for 2 years increased trabecular BVF by 9.6% (P < .0001), trabecular thickness by 2.6% (P < .001), and trabecular AS by 9.8% (P < .001). In contrast, testosterone treatment in all subjects significantly increased cortical thickness by 2.4% (P < .01) but decreased cortical BVF by −4.7% (P < .01) and cortical AS by −6.9% (P < .01).
Conclusion:
Combined T and GH treatment of men with hypopituitarism for 2 years did not improve the measured structural or mechanical parameters of the distal tibia more than T alone. However, testosterone significantly increased the structural and mechanical properties of trabecular bone but decreased most of these properties of cortical bone, illustrating the potential importance of assessing trabecular and cortical bone separately in future studies of the effect of testosterone on bone.
Testosterone deficiency is a well-known cause of osteoporosis in men. Severe testosterone deficiency leads to a decrease in bone mineral density (BMD) (1–3) and trabecular architecture (4, 5) and increased risk of fracture (6, 7). Testosterone treatment of men with severe testosterone deficiency improves their BMD (8, 9), trabecular architecture (10), and the mechanical properties of their bone (11). These changes are mediated, at least in part, by conversion of testosterone to estradiol (12–14).
GH deficiency has also been associated with low BMD (15) and increased risk of fracture (16, 17). GH replacement in men with GH deficiency improves BMD (18–20). Testosterone and GH appear to work synergistically to exert some anabolic effects, including those on lean body mass (21), protein synthesis (22), and sexual hair (23). Whether or not testosterone and GH act synergistically on bone has not been explored. The differential effects of testosterone and GH on trabecular and cortical bone have also not been explored.
In this study, we report the effects of testosterone alone and combined with GH on structural and mechanical properties of bone in men with severe deficiencies of both hormones, as determined by magnetic resonance microimaging (μMRI) and finite element analysis (FEA). μMRI allows noninvasive evaluation of trabecular microarchitecture as well as determination of the mechanical properties of trabecular and cortical bone separately. μMRI-based FEA has been shown to be a valuable tool in monitoring treatment effects of testosterone and estradiol (11, 24). In addition, μMRI-derived structural parameters have been shown to correlate better with vertebral deformity than BMD by DXA (25, 26). μMRI-derived axial stiffness (AS), a measure of bone strength, has been validated using cadaveric bone and has been shown to be strongly correlated with μCT-derived AS (27). Furthermore, μMRI-based AS can be determined on cortical and trabecular bone separately (28).
The primary end point of the study was to determine whether GH would potentiate the effect of testosterone on bone as determined by μMRI of distal tibia; it did not. We did find, however, that testosterone, with or without GH, had previously unsuspected differential effects on the structural and mechanical parameters of trabecular bone compared with cortical bone.
Subjects and Methods
Subject selection
The Institutional Review Board at the University of Pennsylvania approved this study, and each subject gave written, informed consent. Thirty-two men, 25 to 79 years of age, with panhypopituitarism were recruited from the Penn Pituitary Center. Inclusion criteria included male gender, hypothalamic or pituitary disease, hypogonadism (8:00 am serum testosterone <250 ng/dL twice) for at least 2 years and GH deficiency (serum IGF-1 below age-specific normal or a maximum GH response to arginine-GHRH <4.1 ng/mL) (29) for at least 2 years. Subjects had not received treatment with testosterone or GH for at least 2 years. Deficiencies of T4 and cortisol occurred with similar frequency in both groups (Table 1) and were stably replaced. Replacement of T4 was physiologic by serum T4 concentration. Replacement of hydrocortisone was physiologic clinically. Exclusion criteria included illnesses that can affect bone or in which testosterone or GH replacement are contraindicated as well as medications that affect bone and conditions that preclude performing MRI.
Table 1.
Characteristics of Subjects at Baselinea
| Characteristic | Testosterone | Testosterone and GH | P Value |
|---|---|---|---|
| Number of men | 15 | 17 | |
| Age, y | 47.2 ± 4.7 (25–70) | 52.9 ± 2.9 (25–79) | .30 |
| BMI, kg/m2 | 31.6 ± 1.8 | 31.3 ± 1.6 | .91 |
| Testosterone, ng/dL | 72.2 ± 22.2 | 87.0 ± 24.2 | .66 |
| Estradiol, pg/mL | 5.3 ± 1.3 | 4.9 ± 1.0 | .82 |
| IGF-1, ng/mL | 41.7 ± 5.9 | 59.6 ± 6.0 | .04 |
| Levothyroxine Rx (n) | 11 | 11 | .60 |
| Hydrocortisone Rx (n) | 13 | 15 | .89 |
| BMD, g/cm2 | |||
| Lumbar spine | 0.955 ± 0.061 | 0.938 ± 0.042 | .82 |
| Femoral neck | 0.762 ± 0.034 | 0.761 ± 0.035 | .98 |
| Total hip | 0.916 ± 0.042 | 0.915 ± 0.030 | .99 |
| μMRI-derived structural parameter at distal tibia | |||
| Trabecular BVF | 0.104 ± 0.004 | 0.112 ± 0.004 | .19 |
| Tb.Th, mm | 0.107 ± 0.001 | 0.108 ± 0.001 | .58 |
| Cortical BVF | 0.535 ± 0.017 | 0.519 ± 0.016 | .48 |
| Ct.Th, mm | 1.371 ± 0.118 | 1.336 ± 0.099 | .82 |
| μMRI-derived mechanical parameter at distal tibia | |||
| Trabecular bone, GPa | 1.34 ± 0.13 | 1.31 ± 0.10 | .87 |
| Cortical bone, GPa | 0.64 ± 0.07 | 0.59 ± 0.04 | .55 |
| Whole bone, GPa | 2.12 ± 0.14 | 1.97 ± 0.10 | .41 |
Unless otherwise indicated, all values are mean ± SE.
Treatment
Subjects were randomly assigned to receive testosterone alone (T-only) or testosterone plus GH (T+GH). The testosterone preparation was AndroGel 1% (AbbVie). The dose was initially 5g daily and was adjusted to keep the serum testosterone within the normal range for young men, 300–800 ng/dL. The GH preparation was HumatroPen (recombinant hGH; Eli Lilly). The dose was initially 2 μg/kg body weight and was adjusted to keep serum IGF-1 in the age- and gender-specific normal range. All subjects except for one in each treatment arm were prescribed CaCO3-vitamin D supplementation to increase their calcium intake to 1000 mg/d.
Assays of testosterone, estradiol, IGF-1, PTH, C-telopeptide, and bone-specific alkaline phosphatase
Sera from each visit were stored at −80°C. At the end of treatment, all samples from each subject were assayed in the same assay run. Serum testosterone and estradiol concentrations were assayed at the Endocrine and Metabolic Research Laboratory, Harbor-UCLA Medical Center, using liquid chromatography tandem mass spectrometry. The intra- and interassay coefficients of variation (CV) were both <5% for testosterone and <5% and <10% for estradiol. The lower limits of quantification were 1 ng/dL for testosterone (30) and 2 pg/mL for estradiol (31). Normal ranges for young men are 288 to 1004 ng/dL for testosterone and 9.9 to 32.8 pg/mL for estradiol.
Serum IGF-1, C-telopeptide (CTX), bone-specific alkaline phosphatase (BSAP) and PTH were measured in the University of Pennsylvania Clinical and Translational Research Center Core Laboratory. IGF-1 was measured by an ELISA using R&D Systems reagents. The mean intra- and interassay CV were <5.1%. CTX was measured by RIA using Orion Diagnostica reagents. The intra- and interassay CV were <8%. BSAP was measured by ELISA using MicroVue reagents. The intra- and interassay CV were <6%. Intact PTH was measured by immunoradiometric assay using Scantibodies Laboratory reagents. The intra- and interassay CVs were <2.4%.
DXA measurements
BMD of the anterior-posterior lumbar spine (L1–L4) and hip was determined by DXA using a Hologic densitometer. Three subjects did not have BMD measurements because of weight restriction. The lumbar spine could not be analyzed in 2 subjects because of severe scoliosis. Individual lumbar vertebrae were excluded from analysis if there was an anatomical deformity or more than 1.0 Z-score difference in the vertebrae in question and adjacent vertebrae. Nine subjects had 1 vertebra excluded, and 2 subjects had 2 excluded. A total of 29 subjects (14 T-only; 15 T+GH) had hip scans and 27 (12 T-only; 15 T+GH) spine scans. The operating system changed from Delphi A (version 11.2) in the first year to Discovery A (versions 12.4 and 12.6) thereafter. Analysis of scans in both software versions found no significant difference. All scans were analyzed using the compare function and reviewed for quality and consistency. The CV of daily measurements of a spine phantom was 0.4%.
Micro-MRI
Data acquisition
μMRI of the right distal tibia was performed using a 1.5-T whole-body MRI scanner. The first 12 subjects (5 T-only; 7 T+GH) were scanned on the GE instrument, and the next 20 (10 in each group) on a Siemens Sonata. Each subject was scanned with the same scanner at each visit. The change in scanner did not affect the changes in trabecular and cortical measurements. A custom-designed, 2-element receive-only surface coil was placed anteriorly over the immobilized right tibia 1 cm proximal to the medial malleolus midpoint. Using a fast large-angle spin-echo (FLASE) pulse sequence (32), 32 contiguous transaxial images were obtained with an acquisition voxel size of 137 × 137× 410 μm3.
Image processing
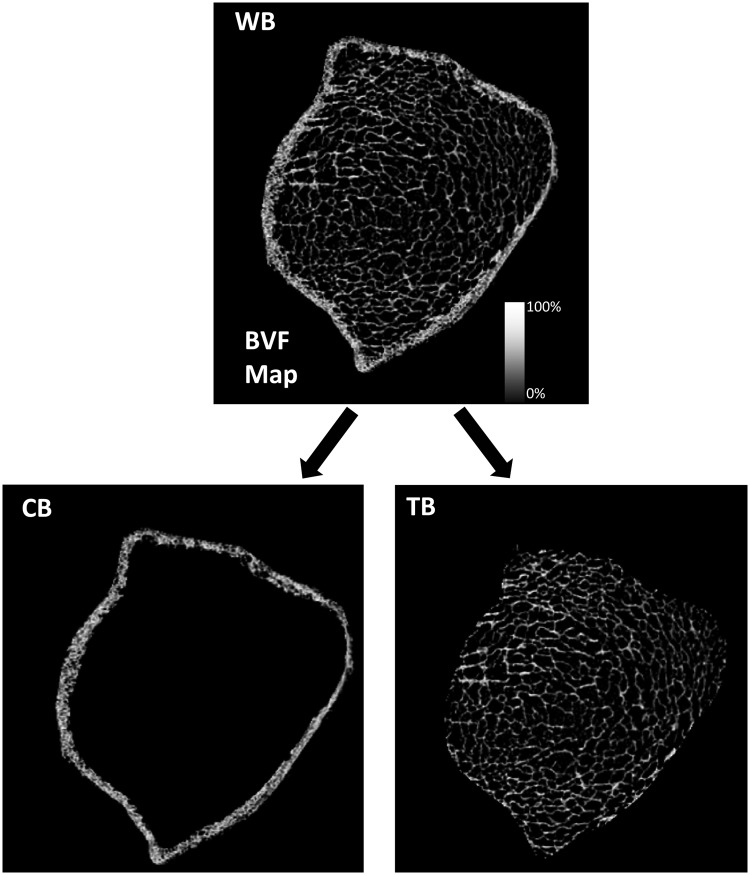
The raw μMRI data were corrected for involuntary subject motion using the navigator data during the FLASE acquisition (33) and by autofocusing (34). A local thresholding algorithm was used to correct heterogeneity in image intensity (35). Images were converted to 3-dimensional maps by assigning to each voxel a bone volume fraction (BVF) from 0% (pure bone marrow) to 100% (pure bone), depending on how much bone was present in the voxel. Using an operator-guided segmentation program, a single operator blinded to the treatment delineated the endosteal and periosteal boundaries of cortical bone to separate cortical bone from surrounding soft tissue and trabecular bone from the cortex, generating 3 sets of 3-dimensional volumes: whole bone, cortical bone, and trabecular bone (Figure 1). To ensure that the same portion of bone was compared in each subject at each time point, common transaxial slices were extracted from the dataset at each time point for each subject (Supplemental Figure 1, published on The Endocrine Society's Journals Online website at http://jcem.endojournals.org). The intra-observer reproducibility of the μMRI parameters was determined by segmenting 10 randomly selected cases 3 times each and calculating the mean CV.
Figure 1.
Separation of cortical bone from trabecular bone. BVF maps were derived from midaxial FLASE μMRI images obtained from the right tibial metaphysis. BVF represents fractional occupancy of bone linearly scaled between 0% (pure marrow, shown as black) and 100% (pure bone, shown as white) in each voxel. A single operator, starting with whole bone (WB), delineated the endosteal and periosteal boundaries of cortical bone to separate cortical bone (CB) from surrounding soft tissue and trabecular bone (TB) from the cortex.
Structural parameters
BVF of trabecular bone was derived from averaging 3D BVF of all voxels in the trabecular bone compartment, ie, the ratio of bone volume to total volume (BV/TV) (35). Cortical BVF was computed in a similar manner from the cortical bone compartment. Trabecular thickness (Tb.Th) was computed by the method of fuzzy-distance transform (36). Cortical thickness (Ct.Th) was calculated from the mean differences of radii of periosteal and endosteal boundaries on each axial slice. The radii were estimated by assuming the respective encompassed areas were concentric circles (28). The mean intra-observer CV for the structural parameters were 0.8% for trabecular BVF, 2.6% for cortical BVF, 0.2% for Tb.Th, and 5.8% for Ct.Th.
Micro-FEA
To create an finite element (FE) model, each voxel was converted to a hexahedral FE with the same dimensions. Bone was assumed to have a tissue modulus of 15 GPa and a Poisson's ratio of 0.3 (37). The tissue modulus of each FE was linearly adjusted by BVF to account for the fractional bone volume content in each voxel. This process resulted in the generation of 3 sets of FE models (whole, cortical, and trabecular bone) for each subject at each time point (27).
AS was estimated by simulated compressive loading along the superior-inferior direction for each FE model (whole bone, cortical bone and trabecular bone) separately (27, 28). One percent axial strain was applied separately to the proximal face of each of the three models whereas the distal face was constrained in the axial direction. AS was calculated as the ratio of the stress on the proximal face to the applied strain. The mean intra-observer CV values for the mechanical parameters were 2.9% for whole-bone AS, 3.4% for cortical AS, and 1.3% for trabecular AS.
Bone strength was also measured in terms of failure load, as described in Supplemental Figure 2.
Statistical analysis
Sample size estimation was based on trabecular BVF as the primary endpoint. We assumed an α (type I error rate) of 0.05 (two-tailed) and a ß of 0.2 (80% power). We based the SD of the change in BVF during 24 months of treatment on that in one of our previous studies (5). The estimate of the difference between the means of the 2 treatment arms (μ1 − μ2) was based on the effect of GH on bone in men (19). Using these assumptions and values, we calculated that we would need 15 men in each treatment arm, or 30 total men, to complete 24 months of treatment. In fact, 32 men completed 24 months. To account for multiple comparisons, repeated-measures ANOVA was performed first to determine whether there was an overall significant change in outcome with treatment. If so, further analyses were performed on percent changes from baseline to 12 or 24 months and from 12 to 24 months using both the paired t tests and Wilcoxon's signed-rank tests. The percent changes at 12 and 24 months were compared between the 2 treatment groups (T-only and T+GH) using the unpaired 2-sample t test and Wilcoxon's rank-sum test. Because the results were similar, only the t test results are reported. A type I error rate of 0.05 was used for statistical significance. STATA statistical software version 12 was used for all analyses.
Results
Subjects
Thirty-five subjects were randomized, 17 to the T-only group and 18 to the T+GH group. Thirty-one completed 24 months of treatment. Two did not begin treatment, and 1 did not return after beginning treatment. Treatment was terminated in 1 subject after the 12-month visit when he developed prostate cancer. Data from 32 subjects (15 T-only and 17 T+GH), the 31 who completed 24 months and the 1 who discontinued at 12 months, are presented.
The T-only and T+GH groups were similar in baseline age, BMI, serum testosterone, and estradiol concentrations, BMD, and μMRI-derived structural and mechanical parameters (Table 1). The IGF-1 concentrations were statistically different, but both were low.
Serum testosterone, estradiol, IGF-1, CTX, BSAP, and PTH concentrations
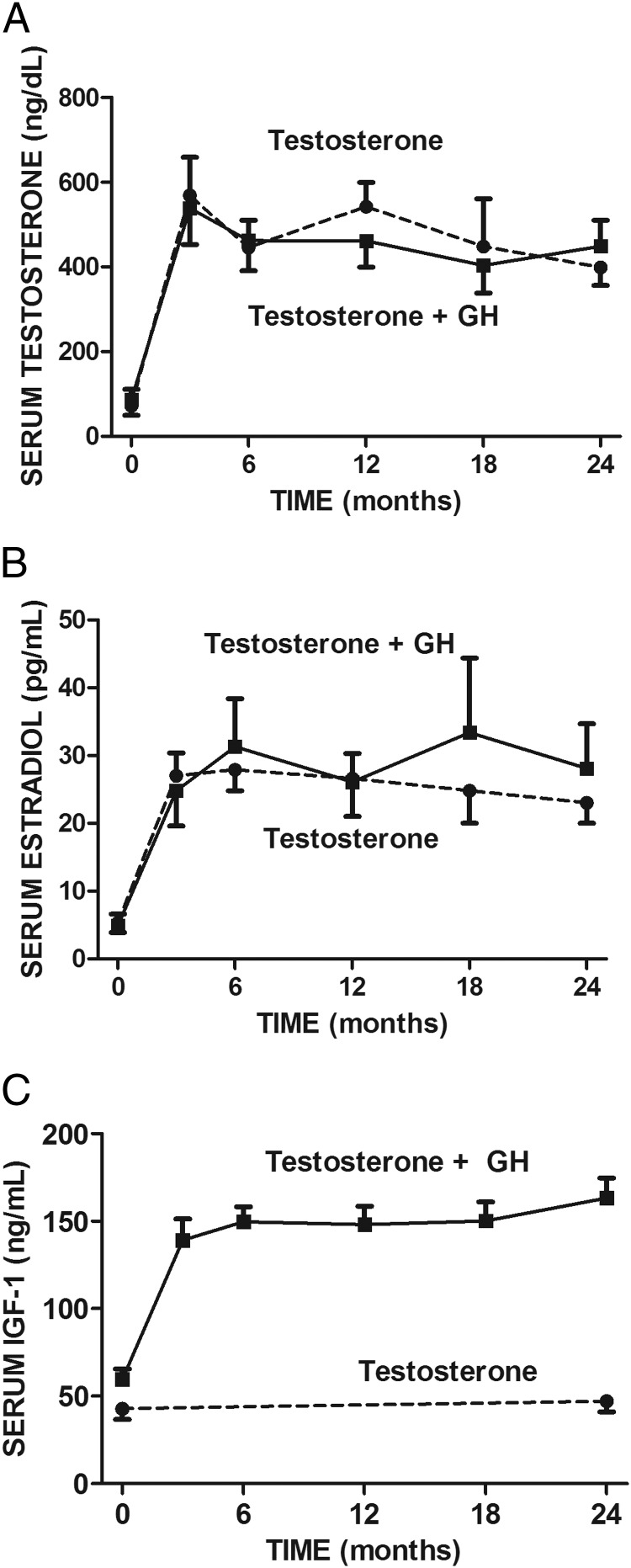
The serum testosterone and estradiol concentrations increased in both treatment groups from low at baseline to midnormal for young men by month 3 and remained normal for the remainder of treatment (Figure 2, A and B). The mean IGF-1 concentration in the T-only group did not change from baseline to 24 months (Figure 2C). The mean serum IGF-1 in the T+GH group increased by month 3 of treatment and remained stable through month 24. Neither serum CTX nor BSAP changed during treatment in either group, nor did the treatment groups differ from each other in either parameter at any time. The mean intact PTH concentration was normal at baseline and did not change during treatment in either treatment group and did not differ between the treatment groups at any time (data not shown).
Figure 2.
Serum concentrations of testosterone (A), estradiol (B), and IGF-1 (C) at baseline and after 3, 6, 12, 18, and 24 months of treatment with testosterone (n = 15) or testosterone and growth hormone (n = 17).
DXA results
Repeated-measures ANOVA showed a highly significant overall treatment effect on areal BMD of the lumbar spine (P < .0001), femoral neck (P < .0001), and total hip (P < .0001) (Table 2). Areal BMD at all 3 sites increased significantly from baseline to 12 and 24 months in both groups but did not differ between the 2 groups at any site at any time (Supplemental Figure 3). At the lumbar spine, the mean (±SE) increases in the T-only and T-GH groups at 24 months were 6.3% ± 1.9% (P < .01) and 7.2% ± 1.1% (P < .001). At the femoral neck, the mean increases were 4.5% ± 1.1% (P < .01) and 5.2% ± 1.3% (P < .01). At the total hip, the mean increases were 5.4% ± 1.2% (P < .001) and 5.7% ± 1.5% (P < .01).
Table 2.
Areal BMD by DXA and μMRI-derived Structural and Mechanical Parameters of the Distal Tibia Before and During Treatment of Men With Hypopituitarism With Either Testosterone Alone (n = 15) or a Combination of Testosterone and GH (n = 17) and All Subjects (n = 32)a
| Testosterone |
Testosterone and GH |
All Subjects |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 mo | 24 mo | Baseline | 12 mo | 24 mo | Baseline | 12 mo | 24 mo | |
| Areal BMD by DXA | |||||||||
| Lumbar spine, g/cm2b | 0.955 ± 0.061 | 1.001 ± 0.062 | 1.012 ± 0.065 | 0.938 ± 0.042 | 0.970 ± 0.050 | 0.996 ± 0.052 | 0.946 ± 0.035 | 0.984 ± 0.038 | 1.004 ± 0.040 |
| Femoral neck, g/cm2b | 0.762 ± 0.034 | 0.783 ± 0.034 | 0.797 ± 0.038 | 0.761 ± 0.035 | 0.785 ± 0.036 | 0.804 ± 0.038 | 0.761 ± 0.024 | 0.784 ± 0.024 | 0.800 ± 0.026 |
| Total hip, g/cm2b | 0.916 ± 0.042 | 0.952 ± 0.040 | 0.961 ± 0.039 | 0.915 ± 0.030 | 0.950 ± 0.031 | 0.965 ± 0.038 | 0.915 ± 0.025 | 0.950 ± 0.025 | 0.963 ± 0.027 |
| Structural parameters | |||||||||
| Trabecular BVFb | 0.104 ± 0.004 | 0.111 ± 0.006 | 0.114 ± 0.005 | 0.112 ± 0.004 | 0.119 ± 0.004 | 0.123 ± 0.005 | 0.109 ± 0.003 | 0.115 ± 0.003 | 0.119 ± 0.004 |
| Tb.Th, mmb | 0.107 ± 0.001 | 0.108 ± 0.001 | 0.109 ± 0.001 | 0.108 ± 0.001 | 0.110 ± 0.001 | 0.119 ± 0.001 | 0.108 ± 0.001 | 0.109 ± 0.001 | 0.111 ± 0.001 |
| Cortical BVFb | 0.535 ± 0.017 | 0.499 ± 0.020 | 0.501 ± 0.015 | 0.519 ± 0.016 | 0.480 ± 0.015 | 0.498 ± 0.014 | 0.527 ± 0.017 | 0.489 ± 0.012 | 0.499 ± 0.010 |
| Ct.Th, mmb | 1.371 ± 0.118 | 1.398 ± 0.120 | 1.402 ± 0.120 | 1.336 ± 0.099 | 1.379 ± 0.103 | 1.354 ± 0.106 | 1.353 ± 0.075 | 1.388 ± 0.077 | 1.377 ± 0.079 |
| Mechanical parameters (AS) | |||||||||
| Trabecular bone, GPac | 1.34 ± 0.13 | 1.43 ± 0.16 | 1.48 ± 0.17 | 1.31 ± 0.10 | 1.39 ± 0.10 | 1.43 ± 0.12 | 1.33 ± 0.08 | 1.41 ± 0.09 | 1.45 ± 0.10 |
| Cortical BOne, GPac | 0.64 ± 0.07 | 0.60 ± 0.07 | 0.58 ± 0.07 | 0.59 ± 0.04 | 0.53 ± 0.05 | 0.55 ± 0.04 | 0.61 ± 0.04 | 0.56 ± 0.04 | 0.57 ± 0.04 |
| Whole bone, GPad | 2.12 ± 0.14 | 2.17 ± 0.17 | 2.21 ± 0.18 | 1.97 ± 0.10 | 2.00 ± 0.11 | 2.04 ± 0.11 | 2.04 ± 0.09 | 2.08 ± 0.10 | 2.12 ± 0.10 |
All values are mean ± SE.
Overall trend, P < .0001.
Overall trend, P < .0005.
Overall trend, P < .005.
Micro-MRI results
Trabecular and cortical BVFs
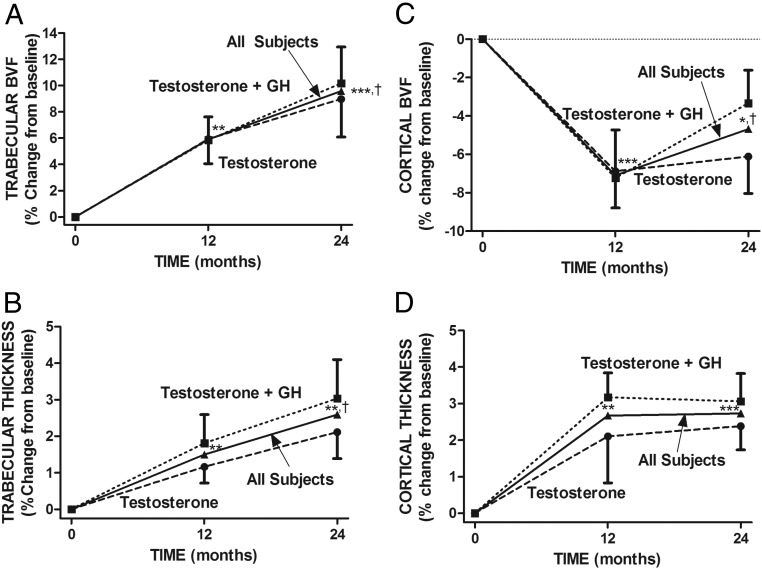
Repeated-measures ANOVA showed a highly significant overall treatment effect on both trabecular and cortical BVF (P < .0001 each). Mean (±SE) trabecular BVF increased significantly from baseline in both groups at 12 and 24 months (Table 2 and Figure 3A). In contrast, mean (±SE) cortical BVF decreased significantly from baseline to 12 months in both groups. At 24 months, cortical BVF in the T-only group remained significantly lower than baseline, but that in the T+GH group did not. From 12 to 24 months, cortical BVF did not change significantly in the T-only group but increased in the T+GH group (Table 2 and Figure 3C). The changes in cortical bone with time can be appreciated visually in the insets in Supplemental Figure 1. There was no significant difference between the 2 treatment groups at any time. In all subjects combined, mean trabecular BVF increased significantly from baseline to 12 (5.9% ± 1.3%, P < .001) and 24 months (9.6% ± 2.0%, P < .0001) (Figure 3C). In contrast, mean (±SE) cortical BVF decreased significantly from baseline to 12 (−7.1% ± 1.3%, P < .0001) and 24 months (−4.7% ± 1.3, P < .01).
Figure 3.
Contrast between the effects of testosterone and GH on trabecular bone structure (A and B) and their effects on cortical bone structure (C and D). Illustrated are the percent changes in trabecular BVF (A), Tb.Th (B), cortical BVF (C), and Ct.Th (D) of the distal tibia from baseline to 12 and 24 months after treatment with testosterone (n = 15) and testosterone and GH (n = 17) and in all subjects (n = 32). There was no significant difference between the 2 treatment groups at any time point. *, P < .01; **, P < .001; ***, P < .0001 compared with baseline; †, P < .05, 24 months compared with 12 months in all subjects.
Whole bone and trabecular areas
Whole bone area, determined by periosteal perimeter, increased significantly at 24 months (1.6%, P < .01). Trabecular area, determined by endocortical perimeter, did not increase significantly at 24 months (1.2%, not significant). There was no difference in whole bone area and endocortical trabecular area between the treatment groups.
Trabecular and cortical thickness
Repeated-measures ANOVA showed highly significant overall treatment effects on both trabecular and cortical thickness (P < .0001 each). Mean Tb.Th increased significantly in both groups from baseline to 12 and 24 months (Table 2 and Figure 3B). Mean Ct.Th increased significantly in both groups from baseline to 12 and 24 months (Table 2 and Figure 3D). There was no significant difference between the 2 groups at any time. In all subjects combined, mean (±SE) Tb.Th increased significantly from baseline to 12 (1.5% ± 0.5%, P < .001) and 24 months (2.6% ± 0.7%, P < .001), and mean Ct.Th increased significantly from baseline to 12 (2.7% ± 0.6%, P < .001) and 24 (2.7% ± 0.5%, P < .0001) months (Figure 3C).
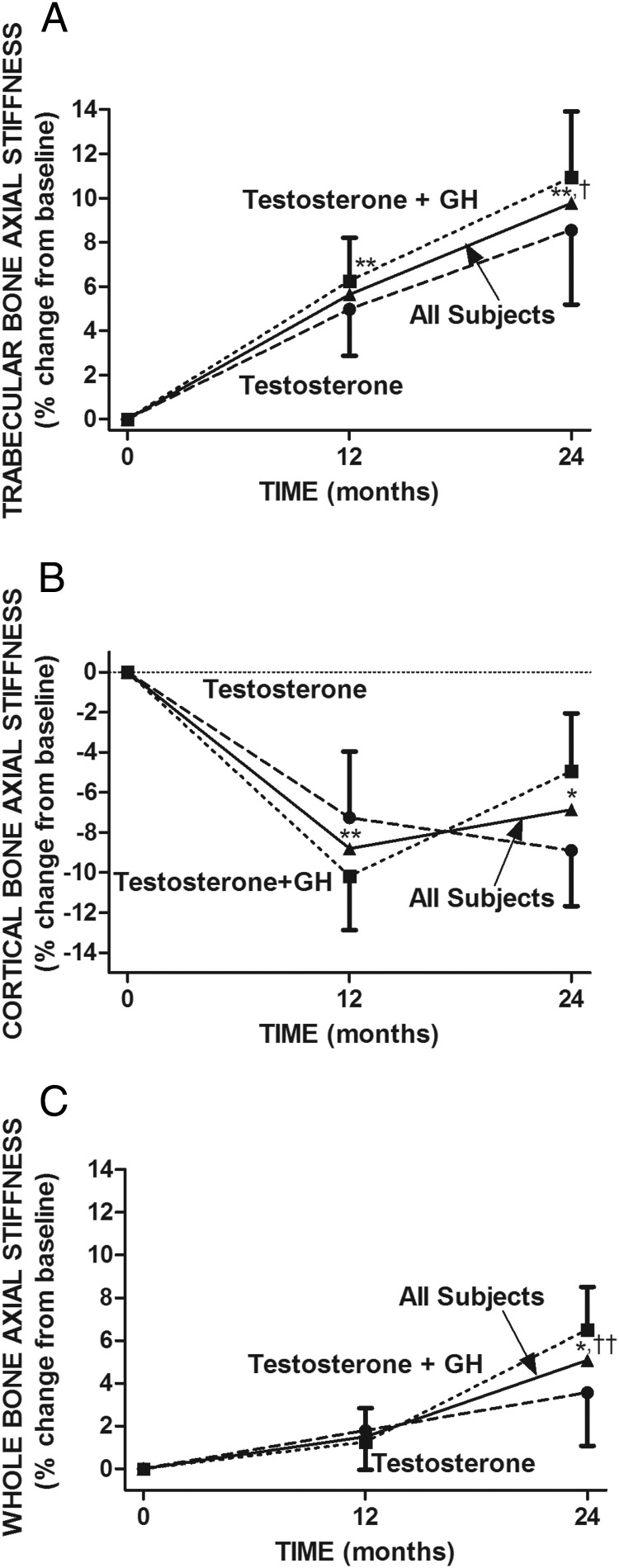
Axial stiffness
Repeated-measures ANOVA showed highly significant overall treatment effects on trabecular and cortical AS (P < .0005 each). Mean trabecular AS increased significantly in both groups from baseline to 12 and 24 months (Table 2 and Figure 4A). The increase was not statistically significant between the 2 treatment groups. In contrast, cortical AS decreased significantly in both groups from baseline to 12 months (Table 2 and Figure 4B). From 12 to 24 months, cortical AS tended to decrease further in the T-only group but increased in the T+GH group. At 24 months, cortical AS in the T-only group remained significantly lower than baseline but not in the T+GH group. There was no significant difference between the 2 groups at any time point. In all subjects combined, mean (±SE) trabecular AS increased significantly from baseline to 12 (5.7% ± 1.4%, P < .001) and 24 (9.8% ± 2.2%, P < .001) months (Figure 4C). In contrast, mean cortical AS decreased significantly from baseline to 12 months (−8.8% ± 2.1%, P < .001). At 24 months, cortical AS tended to increase compared with 12 months (3.2% ± 2.2%, NS), but at 24 months, it remained significantly lower than baseline (−6.9% ± 2.0%, P < .01).
Figure 4.
Comparison of the effects of testosterone and GH on trabecular bone strength (A), cortical bone strength (B), and whole bone strength (C). Illustrated are the percent changes in AS of the distal tibia from baseline to 12 and 24 months after treatment with testosterone (n = 15) and testosterone and GH (n = 17) and in all subjects (n = 32). AS was determined by μMRI-based FEA of trabecular bone (A), cortical bone (B), and whole bone (C). There was no significant difference between the 2 treatment groups at any time point. *, P < .01; **, P < .001, compared with baseline; †, P < .05; ††, P < .001, 24 months compared with 12 months in all subjects.
Repeated-measures ANOVA showed a highly significant overall treatment effect on whole-bone AS (P < .005). Whole-bone AS tended to increase in both treatment groups at 12 months and increased further by 24 months (Table 2 and Figure 4C). In all subjects combined, mean whole-bone AS increased from baseline to 12 months (1.5% ± 1.2%, not significant) and baseline to 24 months (5.1% ± 1.6%, P < .01) (Figure 4C).
Results of failure load assessment are shown in Supplemental Table 1 and Supplemental Figure 2.
Discussion
Treatment for 2 years of men who had severe hypopituitarism with both testosterone and GH significantly altered the structural and mechanical parameters of their distal tibia, as determined by μMRI, but not to a significantly greater degree than testosterone treatment alone. These results were surprising, because previous studies showed that GH treatment for 2 years of similar men increased their lumbar spine BMD (19, 20). In one study, GH treatment increased BMD of the lumbar spine by about 4% compared with placebo (20). In the present study, however, the combination of GH and testosterone did not increase lumbar spine BMD significantly more than testosterone alone. Perhaps the difference in the results of the 2 studies was related to the fact that in the previous study, the men had been stably treated with testosterone before GH was added. Another possible explanation is that the antiresorptive action of testosterone attenuated the anabolic effect of GH, similar to antiresorptive drugs attenuating the anabolic effect of teriparatide (38). A third possibility is that GH would have demonstrated an effect in a larger number of subjects.
Unexpectedly, testosterone treatment had a markedly different effect on trabecular bone than on cortical bone, a finding made possible by technical advances during the course of the trial that allowed analysis of the structural and mechanical properties of trabecular and cortical bone separately. The effect of testosterone treatment on trabecular bone was consistently positive, significantly increasing both the structural (BVF and Tb.Th) and mechanical (AS, a measure of strength) parameters of trabecular bone linearly during the 2 years of treatment. The increase in the structural parameters is consistent with, and appears to explain, the increase in apparent trabecular strength. In contrast, testosterone treatment had mixed effects on parameters of cortical bone, increasing Ct.Th but decreasing cortical BVF and cortical AS, especially in the first year of treatment. The decrease in cortical BVF is consistent with, and probably explains, the decrease in measured cortical bone strength (AS).
Although the current results were obtained at the distal tibia, which is not a common fracture site, a recent study demonstrated that structural and mechanical properties of trabecular and cortical bone at the distal tibia, determined by high-resolution peripheral quantitative computed tomography, were significantly decreased in patients with a history of low-trauma fracture (39).
Because withholding testosterone from men who were so severely hypogonadal would have been unethical, we could not compare hypogonadal subjects untreated for 2 years to the testosterone-treated men. However, in a previous study in men of similar age and severity of hypogonadism, treatment for 2 years also increased BVF and Tb.Th significantly in a 2-year period, and yet age-matched eugonadal men, followed simultaneously, exhibited no change in BVF or Tb.Th (10).
The effects of testosterone on cortical bone are similar to those of teriparatide, which increases not only Ct.Th but also cortical porosity. When teriparatide was administered to 18 postmenopausal women for 18 months, tibial Ct.Th increased by 3.8% and cortical porosity, determined by 1 − BVF, by 13.0% (40). In the present study, testosterone was associated with a decrease in cortical BVF, which is equivalent to an increase in cortical porosity. We explored the possibility that the effect of testosterone on cortical bone was exerted via PTH, but serum PTH concentrations did not change during testosterone treatment. The mechanism by which testosterone exerted its differential effects on trabecular and cortical bone is not certain. The effects of testosterone on bone are at least partly mediated by its conversion to estradiol (13), but serum estradiol concentrations were similar in the 2 groups before and during treatment.
The biologic significance of these results is that they demonstrate the early effects of testosterone on bone remodeling in men with longstanding and severe testosterone deficiency. The practical significance is that assessment of whole-bone structural and mechanical properties in response to a treatment does not necessarily reflect the effects of the treatment on cortical and trabecular bone separately. Future studies of the effects of testosterone, and other agents, on bone should also evaluate these separately. In addition, for testosterone, observation for more than 2 years will be necessary to determine its long-term effects.
In summary, treatment of men who had hypopituitarism with the combination of testosterone and GH did not affect the structural and mechanical parameters of bone at the distal tibia differently from treatment with testosterone alone. Unexpectedly, however, testosterone treatment had very different early effects on the structural and mechanical parameters of cortical bone than on those of trabecular bone. Testosterone treatment for 2 years significantly increased both types of parameters of trabecular bone but, in the first year, significantly decreased those parameters of cortical bone. These results show the early effects of testosterone on bone remodeling and the importance of evaluating trabecular and cortical bone separately in future studies.
Acknowledgments
We thank AbbVie Laboratories for the donation of AndroGel and Eli Lilly for the donation of HumatroPen. We also thank Dr Laurence Kennedy of the University of Florida for recruitment of subjects, Donna Paulhamus-Giordano for reviewing DXA scans, and the nurses and research technicians of the Clinical and Translational Research Center for caring for the subjects and acquiring research data. We also thank the members of the Data Safety Monitoring Board: Drs Clifford Rosen (chair), Lawrence Frohman, Siu Lui Hui, and Christina Wang.
This study was supported by the National Institutes of Health (RO1-AR-050618 to P.J.S. and RO1-AR-55647 to F.W.W.) and Clinical and Translational Science Award UL1-TR000003. Salary support for M.A. was in part from the Institute for the Translational Medicine and Therapeutics of the University of Pennsylvania (UL1-TR000003) and for C.S.R. was from the National Institutes of Health (K25-AR-060283).
Current address for Y.A.B.: Samsung R&D Institute America, Richardson, Texas.
Current address for S.O.L.: Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
Disclosure Summary: P.J.S. has received research funding from AbbVie Laboratories. The other authors have nothing to disclose.
Footnotes
- AS
- axial stiffness
- BMD
- bone mineral density
- BSAP
- bone-specific alkaline phosphatase
- BVF
- bone volume fraction
- Ct.Th
- cortical thickness
- CTX
- C-telopeptide
- CV
- coefficients of variation
- FE
- finite element
- FEA
- FE analysis
- FLASE
- fast large-angle spin-echo
- μMRI
- magnetic resonance microimaging
- Tb.Th
- trabecular thickness
- T-only
- testosterone alone.
References
- 1. Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90:6410–6417 [DOI] [PubMed] [Google Scholar]
- 2. Stepán JJ, Lachman M, Zverina J, Pacovský V, Baylink DJ. Castrated men exhibit bone loss: effect of calcitonin treatment on biochemical indices of bone remodeling. J Clin Endocrinol Metab. 1989;69:523–527 [DOI] [PubMed] [Google Scholar]
- 3. Wadhwa VK, Weston R, Mistry R, Parr NJ. Long-term changes in bone mineral density and predicted fracture risk in patients receiving androgen-deprivation therapy for prostate cancer, with stratification of treatment based on presenting values. BJU Int. 2009;104:800–805 [DOI] [PubMed] [Google Scholar]
- 4. Hamilton EJ, Ghasem-Zadeh A, Gianatti E, et al. Structural decay of bone microarchitecture in men with prostate cancer treated with androgen deprivation therapy. J Clin Endocrinol Metab. 2010;95:E456–E463 [DOI] [PubMed] [Google Scholar]
- 5. Benito M, Gomberg B, Wehrli FW, et al. Deterioration of trabecular architecture in hypogonadal men. J Clin Endocrinol Metab. 2003;88:1497–1502 [DOI] [PubMed] [Google Scholar]
- 6. Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164 [DOI] [PubMed] [Google Scholar]
- 7. Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–7903 [DOI] [PubMed] [Google Scholar]
- 8. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–2677 [DOI] [PubMed] [Google Scholar]
- 9. Leifke E, Körner HC, Link TM, Behre HM, Peters PE, Nieschlag E. Effects of testosterone replacement therapy on cortical and trabecular bone mineral density, vertebral body area and paraspinal muscle area in hypogonadal men. Eur J Endocrinol. 1998;138:51–58 [DOI] [PubMed] [Google Scholar]
- 10. Benito M, Vasilic B, Wehrli FW, et al. Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res. 2005;20:1785–1791 [DOI] [PubMed] [Google Scholar]
- 11. Zhang XH, Liu XS, Vasilic B, et al. In vivo microMRI-based finite element and morphological analyses of tibial trabecular bone in eugonadal and hypogonadal men before and after testosterone treatment. J Bone Miner Res. 2008;23:1426–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khosla S, Melton LJ, 3rd, Riggs BL. Clinical review 144: Estrogen and the male skeleton. J Clin Endocrinol Metab. 2002;87:1443–1450 [DOI] [PubMed] [Google Scholar]
- 13. Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab. 2003;88:204–210 [DOI] [PubMed] [Google Scholar]
- 15. Tritos NA, Greenspan SL, King D, et al. Unreplaced sex steroid deficiency, corticotropin deficiency, and lower IGF-I are associated with lower bone mineral density in adults with growth hormone deficiency: a KIMS database analysis. J Clin Endocrinol Metab. 2011;96:1516–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosén T, Wilhelmsen L, Landin-Wilhelmsen K, Lappas G, Bengtsson BA. Increased fracture frequency in adult patients with hypopituitarism and GH deficiency. Eur J Endocrinol. 1997;137:240–245 [DOI] [PubMed] [Google Scholar]
- 17. Wüster C, Abs R, Bengtsson BA, et al. The influence of growth hormone deficiency, growth hormone replacement therapy, and other aspects of hypopituitarism on fracture rate and bone mineral density. J Bone Miner Res. 2001;16:398–405 [DOI] [PubMed] [Google Scholar]
- 18. Bex M, Abs R, Maiter D, Beckers A, Lamberigts G, Bouillon R. The effects of growth hormone replacement therapy on bone metabolism in adult-onset growth hormone deficiency: a 2-year open randomized controlled multicenter trial. J Bone Miner Res. 2002;17:1081–1094 [DOI] [PubMed] [Google Scholar]
- 19. Baum HB, Biller BM, Finkelstein JS, et al. Effects of physiologic growth hormone therapy on bone density and body composition in patients with adult-onset growth hormone deficiency. A randomized, placebo-controlled trial. Ann Intern Med. 1996;125:883–890 [DOI] [PubMed] [Google Scholar]
- 20. Snyder PJ, Biller BM, Zagar A, et al. Effect of growth hormone replacement on BMD in adult-onset growth hormone deficiency. J Bone Miner Res. 2007;22:762–770 [DOI] [PubMed] [Google Scholar]
- 21. Blackman MR, Sorkin JD, Münzer T, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–2292 [DOI] [PubMed] [Google Scholar]
- 22. Mauras N, Rini A, Welch S, Sager B, Murphy SP. Synergistic effects of testosterone and growth hormone on protein metabolism and body composition in prepubertal boys. Metabolism. 2003;52:964–969 [DOI] [PubMed] [Google Scholar]
- 23. Blok GJ, de Boer H, Gooren LJ, van der Veen EA. Growth hormone substitution in adult growth hormone-deficient men augments androgen effects on the skin. Clin Endocrinol (Oxf). 1997;47:29–36 [DOI] [PubMed] [Google Scholar]
- 24. Wehrli FW, Rajapakse CS, Magland JF, Snyder PJ. Mechanical implications of estrogen supplementation in early postmenopausal women. J Bone Miner Res. 2010;25:1406–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ladinsky GA, Vasilic B, Popescu AM, et al. Trabecular structure quantified with the MRI-based virtual bone biopsy in postmenopausal women contributes to vertebral deformity burden independent of areal vertebral BMD. J Bone Miner Res. 2008;23:64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wehrli FW, Gomberg BR, Saha PK, Song HK, Hwang SN, Snyder PJ. Digital topological analysis of in vivo magnetic resonance microimages of trabecular bone reveals structural implications of osteoporosis. J Bone Miner Res. 2001;16:1520–1531 [DOI] [PubMed] [Google Scholar]
- 27. Rajapakse CS, Magland JF, Wald MJ, et al. Computational biomechanics of the distal tibia from high-resolution MR and micro-CT images. Bone. 2010;47:556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rajapakse CS, Leonard MB, Bhagat YA, Sun W, Magland JF, Wehrli FW. Micro-MR imaging-based computational biomechanics demonstrates reduction in cortical and trabecular bone strength after renal transplantation. Radiology. 2012;262:912–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Biller BM, Samuels MH, Zagar A, et al. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab. 2002;87:2067–2079 [DOI] [PubMed] [Google Scholar]
- 30. Shiraishi S, Lee PW, Leung A, Goh VH, Swerdloff RS, Wang C. Simultaneous measurement of serum testosterone and dihydrotestosterone by liquid chromatography-tandem mass spectrometry. Clin Chem. 2008;54:1855–1863 [DOI] [PubMed] [Google Scholar]
- 31. Rothman MS, Carlson NE, Xu M, et al. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry. Steroids. 2011;76:177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Magland JF, Wald MJ, Wehrli FW. Spin-echo micro-MRI of trabecular bone using improved 3D fast large-angle spin-echo (FLASE). Magn Reson Med. 2009;61:1114–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song HK, Wehrli FW. In vivo micro-imaging using alternating navigator echoes with applications to cancellous bone structural analysis. Magn Reson Med. 1999;41:947–953 [DOI] [PubMed] [Google Scholar]
- 34. Lin W, Ladinsky GA, Wehrli FW, Song HK. Image metric-based correction (autofocusing) of motion artifacts in high-resolution trabecular bone imaging. J Magn Reson Imaging. 2007;26:191–197 [DOI] [PubMed] [Google Scholar]
- 35. Vasilic B, Wehrli FW. A novel local thresholding algorithm for trabecular bone volume fraction mapping in the limited spatial resolution regime of in vivo MRI. IEEE Trans Med Imaging. 2005;24:1574–1585 [DOI] [PubMed] [Google Scholar]
- 36. Saha PK, Wehrli FW. Measurement of trabecular bone thickness in the limited resolution regime of in vivo MRI by fuzzy distance transform. IEEE Trans Med Imaging. 2004;23:53–62 [DOI] [PubMed] [Google Scholar]
- 37. Zysset PK, Guo XE, Hoffler CE, Moore KE, Goldstein SA. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J Biomech. 1999;32:1005–1012 [DOI] [PubMed] [Google Scholar]
- 38. Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215 [DOI] [PubMed] [Google Scholar]
- 39. Stein EM, Liu XS, Nickolas TL, et al. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res. 2010;25:2572–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hansen S, Hauge EM, Beck Jensen JE, Brixen K. Differing effects of PTH 1–34, PTH 1–84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2013;28:736–745 [DOI] [PubMed] [Google Scholar]