Abstract
Context:
Anorexia nervosa (AN), a prevalent psychiatric disorder predominantly affecting women, is characterized by self-induced starvation and low body weight. Increased clinical fractures are common, and most women have low bone mineral density (BMD). Previously investigated treatments have led to no or modest increases in BMD in AN.
Objective:
Our objective was to investigate the effect of teriparatide (TPT; human PTH[1–34]), an anabolic agent, on low bone mass in women with AN.
Design, Setting, and Patients:
This randomized, placebo-controlled trial at a clinical research center included 21 women with AN: 10 (mean age ± SEM, 47 ± 2.7 years) treated with TPT and 11 (47.1 ± 2.3 years) treated with placebo.
Interventions:
TPT (20 μg SC) or placebo was administered for 6 months.
Main Outcome Measures:
Our primary outcome measure was change in BMD of the spine and hip by dual-energy x-ray absorptiometry. Secondary outcome measures included changes in serum N-terminal propeptide of type 1 procollagen (P1NP), C-terminal collagen cross-links, sclerostin, and IGF-1 levels.
Results:
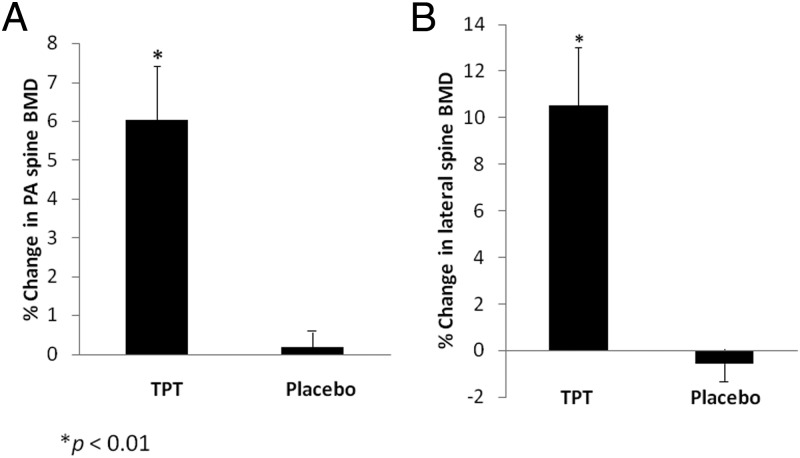
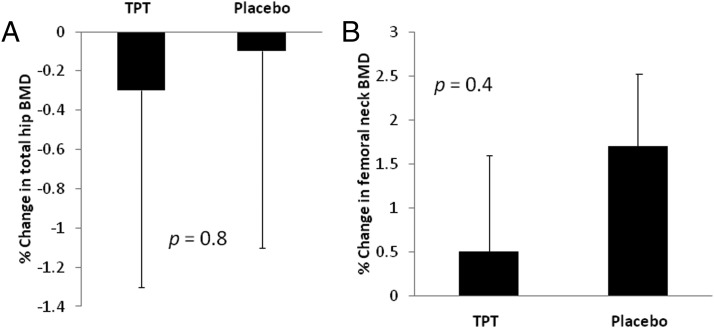
At 6 months, spine BMD increased significantly more with TPT (posteroanterior spine, 6.0% ± 1.4%; lateral spine, 10.5% ± 2.5%) compared with placebo (posteroanterior spine, 0.2% ± 0.7%, P < .01; lateral spine, −0.6% ± 1.0%; P < .01). The results remained significant after controlling for baseline body mass index, P1NP, and IGF-1. Changes in femoral neck (P = .4) and total hip (P = 0.8) BMD were comparable in both groups, as were changes in weight. Serum P1NP levels increased after 3 months of TPT treatment and remained at this higher level at 6 months, whereas P1NP levels were unchanged in the placebo group (P = .02). TPT was well-tolerated by all subjects.
Conclusions:
This study demonstrates that TPT administration increases spine BMD substantially after only 6 months of therapy in women with AN.
Anorexia nervosa (AN) is a psychiatric disorder affecting predominantly young women with a lifetime prevalence approaching 2.2% (1). The disease is characterized by self-induced starvation and the inability to maintain a normal weight for height (2) and is associated with a number of severe medical comorbidities, including clinical fractures. More than 85% of women with AN have bone mineral density (BMD) values more than 1 SD below an age-comparable mean (3, 4), and importantly, there is a 7-fold increased risk of fracture (5). However, there are currently no Food and Drug Administration-approved treatments for low bone mass in patients with AN.
Although adolescents and young women are the group most often affected by AN, an increasing number of women over the age of 35 have been entering programs for the treatment of AN (6). The relapse rate of AN is high with only approximately 50% of patients recovering after 21 years (7), and therefore, the increasing prevalence of AN in women over 35 is thought to be due primarily to persistent disease (8). However, new-onset AN is also noted among older women (9–11) and is associated with more significant weight loss (10). Therefore, a group of women with AN over 35 would be expected to have more significant bone loss.
Previous studies have investigated the effects of a number of therapies, including oral contraceptives (OCs) (12, 13), bisphosphonates (14), testosterone (14), dehydroepiandrosterone (DHEA) (15–17), and recombinant human (rh)IGF-1 (13) on BMD in women with AN. OCs (12, 13), DHEA (15, 16), and testosterone (14) did not increase BMD in women with AN. The only agents that have resulted in increases in BMD in AN include an anabolic agent, rhIGF-1, in combination with OCs (13) and bisphosphonates, which increase BMD by 2% to 4% over 1 year, the greatest reported increase in BMD to date in women with AN (14). Because bone loss in AN, unlike other estrogen-deficient states, is characterized by decreased bone formation in the setting of undernutrition (18–20), a potent anabolic agent, such as teriparatide (TPT; human PTH[1–34]), would be a logical treatment choice. However, TPT-induced bone formation is mediated, in part, by local IGF-1 production (21, 22), and systemic levels of IGF-1 (23) and IGF-1 production in response to GH are reduced in AN (24). Therefore, whether TPT would be an effective treatment in AN is not known. We hypothesized that the profound low bone formation state in AN would respond to TPT administration by an increase in BMD. We studied the effects of TPT on a group of women with AN and low BMD by 1) comparing changes in BMD after 6 months of treatment with TPT or placebo and 2) comparing changes in markers of bone turnover over the 6-month study period.
Subjects and Methods
Subjects
Twenty-one women (mean age ± SEM, 47 ± 1.7 years) who met DSM IV weight and psychiatric criteria for AN and who had a T-score of ≤−2.5 at any site were recruited through referrals from local eating disorder providers, endocrinologists, and online advertisements. Subjects were randomized to receive either TPT (Eli Lilly and Company) or identical placebo injections. Subjects in the TPT group were 69% to 85% of ideal body weight at the time of the screening visit, and subjects in the placebo group were 61% to 85% of ideal body weight. Subjects with abnormal thyroid function tests, chronic diseases known to affect BMD (other than AN), or diabetes mellitus were excluded from participation. None of the subjects were treated with oral bisphosphonates within 12 months of the study or iv bisphosphonates within 3 years of initiating the study. Exclusion criteria also included the use of medications known to affect bone metabolism, including estrogen, in the 3 months preceding the study. The protocol was approved by the Partners Institutional Review Board and complied with the Health Insurance Portability and Accountability Act (HIPAA) guidelines. Written informed consent was obtained from all subjects.
Experimental protocol
After a screening visit during which a history, physical examination, and studies to determine eligibility were performed, subjects presented for the baseline visit. Clinical and nutritional evaluations and laboratory and radiologic studies were performed at this visit, and subjects were taught to self-administer the study medication. Calcium and vitamin D intake was assessed by a bionutritionist from the Clinical Research Center at the Massachusetts General Hospital, and if self-reported intake was less than 1200 mg daily of calcium, subjects were provided with supplements to achieve a daily intake of 1200 mg of calcium. All subjects were provided with a daily vitamin containing 400 IU of vitamin D.
Study participants self-administered 20 μg of TPT or placebo once daily sc using a 31-gauge pen needle in the abdomen for 6 months. Participants were seen every month at the Massachusetts General Hospital Clinical Research Center at which time a physical examination and laboratory studies were performed.
Anthropometric measurements
Subjects were weighed on an electronic scale while wearing a hospital gown, and height was measured as the average of 3 readings on a single stadiometer. Elbow breadth (for estimation of frame size) was measured using calipers and compared with norms based on NHANES-I data. Body mass index (BMI) was calculated using the formula [weight (kilograms)/height (meters)2], and the percentage of ideal body weight was calculated based on 1983 Metropolitan Life Height and Weight tables (25).
Biochemical assessment
Serum IGF-1, N-terminal propeptide of type 1 procollagen (P1NP), and C-terminal collagen cross-links (CTX) were measured by a luminescent immunoassay analyzer (ISYS Analyzer; Immunodiagnostics Corporation). The detection limit for IGF-1 was 4.4 ng/mL with an intra-assay coefficient of variation (CV) of 2.2% and an interassay CV of 5.1%. The detection limit for P1NP was 1 ng/mL, with an intra-assay CV of 2.9% and an interassay CV of 4.6%. The detection limit for CTX was 0.023 ng/mL with an intra-assay CV of 3.2% and an interassay CV of 6.2%. Serum sclerostin levels were measured by ELISA (TECOmedical Group, Quidel Corporation) with a detection limit of 0.170 ng/mL and an intra-assay CV of 3.1% and interassay CV of 3.5%.
Radiologic imaging
All subjects underwent dual-energy x-ray absorptiometry imaging to measure BMD of the posteroanterior (PA) lumbar spine (L1–L4), lateral spine (L2–L4), total hip, and femoral neck using a Hologic Discovery A densitometer (Hologic Inc). CV of dual-energy x-ray absorptiometry have been reported as <1% for bone (26).
Statistical analysis
Statistical analysis was performed using JMP Pro version 10.0 (SAS Institute) software. Means and the SEM are reported. Means were compared using the Student's t test unless the distribution was non-normal, in which case the Wilcoxon test was used. Proportions were compared using the Fisher's exact test. Pearson correlation coefficients, or if the data were non-normal, Spearman's coefficients, were calculated to assess univariate relationships. Multivariable analyses were performed using least-squares linear regression to control for confounders. Repeated-measures analysis was used to investigate the group × time interaction for P1NP, CTX, IGF-1, and sclerostin levels measured at baseline and 3 and 6 months. Only individuals with complete data (n = 19 for P1NP, CTX, and IGF-1; n = 20 for sclerostin) were included in the repeated-measures analysis. A P value of <.05 on a two-tailed test was used to indicate significance.
Results
Baseline characteristics
Baseline characteristics of the study subjects are listed in Table 1. Baseline characteristics were similar in both groups except for serum IGF-1 and P1NP levels, which were significantly higher in the TPT group as compared with the placebo group at baseline. Both groups had low bone mass as per protocol; the mean lateral spine T-score was <−3.0 in both groups. All of the subjects completed the study and remained on their assigned treatment for the duration of the study.
Table 1.
Baseline Characteristics of the Study Participants
| Teriparatide (n = 10) | Placebo (n = 11) | P Value | |
|---|---|---|---|
| Age, y | 47.0 ± 2.7 | 47.1 ± 2.3 | .98 |
| BMI, kg/m2 | 17.6 ± 0.4 | 16.6 ± 0.4 | .08 |
| Weight, kg | 47.2 ± 2.1 | 45.4 ± 1.4 | .47 |
| Height, cma | 163.5 ± 2.3 | 165.5 ± 1.8 | .86 |
| Percentage of ideal body weight | 80.1 ± 2 | 74.7 ± 1.8 | .06 |
| Years since onset of anorexia nervosa (self-reported) | 20.4 ± 3.7 | 18.0 ± 4.3 | .70 |
| Mean systolic blood pressure, mm Hg | 106 ± 5 | 105 ± 4 | .90 |
| Mean diastolic blood pressure, mm Hg | 67 ± 4 | 69 ± 3 | .75 |
| Self-reported hours of exercise per weeka | 7.2 ± 2.3 | 8.4 ± 2.9 | .97 |
| Amenorrhea, n | 8 | 7 | .64 |
| Subjects reporting current use of antidepressant medications, n | 8 | 7 | .64 |
| Subjects obtaining <1200 mg Ca daily (through diet and/or supplements), n | 2 | 3 | .99 |
| Subjects obtaining <400 IU vitamin D daily (through diet and/or supplements), n | 2 | 5 | .35 |
| BMD of PA spine, g/cm2 | 0.77 ± 0.02 | 0.81 ± 0.03 | .28 |
| PA spine T-score | −2.6 ± 0.2 | −2.1 ± 0.3 | .29 |
| BMD of lateral spine, g/cm2 | 0.53 ± 0.03 | 0.57 ± 0.01 | .22 |
| Lateral spine T-score | −3.5 ± 0.3 | −3.0 ± 0.2 | .24 |
| BMD of total hip, g/cm2 | 0.71 ± 0.04 | 0.69 ± 0.02 | .72 |
| Total hip T-scorea | −1.9 ± 0.4 | −2.0 ± 0.1 | .60 |
| BMD of femoral neck, g/cm2a | 0.60 ± 0.03 | 0.60 ± 0.02 | .70 |
| Femoral neck T-scorea | −2.3 ± 0.3 | −2.3 ± 0.1 | .57 |
| Past use of bisphosphonates, n | 4 | 5 | .99 |
| 25-OH vitamin D, ng/mL | 31.1 ± 3.3 | 40.4 ± 4.2 | .1 |
| IGF-1, ng/mL | 152 ± 18 | 104 ± 9 | .03 |
| P1NP, ng/mLa | 63 ± 8 | 37 ± 6 | .02 |
| CTX, ng/mL | 0.55 ± 0.12 | 0.53 ± 0.08 | .88 |
| Sclerostin, ng/mL | 0.59 ± 0.05 | 0.69 ± 0.10 | .39 |
Wilcoxon test used to analyze between-group differences.
Changes in BMD
After 6 months, there was a 6.0% ± 1.4% increase in BMD at the PA spine and an absolute increase of 0.05 ± 0.01 g/cm2 in the TPT group compared with a 0.2% ± 0.7% increase (absolute increase, 0.002 ± 0.005 g/cm2) in the placebo group (P < .01 for both percent change and absolute change in BMD) (Figure 1A). These differences remained significant after controlling for baseline percentage of ideal body weight (or BMI), baseline IGF-1, and baseline P1NP levels (P < .01 for both percent change and absolute change in BMD). Changes in body weight over the 6-month study period were not significantly different between the groups, although there was a trend toward significance as the placebo group gained more weight than the TPT group (placebo, 1.8% ± 1.6%, vs TPT, −2.4% ± 1.7%; P = .09).
Figure 1.
There was a significantly greater increase in BMD after 6 months of TPT as compared with placebo in the PA spine (P < .01) (A) and lateral spine (P < .01) (B).
At 6 months, BMD also increased significantly more with TPT than placebo at the lateral spine. There was a 10.5% ± 2.5% increase in BMD at the lateral spine and an absolute increase of 0.05 ± 0.01 g/cm2 in the TPT group as compared with a −0.6% ± 1.0% decrease (absolute change, −0.003 ± 0.005 g/cm2) in the placebo group (P < .01 for both percent change and absolute change in BMD) (Figure 1B). These differences remained significant after controlling for baseline percentage of ideal body weight (or BMI) and baseline IGF-1 and P1NP levels (P ≤ .001 for both percent change and absolute change in BMD).
Changes in BMD of the hip and femoral neck were similar in both groups after 6 months. There were no changes in total hip BMD in either group (TPT, −0.3% ± 0.9% [absolute change, −0.003 ± 0.006 g/cm2], vs placebo, −0.1% ± 1.0% [absolute change, −0.001 ± 0.007 g/cm2]; P = .8 for both percent change and absolute change in BMD) (Figure 2A). Changes in femoral neck BMD were also similar in both groups (TPT, 0.5% ± 1.1% [absolute change, 0.001 ± 0.007 g/cm2], vs placebo, 1.7% ± 0.8% [absolute change, 0.01 ± 0.005 g/cm2]; P > .2 for both percent change and absolute change in BMD) (Figure 2B).
Figure 2.
After 6 months of treatment, changes in BMD in the total hip (A) and femoral neck (B) were similar in the TPT and placebo groups.
Changes in levels of markers of bone turnover and sclerostin
P1NP
In the TPT group, levels of P1NP, a marker of bone formation, increased 136% ± 21.9% during the first 3 months of treatment and remained at this increased level through 6 months. In comparison, P1NP levels remained stable over the entire 6-month study in the placebo group. This difference in the pattern in which P1NP levels changed in the 2 groups was statistically significant (P = .02) (Figure 3A).
Figure 3.
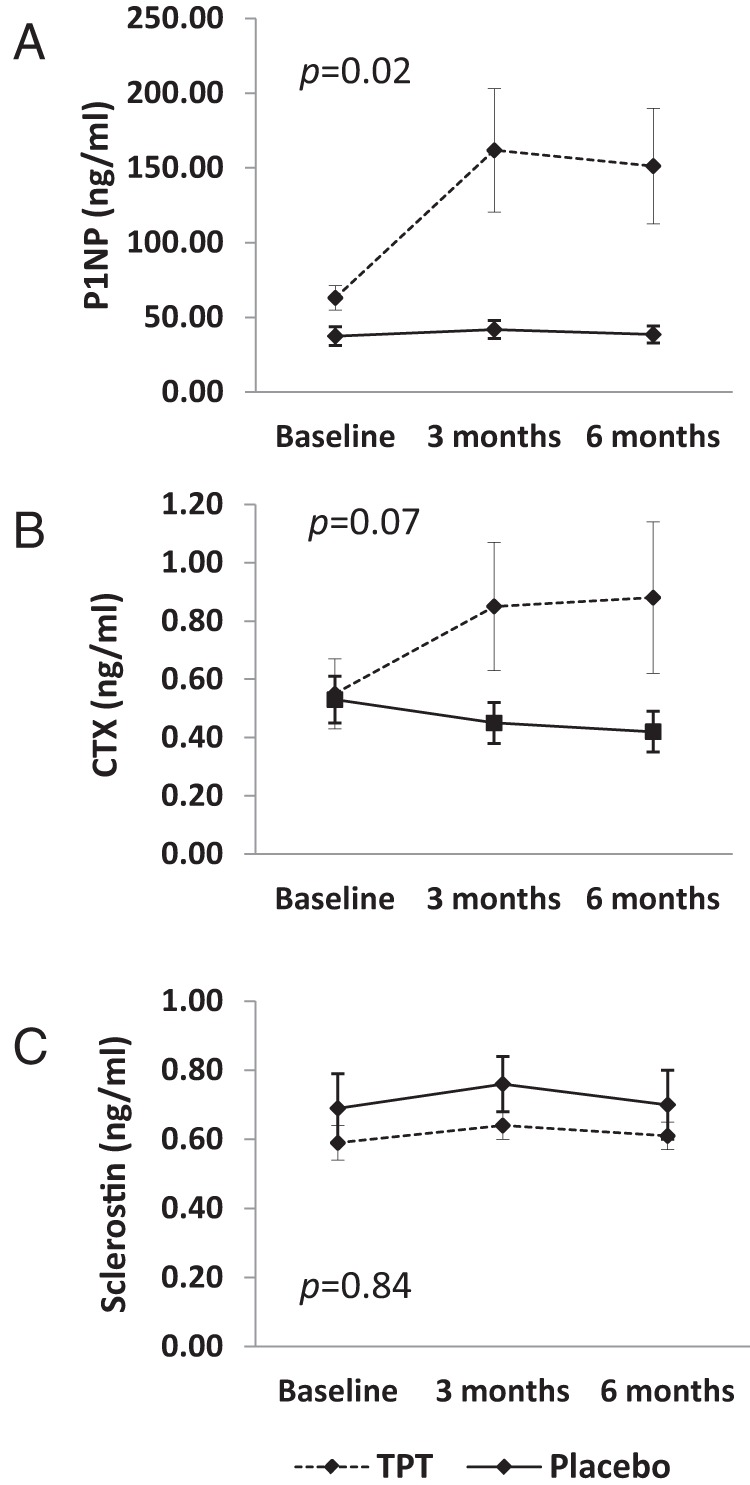
A, P1NP levels increased after 3 months and remained at this higher level in the group receiving TPT, whereas levels remained stable in the group receiving placebo (P = .02 for group × time interaction). B, CTX levels also increased after 3 months of TPT and remained at this higher level at 6 months, whereas they remained stable in the placebo group; however, this difference in the pattern of change of CTX levels was not statistically significant (P = .07 for group × time interaction). C,Serum sclerostin levels remained stable in both groups over the 6-month study period. All P values represent group × time interaction.
Increases in P1NP levels during the first 3 months of treatment predicted increases in PA spine and lateral spine BMD over the 6-month study period. In the group as a whole, there was a significant association between change in P1NP levels during months 1 to 3 of treatment and the 6-month percent change in PA spine BMD (R = 0.64; P < .01) and lateral spine BMD (R = 0.73; P < .001). In the TPT group (n = 10), there was also a significant association between change in P1NP levels during the first 3 months of treatment and the 6-month percent change in PA spine BMD (R = 0.68; P = .04).
CTX
Levels of CTX, a marker of bone resorption, also increased during the first 3 months of treatment with TPT (45.5% ± 26.6%) and remained at this higher level through the 6-month study period. In the placebo group, CTX levels remained stable over the 6-month study (Figure 3B). However, this difference in CTX patterns between groups did not reach statistical significance (P = .07).
Sclerostin
Levels of sclerostin, a Wnt signaling pathway inhibitor and negative regulator of bone formation, were stable in both the TPT and placebo group over the 6-month study period (Figure 3C).
Relationship between baseline IGF-1 levels and changes in BMD
IGF-1 levels remained stable in both the TPT group and the placebo group over the 6-month study period (Figure 4A).
Figure 4.

A, IGF-1 levels remained stable in both groups over the 6-month study period (P = .15 for group × time interaction). B, There was a significant inverse association between baseline IGF-1 level and 6-month change in PA spine BMD (R = −0.74; P = .02).
We performed an exploratory analysis to investigate whether serum IGF-1 levels predict changes in BMD in individuals treated with TPT. We examined the relationship between baseline IGF-1 levels and 6-month changes in BMD in the PA spine and lateral spine in the group of women who received TPT (n = 10). There was an inverse association between baseline IGF-1 level and both percent change in PA spine BMD (R = −0.74; P = .02) and absolute change in PA spine BMD (R = −0.72; P = .02), such that individuals with the lower baseline IGF-1 levels had the greatest increase in BMD after 6 months of treatment with TPT (Figure 4B). This relationship held after controlling for changes in weight over the 6-month study period (P = .03 for both percent change and absolute change in BMD).
There was also an inverse relationship between baseline IGF-1 level and percent change and absolute change in lateral spine BMD in the TPT group, but this relationship was not statistically significant (R = −0.39; P = .3 for percent change in lateral spine BMD; and R = −0.44; P = .2 for absolute change in lateral spine BMD).
Adverse effects
TPT was well-tolerated by all study participants. None of the subjects dropped out of the study, and there were no serious side effects or adverse events. Table 2 lists adverse events reported by subjects in the TPT and placebo groups. A similar number of subjects in each treatment group reported lightheadedness, muscle pain or spasm, and headaches. More subjects in the placebo group reported nausea and vomiting as compared with subjects in the TPT group. Nine subjects in the placebo group and 6 subjects in the TPT group reported the development of ecchymoses, bruising, or erythematous lesions at the injection sites that resolved without further treatment. Two subjects in the TPT group had an increase in serum calcium levels to above the normal range. One of the subjects had a serum calcium level of 10.3 mg/dL (normal range, 8.7–10.2 mg/dL) at her month 1 study visit. Her calcium supplement intake was decreased by 50%, and her serum calcium levels subsequently remained within the normal range for the remainder of the study. The second subject had a serum calcium level of 11.4 mg/dL (normal range, 8.7–10.2 mg/dL) at her month 3 study visit. We recommended that she stop all calcium supplementation, and her subsequent serum calcium levels were within the normal range for the remainder of the study. Three subjects in the TPT group had 24-hour urine calcium levels greater than 400 mg (all were less than 500 mg). All urine calcium levels normalized after subjects decreased their calcium supplement intake. None of the subjects stopped use of the study medication.
Table 2.
Number of Subjects in Each Randomization Group Experiencing Adverse Effects
| No. of Subjects |
||
|---|---|---|
| Teriparatide (n = 10) | Placebo (n = 11) | |
| Lightheadedness | 4 | 6 |
| Muscle pain or spasm | 3 | 4 |
| Nausea | 1 | 5 |
| Vomiting | 1 | 3 |
| Headache | 6 | 5 |
| Injection site ecchymoses, bruising, erythema | 6 | 9 |
| Serum Ca above normal range | 2 | 0 |
| 24-h urine calcium >400 mg | 3 | 1 |
Discussion
We have shown that 6 months of treatment with TPT significantly increases BMD at the PA spine and lateral spine in women with AN with minimal adverse effects. This is the first reported randomized placebo-controlled study of TPT in AN, and the only therapy to demonstrate this degree of dramatic improvement at the PA spine. The increases in BMD appear to be mediated by an increase in bone formation as evidenced by the fact that changes in P1NP levels during the first 3 months of treatment strongly predict changes in spine BMD over the study duration.
AN has a lifetime prevalence of 2.2% (1), and only approximately 50% of women with AN recover even many years after their initial diagnosis (7). Low BMD is the most common medical comorbidity associated with AN. In women who are able to gain weight and resume their menses, the mean annual increase in BMD is 1.8% at the hip and 3.1% at the spine (27). In individuals who remain low weight and amenorrheic, the annual rate of bone loss is −2.4% at the hip and −2.6% at the spine (27). Importantly, this bone loss is associated with an increased risk of fracture. Young women with AN have a 7-fold increased risk of fracture compared with age-matched controls (5), and given the chronicity of the disease, the incidence of fracture has been shown to remain elevated at 57%, even many years after the diagnosis of AN (28). Therefore, bone loss and increased fracture risk can persist and lead to life-long morbidity, and finding a beneficial treatment for the bone loss associated with AN is critical.
Low bone mass in AN is characterized by an uncoupling of bone formation and bone resorption (18–20). Previous studies have investigated the use of predominantly antiresorptive agents for the treatment of low bone mass in AN. Estrogen administered as an OC pill does not result in an increase in BMD in AN (12, 13). In contrast, physiologic doses of estrogen, primarily administered transdermally in adolescent girls with AN, leads to significant improvements in BMD (29). Androgen replacement, in the form of transdermal testosterone (14) or DHEA (15), does not increase BMD in AN, although 18 months of DHEA in conjunction with an OC pill results in maintenance, but not improvement, of BMD in AN (17). Other than physiologic estrogen replacement, which results in a 2.6% increase in spine BMD after 18 months of treatment, the only 2 agents that have demonstrated efficacy in this population include rhIGF-1 in combination with an OC pill, which resulted in a 1.8% increase in spine BMD after 9 months of treatment (13), and risedronate, a bisphosphonate, which is the most effective therapy to date, increasing spine BMD by 3% to 4% after 1 year of treatment (14). Therefore, this is the first treatment study to demonstrate significant increases of 6% to 10% in spine BMD in a period of only 6 months.
Whether TPT would result in an increase in BMD in women with AN was previously unknown, because this population is relatively IGF-1–deficient and IGF-1 is an important mediator of the anabolic effects of TPT on bone. Systemic IGF-1 is predominantly produced by the liver and is regulated by GH and nutritional status, and levels of IGF-1 in women with AN are approximately 50% of those of normal-weight women (23). TPT induces IGF-1 expression and secretion in osteoblasts (30); therefore, local IGF-1 production, in bone, is likely a more important mediator of the effects of TPT than systemic IGF-1. This is supported by studies in mice with a liver-specific deletion of the IGF-1 gene (31), which exhibit significant increases in trabecular number and thickness in response to TPT treatment compared with vehicle-treated mice (31). Our data also demonstrate that systemic levels of IGF-1 do not need to be above a certain threshold for TPT to improve BMD. In fact, in the 10 women who received TPT, the women with the lowest baseline IGF-1 levels had the greatest increases in BMD. This may be due to the fact that women with lower levels of IGF-1 at baseline benefit more from TPT-induced IGF-1 secretion in bone than women with higher baseline levels. Importantly, although additional studies will be needed to better understand the relationship between bone acquisition in response to TPT and IGF-1 in women with AN, our study suggests that women with low systemic IGF-1 levels will demonstrate significant increases in spine BMD in response to treatment with TPT.
We also found that serum sclerostin – a potent inhibitor of Wnt signaling and a negative regulator of bone formation – did not appear to mediate changes in BMD in response to treatment with TPT. Prior studies have shown a decrease (32) or no change (33, 34) in serum sclerostin levels in response to TPT. We have previously shown that sclerostin is not an important mediator of low BMD in adolescent girls with AN, because levels are similar in girls with AN and healthy controls, and changes in sclerostin do not mediate changes in BMD in response to physiologic estrogen replacement (35). Similarly, we found that in women with AN, sclerostin levels did not change in response to TPT treatment and were similar in both the TPT and the placebo groups over the 6-month time course. Therefore, our data suggest that serum sclerostin is not an important mediator of changes in BMD in response to TPT in women with AN.
Whereas significant increases in spine BMD were observed after only 6 months of treatment with TPT, the effect of a longer duration of therapy is unknown except for a case report that demonstrated increases in both spine and hip BMD in response to 2 years of teriparatide in a woman with AN (36). Importantly, although we only studied the effects of 6 months of treatment, the increases in BMD observed in this study are greater than any other increases in BMD in response to treatment in a population with AN. Additional studies will be needed to determine whether longer-term use of TPT will result in BMD gains in the hip and continued improvements at the spine.
In conclusion, 6 months of treatment with TPT led to significant increases in spine BMD in a population of women with AN. Future studies will be needed to better understand the mechanism by which TPT leads to improvements in BMD in this population, as this will allow us to better understand the pathophysiology of bone loss in states of nutritional deprivation.
Acknowledgments
We thank the patients who participated in this study as well as the nurses and bionutritionists of the Massachusetts General Hospital Clinical Research Center for their expert care. We thank Eli Lilly and Company for providing the study medications.
The project described was supported by National Institutes of Health (NIH) Grant K23-DK094820 (Fazeli); the NIH/National Center for Research Resources Grant UL1 RR025758 (to the Harvard Clinical and Translational Science Center); NIH/National Center for Advancing Translational Science Grant UL1 TR000170–05 (to the Harvard Clinical and Translational Science Center); and the Jane Walentas Women's Health Award from the Foundation for Women's Wellness.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Institutes of Health, the Foundation for Women's Wellness, or Eli Lilly and Company.
Disclosure Summary: All study medications (Teriparatide and placebo) were provided by Eli Lilly and Co.
Footnotes
- AN
- anorexia nervosa
- BMD
- bone mineral density
- BMI
- body mass index
- CTX
- C-terminal collagen cross-links
- CV
- coefficient of variation
- DHEA
- dehydroepiandrosterone
- P1NP
- N-terminal propeptide of type 1 procollagen
- rh
- recombinant human
- TPT
- teriparatide.
References
- 1. Keski-Rahkonen A, Hoek HW, Susser ES, et al. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry. 2007;164:1259–1265 [DOI] [PubMed] [Google Scholar]
- 2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th ed Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 3. Grinspoon S, Thomas E, Pitts S, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133:790–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005;165:561–566 [DOI] [PubMed] [Google Scholar]
- 5. Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA. 1991;265:1133–1138 [PubMed] [Google Scholar]
- 6. Trickey H. Eating disorders exact toll on adults, too. CNN website. http://www-cgi.cnn.com/2006/HEALTH/diet.fitness/03/24/hb.adult.eating.disorders/index.html 2006. Accessed October 15, 2013
- 7. Löwe B, Zipfel S, Buchholz C, Dupont Y, Reas DL, Herzog W. Long-term outcome of anorexia nervosa in a prospective 21-year follow-up study. Psychol Med. 2001;31:881–890 [DOI] [PubMed] [Google Scholar]
- 8. Scholtz S, Hill LS, Lacey H. Eating disorders in older women: does late onset anorexia nervosa exist? Int J Eat Disord. 2010;43:393–397 [DOI] [PubMed] [Google Scholar]
- 9. Kellett J, Trimble M, Thorley A. Anorexia nervosa after the menopause. Br J Psychiatry. 1976;128:555–558 [DOI] [PubMed] [Google Scholar]
- 10. Boast N, Coker E, Wakeling A. Anorexia nervosa of late onset. Br J Psychiatry. 1992;160:257–260 [DOI] [PubMed] [Google Scholar]
- 11. Beck D, Casper R, Andersen A. Truly late onset of eating disorders: a study of 11 cases averaging 60 years of age at presentation. Int J Eat Disord. 1996;20:389–395 [DOI] [PubMed] [Google Scholar]
- 12. Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898–904 [DOI] [PubMed] [Google Scholar]
- 13. Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2002;87:2883–2891 [DOI] [PubMed] [Google Scholar]
- 14. Miller KK, Meenaghan E, Lawson EA, et al. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2011;96:2081–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordon CM, Grace E, Emans SJ, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935–4941 [DOI] [PubMed] [Google Scholar]
- 16. Bloch M, Ish-Shalom S, Greenman Y, Klein E, Latzer Y. Dehydroepiandrosterone treatment effects on weight, bone density, bone metabolism and mood in women suffering from anorexia nervosa-a pilot study. Psychiatry Res. 2012;200:544–549 [DOI] [PubMed] [Google Scholar]
- 17. Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism. 2012;61:1010–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grinspoon S, Baum H, Lee K, Anderson E, Herzog D, Klibanski A. Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81:3864–3870 [DOI] [PubMed] [Google Scholar]
- 19. Stefanis N, Mackintosh C, Abraha HD, Treasure J, Moniz C. Dissociation of bone turnover in anorexia nervosa. Ann Clin Biochem. 1998;35(Pt 6):709–716 [DOI] [PubMed] [Google Scholar]
- 20. Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84:4489–4496 [DOI] [PubMed] [Google Scholar]
- 21. Pfeilschifter J, Laukhuf F, Müller-Beckmann B, Blum WF, Pfister T, Ziegler R. Parathyroid hormone increases the concentration of insulin-like growth factor-I and transforming growth factor beta 1 in rat bone. J Clin Invest. 1995;96:767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watson P, Lazowski D, Han V, Fraher L, Steer B, Hodsman A. Parathyroid hormone restores bone mass and enhances osteoblast insulin-like growth factor I gene expression in ovariectomized rats. Bone. 1995;16:357–365 [DOI] [PubMed] [Google Scholar]
- 23. Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GB., Jr The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab. 1992;75:762–767 [DOI] [PubMed] [Google Scholar]
- 24. Fazeli PK, Lawson EA, Prabhakaran R, et al. Effects of recombinant human growth hormone in anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:4889–4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Metropolitan Life Insurance Company. Metropolitan height and weight tables. Metropolitan Stat Bull. 1983;64:2–9 [PubMed] [Google Scholar]
- 26. Barthe N, Braillon P, Ducassou D, Basse-Cathalinat B. Comparison of two Hologic DXA systems (QDR 1000 and QDR 4500/A). Br J Radiol. 1997;70:728–739 [DOI] [PubMed] [Google Scholar]
- 27. Miller KK, Lee EE, Lawson EA, et al. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006;91:2931–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lucas AR, Melton LJ, 3rd, Crowson CS, O'Fallon WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc. 1999;74:972–977 [DOI] [PubMed] [Google Scholar]
- 29. Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26:2430–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCarthy TL, Centrella M, Canalis E. Parathyroid hormone enhances the transcript and polypeptide levels of insulin-like growth factor I in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1989;124:1247–1253 [DOI] [PubMed] [Google Scholar]
- 31. Yakar S, Bouxsein ML, Canalis E, et al. The ternary IGF complex influences postnatal bone acquisition and the skeletal response to intermittent parathyroid hormone. The Journal of Endocrinology. 2006;189:289–299 [DOI] [PubMed] [Google Scholar]
- 32. Drake MT, Srinivasan B, Mödder UI, et al. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010;95:5056–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Polyzos SA, Anastasilakis AD, Bratengeier C, Woloszczuk W, Papatheodorou A, Terpos E. Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women–the six-month effect of risedronate and teriparatide. Osteoporos Int. 2012;23:1171–1176 [DOI] [PubMed] [Google Scholar]
- 34. Gatti D, Viapiana O, Idolazzi L, Fracassi E, Rossini M, Adami S. The waning of teriparatide effect on bone formation markers in postmenopausal osteoporosis is associated with increasing serum levels of DKK1. J Clin Endocrinol Metab. 2011;96:1555–1559 [DOI] [PubMed] [Google Scholar]
- 35. Faje AT, Fazeli PK, Katzman DK, et al. Sclerostin levels and bone turnover markers in adolescents with anorexia nervosa and healthy adolescent girls. Bone. 2012;51:474–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shibli-Rahhal A, McCormick L. Teriparatide treatment of osteoporosis in a patient with anorexia nervosa. Eat Weight Disord. 2013;18:229–231 [DOI] [PubMed] [Google Scholar]