Abstract
Context:
Placental CYP27B1 may contribute to circulating maternal calcitriol concentrations across gestation, but determinants of CYP27B1 and CYP24A1 expression in term human placental tissue are not well established.
Objective:
We hypothesized that higher CYP27B1 protein expression would be associated with increased maternal calcitriol during gestation and that CYP27B1 expression would be impacted by substrate availability.
Design:
This was a prospective, longitudinal study.
Setting:
The study was completed in an urban, prenatal clinic located in Rochester, New York.
Patients:
The study was undertaken in a cohort of 70 pregnant adolescents (≤18 y of age) and their term neonates.
Intervention:
There was no intervention.
Main Outcomes:
Protein and mRNA expressions of CYP27B1, CYP24A1, and vitamin D receptor were measured in term placental tissue and related to 25-hydroxyvitamin D [25(OH)D], 1,25-dihydroxyvitamin D, PTH, serum total calcium, IL-6, leptin, and osteoprotegerin measured in maternal serum at midgestation and delivery and in umbilical cord serum at birth.
Results:
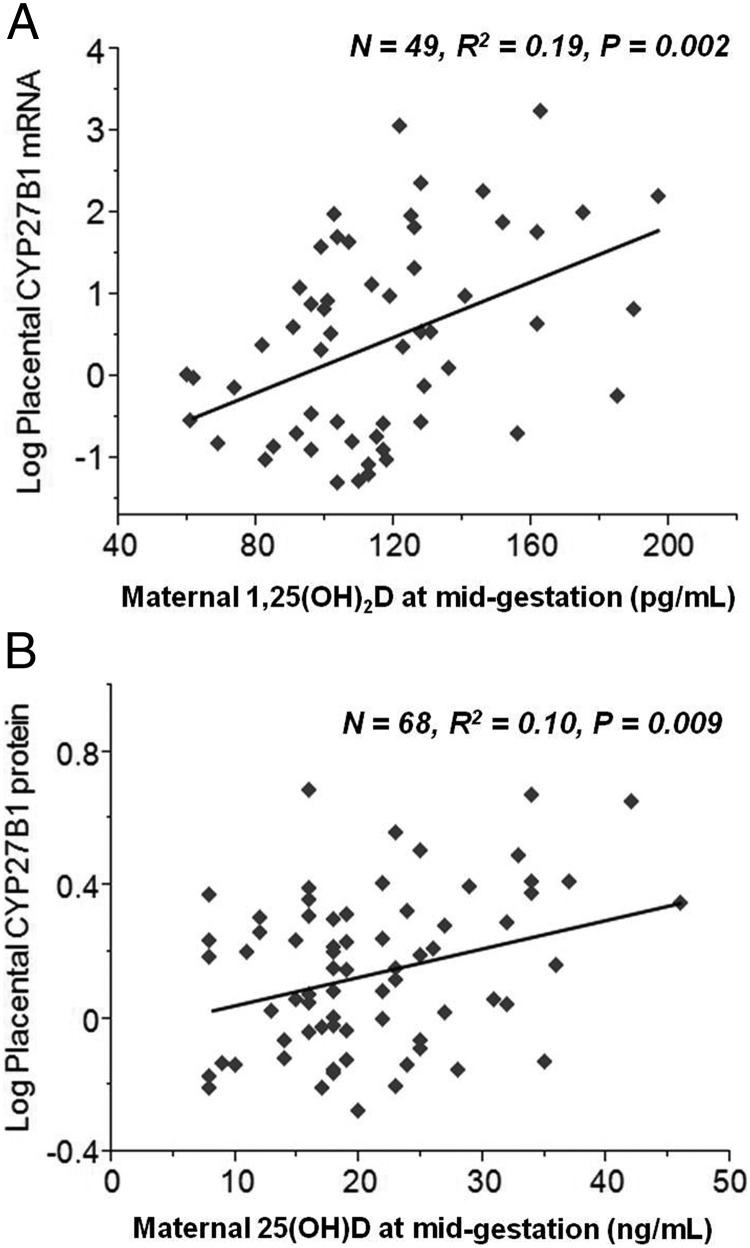
Placental CYP27B1 protein expression was significantly positively associated with maternal 25(OH)D at both midgestation (n = 68, P = .009) and delivery (n=67, P = .006). Maternal serum 1,25-dihydroxyvitamin D concentrations at midgestation were positively correlated with term placental CYP27B1 mRNA expression (n = 49, P = .002). Significant positive associations were evident between placental CYP27B1 and CYP24A1 protein expression (P = .001, n = 70). Maternal PTH concentrations at midgestation or delivery did not significantly impact placental protein or transcript level of either enzyme. Variability in placental CYP27B1 protein expression was best captured by a model that included maternal midgestation 25(OH)D concentration, placental vitamin D receptor protein expression, and maternal midgestation IL-6 concentrations (P = .002, n = 60, R2 = 0.22).
Conclusions:
Higher maternal 25(OH)D during pregnancy was associated with significantly higher placental protein expression of CYP27B1 at term supportive of a link between substrate availability and placental production of calcitriol.
Despite the importance of vitamin D status in maternal and neonatal health, many questions remain regarding vitamin D homeostasis during pregnancy. Suboptimal vitamin D status during pregnancy has been associated with a number of adverse birth outcomes including preeclampsia, gestational diabetes, and primary cesarean sections (1). Maternal vitamin D status during pregnancy has been found to impact fetal bone growth and geometry, bone mass later in childhood, and risk of subsequent chronic diseases in offspring but these associations and the mechanisms responsible for these findings remain controversial (2).
During pregnancy the placenta not only functions as an interface for nutrient and waste transport between the mother and fetus, but this organ also assumes key endocrine roles across gestation. For more than 30 years, it has been known that the placenta expresses both CYP27B1 (1α-hydroxylase) and CYP24A1 (24-hydroxylase), enzymes that are integral to the vitamin D metabolic pathway (3, 4). In addition to the machinery needed to produce and catabolize calcitriol, the placenta also expresses the vitamin D receptor (VDR) (5) in both the decidua (6) and trophoblast (7), suggesting that vitamin D may have regulatory roles in this tissue. At present the degree to which placental CYP27B1 and CYP24A1 activity might contribute to the elevated maternal calcitriol concentrations observed during pregnancy remains controversial. Some data indicate that nearly 50% of maternal calcitriol is obtained from the maternal decidua and placenta (8), whereas others have concluded that the placenta contributes little to the pregnancy-related increase in this hormone (9).
In the kidney, PTH supports calcium and phosphorus homeostasis by enhancing the activity of the CYP27B1 enzyme and inhibiting the activity of the CYP24A1 enzyme (10). The calcitriol produced is then involved in a negative feedback loop to inhibit the renal CYP27B1 enzyme (11) while stimulating expression of the CYP24A1 enzyme (12). In contrast, the CYP27B1 and CYP24A1 enzymes in the placenta are not believed to be regulated by the same negative feedback loops (13), and elevated PTH has not been found to be associated with the increase in calcitriol that is observed during pregnancy (14, 15). Epigenetic studies have found that the placental CYP24A1 gene, but not the VDR or CYP27B1 gene, is methylated, which may serve to silence the expression of this gene in this tissue (16). This placental specific finding is thought to be supportive of the need to limit calcitriol catabolism while promoting higher maternal calcitriol concentrations during gestation.
Recent data have found that elevations in serum calcitriol across pregnancy are influenced by circulating maternal concentrations of 25-hydroxyvitamin D [25(OH)D (calcidiol)] (14, 15), suggesting a link between substrate availability and production of calcitriol. To date, little is known regarding the determinants of the expression of CYP27B1 and CYP24A1 in term placental tissue, nor have possible associations between the expression of these two enzymes been explored in relation to maternal and neonatal 25(OH)D, 1,25-dihydroxyvitamin D [1,25(OH)2D (calcitriol)] and PTH concentrations. To address this issue, we quantified placental transcript and protein expression of both the CYP27B1 and CYP24A1 enzymes in relation to maternal and neonatal hormonal concentrations in a large group of pregnant adolescents at risk for vitamin D insufficiency.
Materials and Methods
Study participants
Pregnant adolescents (n = 168, aged ≤ 18 y) were recruited into this study from the Rochester Adolescent Maternal Program (Rochester, New York). Teens recruited were enrolled in a larger longitudinal study in assessing maternal and fetal bone health and iron status across gestation. Adolescents carrying a single fetus were eligible if otherwise healthy and between 12 and 30 weeks of gestation at entry into the study. Informed written consent was obtained and study procedures were approved by the Institutional Review Boards of the University of Rochester and Cornell University. Results on calcitropic hormones (15), fetal bone growth (17), bone turnover and osteoprotegerin (18), and placental iron transporter expression (19, 20) have been published. Data on placental VDR expression in relation to a dynamic measure of placental calcium transport are under review.
Upon entry, maternal race, ethnicity, prepregnancy weight, and smoking history were self-reported. Each participant attended up to three study visits across gestation. Maternal blood was collected at midgestation and delivery. At birth, infant weight and length were recorded and cord blood was obtained. Serum was stored at −80°C until analysis. At delivery, placental samples were collected from multiple cotyledons, the maternal and fetal membranes were removed, and the samples were cut into small pieces and pooled. Representative aliquots of this mixture were then either flash frozen (for protein analyses) or placed into RNAlater (Ambion) and kept at −80 C until analysis (19). Placental tissue for the CYP27B1 and CYP24A1 analyses were analyzed in a random subset (n = 70) of the 168 teens with quality RNA available and that had delivered infants weighing more than 2500 g at term.
Biochemical analyses in maternal and umbilical cord serum
Biomarkers associated with calcitriol regulation [total serum calcium, 25(OH)D, 1,25(OH)2D, PTH, osteoprotegerin, estradiol] and those associated with body composition or inflammation (leptin, IL-6) were measured as part of the larger study assessing bone health. Serum total calcium was measured using a Modular (P) Chemistry Automated System (Roche Diagnostics). Serum 25(OH)D was measured using the Diasorin RIA (Diasorin Inc) by Quest Laboratories. Quest Laboratories participates in the vitamin D External Quality Assessment Scheme. Calcitriol was analyzed at Boston University in Dr Michael Holick's laboratory (Boston, Massachusetts) using an in-house thymus receptor-binding assay (22). Intact PTH, leptin, and osteoprotegerin (OPG) were analyzed by ELISA (Diagnostic Systems Laboratories, Millipore, and DY805; R&D Systems, respectively). Estradiol was analyzed using a commercially available ELISA from ALPCO Diagnostics. Serum IL-6 in maternal and umbilical cord sera was analyzed by a Multiplex Millipore assay on a Luminex Magpix.
Quantitative real-time PCR analysis of CYP24A1, CYP27B1, and VDR
Placental samples intended for RNA extraction were placed in RNAlater (Invitrogen) at delivery. All RNA samples were extracted using the RNeasy microarray tissue minikit (QIAGEN). Extracted cDNA was synthesized by reverse transcription using the Transcriptor first-strand cDNA synthesis kit (Roche) in a MyCycler personal thermal cycler (Bio-Rad Laboratories). A total of 1000 ng of RNA was used per cDNA reaction. After synthesis, cDNA concentrations were diluted in half by adding 1 vol of water. The RT-PCR primers for CYP24A1 and β-actin were designed through Roche Applied Science Universal Probe Library using the National Center for Biotechnology Information (NCBI) sequence identification number (NM_001101.3, NM_000782.4 for β-actin, and 24-hydroxylase, respectively). The primers for CYP24A1 were as follows: forward primer, 5′-CAT CAT GGC CAT CAA AAC AAT-3′; reverse primer, 5′-GCA GCT CGA CTG GAG TGA C-3′. For β-actin the primers were: forward primer, 5′-CCA ACC GCG AGA AGA TGA-3′; reverse primer, 5′-CCA GAG GCG TAC AGG GAT AG-3′. Forward and reverse primers for CYP27B1 were developed based on data in human decidual cells (23). The primer sequences for CYP27B1 were as follows: forward primer, 5′-CAC CCG ACA CGG AGA CCT T-3′; reverse primer, 5′-TCA ACA GCG TGG ACA CAA ACA-3′. All primers were commercially purchased (IDT).
Expression of VDR placental mRNA was undertaken in 24 random placentas with CYP27B1 and CYP24A1 data. Primers were designed from the VDR NCBI sequence NM_000376.2: forward primer, ACA TCG GCA TGA TGA AGG A; and reverse primer, TTC CGC TTC AGG ATC ATC TC. β-Actin was used as the control gene and primers were designed from the NCBI sequence NM_001101.3. Primers were added to achieve a final molar concentration of 0.7 for both forward and reverse primers. A total of 2 μL of cDNA was added to each reaction and reactions were done in triplicate in 384-well plates. For all PCRs, samples were preincubated at 95°C for 5 minutes, followed by 45 cycles of denaturation at 95°C for 10 seconds, annealing at 60° for 10 seconds, and extension at 72°C for 10 seconds. To differentiate specific from nonspecific products and primer dimers, a melting curve was obtained after amplification by holding the temperature at 95°C for 5 seconds then 65°C for 1 minute followed by a gradual increase in temperature to 97°C at a rate of 0.11°C/s following by cooling at 40°C for 30 seconds (LightCycler 480 SYBR Green I Master; Roche Applied Science). All plates included a positive placental tissue control to correct for plate-to-plate variation and data were expressed relative to β-actin. To control for plate-to-plate variation, ΔΔcycle threshold values were calculated.
Western blot analysis of CYP24A1, CYP27B1, and VDR
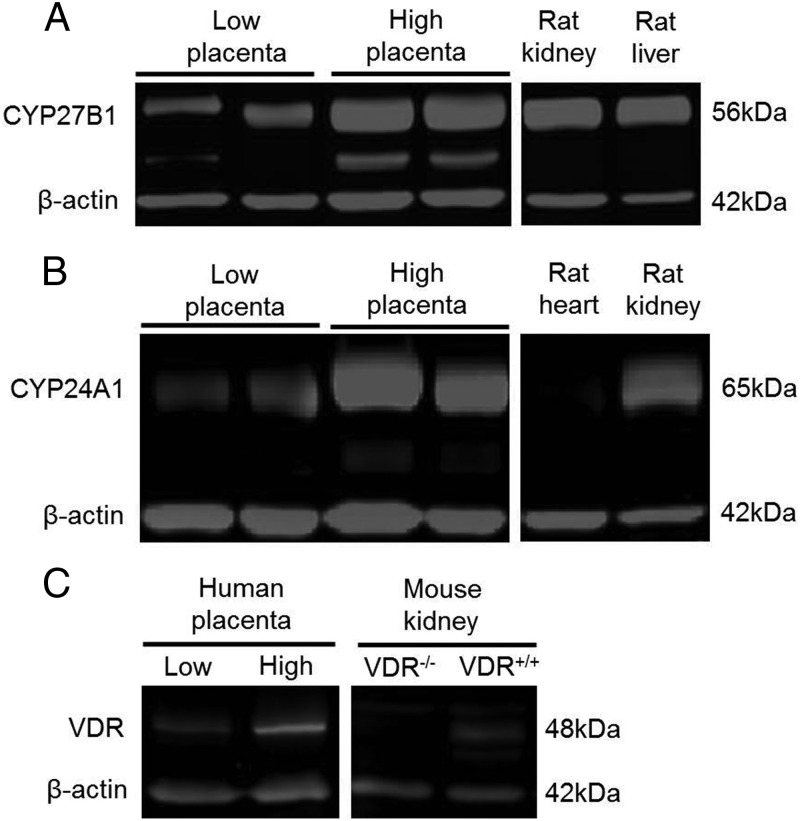
Placental protein lysates (20 μg protein) were mixed with SDS-PAGE and boiled for 5 minutes prior to separation by 10% SDS-PAGE using a triple-wide electrophoresis unit (CBS Scientific). Proteins were electrotransferred to polyvinylidene difluoride membranes (Millipore) and membranes blocked in Odyssey blocking buffer (Li-Cor BioSciences) and then probed with rabbit anti-CYP27B1 (1:750 dilution; sc-67261) and rabbit anti-CYP24A1 (1:750 dilution; sc-66851; Santa Cruz Biotechnology) overnight at 4°C. Protein targets were detected with IR800 goat antirabbit IgG (1:5000 dilution; Li-Cor) and quantified by direct infrared fluorescence using the Li-Cor Odyssey infrared imaging system that allows for simultaneous detection of the target and reference proteins labeled with different infrared dyes. Blots were probed with mouse anti-β-actin (1:3000 dilution; sc-47778; Santa Cruz Biotechnology Inc) to ensure equal loading. Scans were performed at 700 nm (β-actin) and 800 nm (CYP24A1 and CYP27B1) with the Odyssey instrument. Ratios of CYP27B1 and CYP24A1 to β-actin were calculated for each sample. Rat kidney lysate was used as a positive control for both CYP27B1 and CYP24A1 and rat heart lysate was used as a negative control for CYP24A1 (Figure 1). Similar procedures were followed for placental expression of VDR, except membranes were probed with rat anti-VDR (ab8756:Abcam) at a dilution of 2 μg/mL and rabbit anti-β-actin (Abcam) at 10.1 ng/mL overnight at 4°C, and secondary antibodies of goat antirat 800 at a concentration of 0.2 μg/mL and goat antirabbit 680 at 0.1 μg/mL were used (Li-Cor BioSciences). The VDR and β-actin bands were quantified by densitometry at 700 nm (β-actin) and 800 nm (VDR) using the Odyssey instrument, and the ratio of VDR to β-actin calculated. HeLa nuclear lysate (ab14655; Abcam) was used as a positive control for VDR and murine kidney lysates from the Demay VDR−/− animal and wild-type control lysates (provided by Dr James Fleet, Purdue University, West Lafayette, Indiana) were probed for VDR expression as negative controls. Intermembrane variation was controlled for by normalizing the VDR: β-actin ratio of each sample to a standard control placental sample run on every gel.
Figure 1.
Placental CYP27B1 (A) and CYP24A1 (B) were analyzed by Western blot in placental lysates prepared from term deliveries. Shown is a representative blot with individual placental lysate samples selected from those that exhibited either high or low expression of these enzymes. Rat kidney and liver tissue was used as a positive control for CYP27B1; rat kidney was used as a positive control and rat heart tissue was used as a negative control for CYP24A1. Placental VDR expression (C) was also analyzed by Western blot; shown are representative placental samples that exhibited extremes of VDR expression. Murine VDR−/− and VDR+/+ renal tissue lysates were probed for VDR expression as positive and negative controls. Intramembrane variation for each protein was controlled for by normalizing the VDR: β-actin ratio of each sample to a standard control placental sample that was run on every gel.
Statistical methods
All data analyses were conducted using JMP 8.0 (SAS Institute Inc). Data that were not normally distributed [placental CYP27B1 mRNA, placental CYP27B1 protein, placental CYP24A1 mRNA, maternal prepregnancy body mass index (BMI), and maternal midgestation IL-6] were transformed using the natural log prior to statistical testing. Normality of the transformed data was tested using the Shapiro Wilks test. Linear regression analyses were used to examine relationships between CYP24A1 and CYP27B1 and other relevant study outcomes. An ANOVA was used to detect differences between subject groups as a function of race, ethnicity, and vitamin D adequacy. Data were considered significant at P < .05. One subject with a serum midgestation 25(OH)D value greater than 3 SD over the mean was excluded from all analyses involving 25(OH)D. Multivariate models were constructed to model statistical predictors of mRNA and protein expression of placental CYP24A1 and CYP27B1 by including variables that showed significant relationships with the dependent variable in simple linear regression analysis and using a forward stepwise selection strategy for the remaining potential covariates.
Results
Subject characteristics
Characteristics of adolescents that provided placental tissues were compared with the larger study population of 168 adolescents; no significant differences were found for any of the characteristics presented (Table 1). Average calcium intake ingested (928 mg/d) was 85% of the estimated average requirement (1100 mg/d) recommended for pregnancy in those 14–18 years (24).
Table 1.
Characteristics of Participants
| Characteristics | Substudy | Larger Study Cohort |
|---|---|---|
| Total | 70 | 168 |
| Age at delivery, y | 17.5 ± 1.1 (70) | 17.1 ± 1.1 (167) |
| Gestational age at delivery, wk | 40.0 ± 1.2 (70) | 39.2 ± 2.9 (162) |
| Prepregnancy BMI, kg/m2 | 25.7 ± 6.1 (69) | 24.7 ± 5.3 (165) |
| Weight gain over pregnancy, Kg | 17.9 ± 8.9 (69) | 16.9 ± 8.0 (151) |
| Multiparous (parity ≥ 1) | 11.5% (9) | 9.2% (15) |
| Race, % | ||
| African American | 58.6% (41) | 66.1% (111) |
| Caucasian | 41.4% (29) | 33.9% (57) |
| Ethnicity, % | ||
| Hispanic | 31.4% (22) | 24.1% (39) |
| Non-Hispanic | 68.6% (48) | 75.9% (123) |
| Calcium intake, mg/d | 928 ± 347 (70) | 913 ± 414 (162) |
| Vitamin D intake, μg/d | 5.2 ± 2.7 (70) | 5.4 ± 3.4 (162) |
| Birth weight, g | 3362 ± 458 (70) | 3236 ± 592 (159) |
| Season at birth | ||
| Winter | 42.9% (30) | 33.3% (55) |
| Spring | 11.4% (8) | 14.5% (24) |
| Summer | 31.4% (22) | 35.8% (59) |
| Fall | 14.3% (10) | 16.4% (27) |
Data are presented as mean ± SD (n) or percentage (n) where indicated.
Calcitropic hormone concentrations in adolescents and neonates
In these pregnant adolescents, 58% had 25(OH)D concentrations of 20 ng/mL or less, and 16% had 25(OH)D concentrations less than 12 ng/mL at midgestation (Table 2). Similar to the larger cohort (15), fully 54% of neonates had 25(OH)D of 20 ng/mL or less at birth. Maternal 25(OH)D at both midgestation (P = .0003, R2 = 0.19, n = 66 pairs) and delivery (P < .0001, R2 = 0.65, n = 65 pairs) was significantly positively correlated with neonatal 25(OH)D. In contrast, no significant relationships were evident between neonatal 1,25(OH)2D and either maternal 1,25(OH)2D or PTH concentrations at either midgestation or delivery in this group of 70 teens similar to that observed in the larger cohort (15).
Table 2.
Biochemical Measurements in Maternal and Cord Blood Serum
| Variable | Midgestation | Delivery | Cord Blood |
|---|---|---|---|
| 25(OH)D, ng/mL | 21.0 ± 9.1 (69)a | 21.1 ± 12.8 (68)a | 20.8 ± 11.3 (67)a |
| Percentage < 20 ng/mL | 56.0a | 47.0a | 53.7a |
| Percentage < 12 ng/mL | 15.9a | 26.5b | 22.4a |
| 1,25(OH)2D, pg/mL | 120.1 ± 31.5 (49)a | 106.1 ± 33.6 (42)b | 47.7 ± 19.7 (40)c |
| PTH, pg/mL | 30.9 ± 19.4 (47)a | 50.4 ± 35.4 (44)a | 18.5 ± 11.9 (20)b |
| Total serum calcium, mg/dL | 9.1 ± 0.3 (70)a | 9.1 ± 0.4 (67)a | 10.5 ± 0.7 (68)b |
| Estradiol, pg/mL | 2863 ± 1581 (63)a | 5925 ± 2841 (66)b | 8815 ± 4092 (64)c |
| IL-6, pg/mL | 6.0 ± 16.5 (63)a | 5.6 ± 6.1 (63)b | 47.0 ± 173.9 (48)c |
| Leptin, μg/L | 31.4 ± 22.5 (63)a | 39.7 ± 25.2 (66)b | 12.0 ± 10.3 (64)c |
| OPG, pg/mL | 1938 ± 835 (62)a | 2947 ± 1288 (62)b | 975 ± 735 (63)c |
Data are presented as the mean ± SD (n). Data indicated with different superscripts within each row indicate values significantly differ (P < .02) between the midgestation, delivery, and cord blood values (paired t test with Bonferroni correction or logistic regression controlling for participant identification as a random effect).
Maternal determinants of placental CYP27B1 mRNA and protein expression
Placental CYP27B1 mRNA expression was found to be significantly, positively correlated with maternal calcitriol at midgestation (P = .002, R2 = 0.20, n = 49; Figure 2A) but not at delivery (P = .15, R2 = 0.05, n = 42). Placental CYP27B1 transcript was negatively correlated with maternal serum total calcium at delivery (P = .04, R2 = 0.07, n = 66) but was not associated with race, ethnicity, neonatal birth weight, gestational age at delivery, maternal age at delivery, maternal prepregnancy BMI, or average maternal calcitriol or vitamin D intake. No associations were found between placental CYP27B1 transcript and maternal or neonatal PTH or 25(OH)D at any time point (Table 3). When all variables were put into a forward stepwise regression model, the best model to capture variability in placental CYP27B1 mRNA expression was one that included maternal calcitriol at midgestation, maternal serum calcium at midgestation, and delivery and estradiol at delivery (P = .0007, R2 = 0.32, n = 47) (Table 4).
Figure 2.
Term placental tissue was probed for 1α-hydroxylase mRNA expression and ΔΔCt values were plotted against maternal concentrations of calcitriol at midgestation (A). Placental CYP27B1 expression was found to be significantly, positively correlated with maternal serum calcitriol at midgestation (P = .002, n = 49, R2 = 0.19). Placental protein expression of CYP27B1 was found to be significantly positively associated with maternal 25(OH)D concentrations at both midgestation (P = .009, n = 68, R2 = 0.10; B) and delivery (P = .006, n = 67, R2 = 0.11, data not shown).
Table 3.
Predictors of Placental CYP27B1 and CYP24A1 Expression
| Parameter | SE | P value | R2 | n | |
|---|---|---|---|---|---|
| CYP27B1 | |||||
| mRNA (log) | |||||
| 1,25(OH)2D at midgestation | .002 | 0.19 | 49 | ||
| Serum total calcium at delivery | .04 | 0.07 | 66 | ||
| Placental VDR | NS | 68 | |||
| Protein (log) | |||||
| 25(OH)D at midgestation | .009 | 0.10 | 68 | ||
| 25(OH)D at delivery | .006 | 0.11 | 67 | ||
| 25(OH)D in cord blood | .06 | 0.05 | 66 | ||
| IL-6 at midgestation | .07 | 0.05 | 63 | ||
| Placental VDR | NS | 68 | |||
| CYP24A1 | |||||
| mRNA (log) | |||||
| ppBMI | .04 | 0.06 | 69 | ||
| BMI at delivery | .08 | 0.05 | 69 | ||
| Leptin at delivery | .03 | 0.08 | 66 | ||
| IL-6 in cord blood | .02 | 0.11 | 48 | ||
| Placental VDR | NS | 68 | |||
| Protein (log) | |||||
| CYP27B1 protein (log) | .004 | 0.17 | 70 | ||
| OPG in cord blood | .003 | 0.14 | 63 | ||
| Placental VDR | NS | 68 |
Abbreviations: NS, not significant; ppBMI, prepregnancy BMI.
Table 4.
Multivariate Models of Placental CYP27B1 and CYP24A1 Expression
| R2 (Model) | β | P Value | |
|---|---|---|---|
| CYP27B1 | |||
| CYP27B1 protein (n = 60) | 0.22 | .0024 | |
| Midgestation 25(OH)D | 0.0089 | .0079 | |
| Midgestation IL-6 | 0.039 | .0222 | |
| Placental VDR protein expression | −0.10 | .0283 | |
| CYP27B1 mRNA (n = 47) | 0.32 | .0007 | |
| Midgestation 1,25(OH)2D | 0.020 | .0003 | |
| Midgestation serum calcium | −1.4 | .0172 | |
| Delivery estradiol | −0.00014 | .0208 | |
| CYP24A1 | |||
| CYP24A1 protein (n = 63) | 0.23 | .0003 | |
| Placental CYP27B1 protein | 0.80 | .0031 | |
| Cord OPG | −0.00023 | .0065 | |
| CYP24A1 mRNA (n = 46) | 0.23 | .0040 | |
| Delivery serum leptin | −0.011 | .0065 | |
| Cord IL-6 | −0.15 | .0240 |
Although mRNA for CYP27B1 was associated with maternal calcitriol, no significant association was found between maternal calcitriol and levels of the CYP27B1 protein. Instead, maternal 25(OH)D at both midgestation (P = .009, n = 68, R2 = 0.10) and delivery (P = .006, n = 67, R2 = 0.11) was the most significant, positively associated determinant of placental CYP27B1 protein expression (Figure 2B). A significantly higher, 1.13-fold expression of this enzyme was evident at midgestation in teens with 25(OH)D of 20 ng/mL or greater (n = 28) compared with those with concentrations less than 20 ng/mL (P = .04, n = 40, two sided t test). A positive association between neonatal 25(OH)D and CYP27B1 protein expression approached significance (P = .06, R2 = 0.05, n = 66) as did a positive relationship between maternal IL-6 at midgestation and placental CYP27B1 protein expression (P = .07, R2 = 0.05, n = 63). Placental CYP27B1 protein expression was not significantly associated with CYP27B1 mRNA expression or with the other maternal or biochemical factors indicated above (Table 3). The best model to capture variability in placental CYP27B1 protein expression included maternal 25(OH)D at midgestation, placental VDR protein expression, and maternal midgestation IL-6 (P = .002, R2 = 0.22, n = 60) (Table 4).
Maternal determinants of placental 24-hydroxylase mRNA and protein expression
Of all variables analyzed, maternal CYP24A1 mRNA was significantly negatively associated with maternal prepregnancy BMI (P = .04, R2 = 0.06, n = 69) and a similar negative relationship approached significance at delivery (P = .08, R2 = 0.05, n = 69) (Table 3). This may be driven by maternal adiposity because maternal BMI at midgestation and delivery was inversely associated with maternal leptin (P < .0001), and a significant negative association was evident between CYP24A1 mRNA and leptin concentrations at delivery (P = .03, R2 = 0.08, n = 66). An index of neonatal inflammation, IL-6, was negatively associated with CYP24A1 mRNA (P = .02, R2 = 0.11, n = 48). Although all placental tissue was obtained at term, gestational age at delivery (across 37.3–41.9 wk) was negatively associated with placental CYP24A1 mRNA expression (P = .045, R2 = 0.06, n = 70) (Table 3). Placental CYP24A1 mRNA expression did not differ by race, ethnicity, age at delivery, neonatal birth weight, or average daily calcitriol or vitamin D intake. No other significant determinants of CYP24A1 mRNA expression were identified. The best model to capture variability in placental CYP24A1 mRNA expression was one that included maternal leptin at delivery and cord IL-6 (P = .004, R2 = 0.23, n = 46) (Table 4).
Placental CYP24A1 protein expression was not significantly associated with CYP24A1 mRNA expression. However, CYP27B1 and CYP24A1 protein expression were significantly positively correlated (P = .004, R2 = 0.17, n = 70). This relationship was not evident for mRNA expression. Placental CYP24A1 protein expression was significantly negatively associated with cord OPG (P = .003, R2 = 0.14, n = 63). Although leptin and inflammation impacted CYP24A1 transcript, no association was noted between these variables and CYP24A1 protein expression. The best model to capture variability in placental CYP24A1 protein expression was one that included placental CYP27B1 protein expression and cord OPG (P = .0003, R2 = 0.23, n = 63) (Table 4).
Discussion
Recent studies have confirmed that serum levels of calcitriol increase markedly across pregnancy in both pregnant adults (14) and adolescents (15), but the degree to which these increases may be impacted by placental CYP27B1 and CYP24A1 activity has not been well described. In this group of pregnant adolescents, term placental CYP27B1 mRNA expression was positively correlated to maternal serum calcitriol at midgestation, and CYP27B1 protein expression was positively correlated to maternal serum 25(OH)D at both midgestation and delivery. Unlike inverse associations observed in the kidney, placental CYP27B1 and CYP24A1 protein expressions were significantly positively related to one another. To our knowledge, this is the first study to assess the expression of both CYP27B1 and CYP24A1 in term placental tissue in relation to concentrations of circulating vitamin D metabolites in maternal/neonatal dyads.
Conflicting data have been published on the ability of the placenta to contribute to the systemic increases in calcitriol that occur across gestation. Several studies using anephric rat models found evidence to support extra renal synthesis of 1,25(OH)2D during pregnancy (3, 25, 26) and concluded that this likely occurs in the fetoplacental unit (25, 26). Studies in CYP27B1 null pigs found 1,25(OH)2D concentrations to be significantly lower across pregnancy when compared with values observed among control sows, even when the fetus was heterozygous for this mutation (27). Although calcitriol concentrations were lower in the CYP27B1 null porcine model, calcitriol was still detectable (67 vs 29 pg/mL), and unlike the situation in nonpregnant sows, serum calcitriol and phosphate were maintained across pregnancy without calcium/phosphate supplementation (27). Human data on these associations are limited and are largely based on a single case report from a pregnant woman with end-stage renal disease who exhibited significantly lower 1,25(OH)2D at week 25 of gestation when compared with normative pregnancy data (28).
In our study, CYP27B1 protein expression was significantly associated with maternal 25(OH)D at both midgestation and delivery. Moreover, when comparing adolescents with sufficient vs insufficient 25(OH)D status at midgestation, teens with 25(OH)D of 20 ng/mL or greater had significantly higher CYP27B1 protein expression at delivery. This finding supports data by Hollis et al (14), in which maximal 1,25(OH)2D concentrations in pregnant women were evident in those with 25(OH)D concentrations above 40 ng/mL, a finding they attributed to both renal and extrarenal 1α-hydroxylase substrate saturation. Although a positive association between circulating 25(OH)D and 1,25(OH)2D concentrations was not observed among our non-vitamin D supplemented adolescents [half of whom had 25(OH)D concentrations; ≤ 20 ng/mL] (15), our results support the hypothesis that placental CYP27B1 activity may be regulated in some fashion by availability of the 25(OH)D substrate (23). No association was found between maternal PTH at any time point measured with the expression of placental CYP27B1 consistent with in vitro human decidual data that found no effect of PTH or phosphate on calcitriol production (29).
We found maternal calcitriol at midgestation to be significantly positively correlated with placental CYP27B1 mRNA expression. Although regulation of the CYP27B1 and CYP24A1 enzymes is known to be tissue dependent (11, 30), our placental data differ from findings in the kidney in which CYP27B1 production is inhibited by calcitriol through a negative feedback mechanism (10). We observed no significant associations between the mRNA and protein expression of either target gene. Human data have found that the CYP24A1 enzyme is methylated and silenced in the placenta (16). Placental-specific methylation of this enzyme is thought to support maximal calcitriol production and availability during pregnancy. Maternal 25(OH)D and 1,25(OH)2D concentrations at either time point measured were not significantly associated with CYP24A1 expression. In our term placental tissue obtained, CYP24A1 mRNA was negatively associated with gestational age across the span of gestation covered (37–41 wk). Other cross-sectional data found no significant gestational changes in placental CYP24A1 mRNA production when comparing first-, second-, and third-trimester placental tissue samples (13).
In contrast to maternal findings, neonatal 25(OH)D and 1,25(OH)2D concentrations at birth were not significantly associated with protein or mRNA expression of CYP27B1 or CYP24A1. This confirms prior data in these adolescents (15) and among adult pregnant women in which neonatal calcitriol concentrations were not associated with maternal concentrations (31–33). A similar lack of association has previously been found between maternal and neonatal 24,25-dihydroxyvitamin D, suggesting that the fetus independently regulates calcitriol production (34). Although neonatal vitamin D status was not associated with placental CYP27B1 or CYP24A1, neonatal IL-6 was significantly inversely associated with CYP24A1 mRNA and a positive association between maternal IL-6 at midgestation and CYP27B1 protein expression approached significance.
Vitamin D is believed to support antiinflammatory effects in the placenta (35), and recent data have linked autocrine calcitriol production with antiinflammatory and antibacterial responses in both placental decidual (36) and trophoblast tissue (37, 38). Placental 24-hydroxylase protein expression was also negatively correlated to neonatal osteoprotegerin concentrations at birth. In this adolescent cohort, we previously found neonatal OPG to be negatively associated with both birth weight z-score and ponderal index, but it was not associated with maternal or neonatal vitamin D status (18). Few data on roles and determinants of neonatal OPG have been published, although murine data have found calcitriol to down-regulate OPG expression by both accelerating the degradation of OPG mRNA and suppressing the OPG promoter activity through the activator protein-1 binding site (39). No association was found between placental VDR and either CYP27B1 or CYP24A1 expression.
Both maternal prepregnancy BMI and leptin were negatively associated with placental CYP24A1 mRNA expression. These associations between placental vitamin D 24-hydroxylase and maternal adiposity are similar to renal findings in which increased renal 24-hydroxylase mRNA expression was observed in leptin-deficient ob/ob mice, and leptin administration resulted in reduction of 24-hydroxylase expression and suppression of renal 24-hydroxylase activity (21). Our results suggest that maternal leptin may play a similar role in inhibition of the placental 24-hydroxylase during pregnancy.
Our data suggest that maternal 25(OH)D concentrations may be important for the optimal placental production of calcitriol during pregnancy. We have also confirmed prior data showing that CYP27B1 expression is not subject to down-regulation by increased maternal calcitriol and is not impacted by maternal PTH. Instead, placental expression of CYP27B1 and CYP24A1 protein are positively associated in term placental tissue. Neonatal calcitriol at birth was not associated with placental activity of either hydroxylase enzyme, supporting our prior data showing a lack of an association between maternal and neonatal calcitriol. These data add to our understanding of the determinants of placental vitamin D enzyme expression in a pregnant adolescent population at risk for calcium and vitamin D inadequacy. Further studies are needed to identify the mechanisms responsible for the associations noted and to confirm the causality of these associations.
Acknowledgments
We thank Highland Hospital staff and the Strong Midwifery Group for their assistance with the clinical components of this research and the adolescents whose efforts made this research possible. We also thank Dr James Fleet (Purdue University) for the provision of murine VDR−/− and control tissues.
Clinical trials registration number is not applicable because subject recruitment began in June 2007.
This work was supported by the National Research Initiative of the US Department of Agriculture, National Institute of Food and Agriculture, Award 2005–35200-15218, and by General Clinical Research Center Grant 5M01-RR 00044 from the National Center for Research Resources, National Institutes of Health (NIH). Additional support was provided by NIH Grant 5T32DK007158-38.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CYP24A1
- 1,25-dihydroxyvitamin D 24-hydroxylase gene
- CYP27B1
- 25-hydroxyvitamin D 1α-hydroxylase gene
- NCBI
- National Center for Biotechnology Information
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D, calcitriol
- 25(OH)D
- 25-hydroxyvitamin D, calcidiol
- 24,25(OH)2D
- 24,25-dihydroxyvitamin D
- OPG
- osteoprotegerin
- VDR
- vitamin D receptor.
References
- 1. Liu NQ, Hewison M. Vitamin D, the placenta and pregnancy. Arch Biochem Biophys. 2012;523:37–47 [DOI] [PubMed] [Google Scholar]
- 2. Brannon PM, Picciano MF. Vitamin D in pregnancy and lactation in humans. Annu Rev Nutr. 2011;31:89–115 [DOI] [PubMed] [Google Scholar]
- 3. Gray TK, Lester GE, Lorenc RS. Evidence for extra-renal 1α-hydroxylation of 25-hydroxyvitamin D3 in pregnancy. Science. 1979;204:1311–1313 [DOI] [PubMed] [Google Scholar]
- 4. Weisman Y, Harell A, Edelstein S, David M, Spirer Z, Golander A. 1α,25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature. 1979;281:317–319 [DOI] [PubMed] [Google Scholar]
- 5. Pospechova K, Rozehnal V, Stejskalova L, et al. Expression and activity of vitamin D receptor in the human placenta and in choriocarcinoma BeWo and JEG-3 cell lines. Mol Cell Endocrinol. 2009;299:178–187 [DOI] [PubMed] [Google Scholar]
- 6. Delvin EE, Gagnon L, Arabian A, Gibb W. Influence of calcitriol on prolactin and prostaglandin production by human decidua. Mol Cell Endocrinol. 1990;71:177–183 [DOI] [PubMed] [Google Scholar]
- 7. Ross R, Florer J, Halbert K, McIntyre L. Characterization of 1,25-dihydroxyvitamin D3 receptors and in vivo targeting of [3H]-1,25(OH)2D3 in the sheep placenta. Placenta. 1989;10:553–567 [DOI] [PubMed] [Google Scholar]
- 8. Delvin EE, Arabian A, Glorieux FH, Mamer OA. In vitro metabolism of 25-hydroxycholecalciferol by isolated cells from human decidua. J Clin Endocrinol Metab. 1985;60:880–885 [DOI] [PubMed] [Google Scholar]
- 9. Kovacs CS. The role of vitamin D in pregnancy and lactation: insights from animal models and clinical studies. Annu Rev Nutr. 2012;32:97–123 [DOI] [PubMed] [Google Scholar]
- 10. Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1α-hydroxylase and vitamin D synthesis. Science. 1997;277:1827–1830 [DOI] [PubMed] [Google Scholar]
- 11. Armbrecht HJ, Wongsurawat N, Zenser TV, Davis BB. Effect of PTH and 1,25(OH)2D3 on renal 25(OH)D3 metabolism, adenylate cyclase, and protein kinase. Am J Physiol. 1984;246:E102–E107 [DOI] [PubMed] [Google Scholar]
- 12. Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523:9–18 [DOI] [PubMed] [Google Scholar]
- 13. Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental-decidual function. J Soc Gynecol Investig. 2004;11:263–271 [DOI] [PubMed] [Google Scholar]
- 14. Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Young BE, McNanley TJ, Cooper EM, et al. Vitamin D insufficiency is prevalent and vitamin D is inversely associated with PTH and calcitriol in pregnant adolescents. J Bone Miner Res. 2012;27:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Novakovic B, Sibson M, Manuelpillai U, et al. Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. J Biol Chem. 2009;284:14838–14848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young BE, McNanley TJ, Cooper EM, et al. Maternal vitamin D status and calcium intake interact to affect fetal skeletal growth in utero in pregnant adolescents. Am J Clin Nutr. 2012;95:1103–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Young BE, McNanley TJ, Cooper EM, et al. Osteoprotegerin (OPG) differs by race and is related to infant birth weight Z-score in pregnant adolescents. J Develop Origins of Health Dis. 2011;2:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Young MF, Pressman E, Foehr ML, et al. Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta. 2010;31:1010–1014 [DOI] [PubMed] [Google Scholar]
- 20. Jaacks LM, Young MF, Essley BV, et al. Placental expression of the heme transporter, feline leukemia virus subgroup C receptor, is related to maternal iron status in pregnant adolescents. J Nutr. 2011;141:1267–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsunuma A, Kawane T, Maeda T, Hamada S, Horiuchi N. Leptin corrects increased gene expression of renal 25-hydroxyvitamin D3–1α-hydroxylase and -24-hydroxylase in leptin-deficient, ob/ob mice. Endocrinology. 2004;145:1367–1375 [DOI] [PubMed] [Google Scholar]
- 22. Chen TC, Turner AK, Holick MF. A method for the determination of the circulating concentration of vitamin D. J Nutr Biochem. 1990;1:272–276 [DOI] [PubMed] [Google Scholar]
- 23. Zehnder D, Evans KN, Kilby MD, et al. The ontogeny of 25-hydroxyvitamin D(3) 1α-hydroxylase expression in human placenta and decidua. Am J Pathol. 2002;161:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011 [PubMed] [Google Scholar]
- 25. Weisman Y, Vargas A, Duckett G, Reiter E, Root AW. Synthesis of 1,25-dihydroxyvitamin D in the nephrectomized pregnant rat. Endocrinology. 1978;103:1992–1996 [DOI] [PubMed] [Google Scholar]
- 26. Blum M, Weisman Y, Turgeman S, et al. Pregnancy decreases immunoreactive parathyroid hormone level in rats with chronic renal failure. Clin Sci (Lond). 1999;96:427–430 [PubMed] [Google Scholar]
- 27. Lachenmaier-Currle U, Breves G, Harmeyer J. Role of 1,25-(OH)2D3 during pregnancy; studies with pigs suffering from pseudo-vitamin D-deficiency rickets, type I. Q J Exp Physiol. 1989;74:875–881 [DOI] [PubMed] [Google Scholar]
- 28. Turner M, Barre PE, Benjamin A, Goltzman D, Gascon-Barre M. Does the maternal kidney contribute to the increased circulating 1,25-dihydroxyvitamin D concentrations during pregnancy? Miner Electrolyte Metab. 1988;14:246–252 [PubMed] [Google Scholar]
- 29. Delvin EE, Arabian A. Kinetics and regulation of 25-hydroxycholecalciferol 1α-hydroxylase from cells isolated from human term decidua. Eur J Biochem. 1987;163:659–662 [DOI] [PubMed] [Google Scholar]
- 30. Henry HL. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab. 2011;25:531–541 [DOI] [PubMed] [Google Scholar]
- 31. Lund B, Selnes A. Plasma 1,25-dihydroxyvitamin D levels in pregnancy and lactation. Acta Endocrinol. 1979;92:330–335 [DOI] [PubMed] [Google Scholar]
- 32. Fleischman AR, Rosen JF, Cole J, Smith CM, Deluca HF. Maternal and fetal serum 1,25-dihydroxyvitamin D levels at term. J Pediatr. 1980;97:640–642 [DOI] [PubMed] [Google Scholar]
- 33. Hollis BW, Pittard WB., III Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab. 1984;59:652–657 [DOI] [PubMed] [Google Scholar]
- 34. Hillman LS, Slatopolsky E, Haddad JG. Perinatal vitamin D metabolism. IV. Maternal and cord serum 24,25-dihydroxyvitamin D concentrations. J Clin Endocrinol Metab. 1978;47:1073–1077 [DOI] [PubMed] [Google Scholar]
- 35. Liu NQ, Kaplan AT, Lagishetty V, et al. Vitamin D and the regulation of placental inflammation. J Immunol. 2011;186:5968–5974 [DOI] [PubMed] [Google Scholar]
- 36. Evans KN, Nguyen L, Chan J, et al. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod. 2006;75:816–822 [DOI] [PubMed] [Google Scholar]
- 37. Liu N, Kaplan AT, Low J, et al. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod. 2009;80:398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diaz L, Noyola-Martinez N, Barrera D, et al. Calcitriol inhibits TNF-α-induced inflammatory cytokines in human trophoblasts. J Reprod Immunol. 2009;81:17–24 [DOI] [PubMed] [Google Scholar]
- 39. Kondo T, Kitazawa R, Maeda S, Kitazawa S. 1α,25 Dihydroxyvitamin D3 rapidly regulates the mouse osteoprotegerin gene through dual pathways. J Bone Miner Res. 2004;19:1411–1419 [DOI] [PubMed] [Google Scholar]