Abstract
The aims of the current work were to evaluate the hepatoprotective effect of calendula flowers and/or thyme leave extracts on aflatoxins (AFs)-induced oxidative stress, genotoxicity and alteration of p53 bax and bcl2 gene expressions. Eighty male Sprague–Dawley rats were divided into eight equal groups including: the control group, the group fed AFs-contaminated diet (2.5 mg/kg diet) for 5 weeks, the groups treated orally with thyme and/or calendula extract (0.5 g/kg b.w) for 6 weeks and the groups pretreated orally with thyme and/or calendula extract 1 week before and during AFs treatment for further 5 weeks. Blood, liver and bone marrow samples were collected for biochemical analysis, gene expression, DNA fragmentation and micronucleus assay. The results showed that AFs induced significant alterations in oxidative stress markers, increased serum AFP and inflammatory cytokine, percentage of DNA fragmentation, the expression of pro-apoptotic gene p53 and bax accompanied with a decrease in the expression of bcl2. Animals treated with the extracts 1 week before AFs treatment showed a significant decrease in oxidative damage markers, micronucleated cells, DNA fragmentation and modulation of the expression of pro-apoptotic genes. These results suggested that both calendula and thyme extracts had anti-genotoxic effects due to their higher content of total phenolic compounds.
Keywords: Aflatoxins, Calendula, Thyme, sqRT-PCR, Gene expression, Liver
Introduction
Aflatoxins (AFs) are biologically active secondary metabolites mostly produced by certain species of Aspergillus molds including Aspergillus parasiticus, Aspergillus nominus, and Aspergillus flavus (Bedard and Massey 2006). There are four naturally occurring AFs, the most hepatotoxic being aflatoxin B1 (AFB1), and three structurally similar compounds namely aflatoxin B2 (AFB2), aflatoxin G1 (AFG1) and aflatoxin G2 (AFG2). AFs not only contaminate food stuffs but are also found in edible tissues, milk and eggs after consumption of contaminated feed by farm animals (Bennett and Klich 2003; Fink-Gremmels 1999). AFB1 is a mutagenic compound and a contaminant of many food sources, especially in some parts of Africa and Asia and are recognized as one of the most potent hepatocarcinogens in humans and many animal species (Abdel-Wahhab et al. 2010; Hassan et al. 2012). Its genotoxicity requires activation by cytochromes P450 and the formation of AFB1-8, 9-epoxides (Gallagher et al. 1996). Although the mechanism underlying the hepatotoxicity of AFs is not fully understood, several reports suggest that toxicity may ensue through the generation of intracellular reactive oxygen species (ROS) like superoxide anion, hydroxyl radical and hydrogen peroxide (H2O2) during the metabolic processing of AFB1 by cytochrome P450 in the liver (Towner et al. 2003; Sohn et al. 2003). These species may attack soluble cell compounds as well as membranes, eventually leading to the impairment of cell functioning and cytolysis (Berg et al. 2004). Moreover, AFB1 carcinogenicity has been associated with altered expression of many p53-target genes and induction of mutations, principally the p53 codon 249 hotspot mutation (Josse et al. 2012).
In recent years, active principles with diverse chemical structures have been isolated from plants reportedly possessing hepatoprotective effects. Thyme (Thymus vulgaris) can be used fresh or dried as a spice. Essential oils extracted from fresh leaves and flowers can be used as aroma additives in food, pharmaceuticals and cosmetics (Javanmardi et al. 2002). Thyme also possesses various beneficial effects, e.g. antiseptic, carminative, antimicrobial and antioxidative properties (Baranauskiene et al. 2003; El-Nekeety et al. 2011). Calendula L. (Asteraceae), usually known as “marigold”, is a reputed medicinal plant with ornamental properties. The yellow or orange-colored flowers are used as food dye, spice, and tea as well as tincture, ointment or cosmetic cream. The genus calendula is usually indigenous to the southern European region (Rejsková et al. 2010). It is grown in northern parts of Africa and is named as “African marigold” (Muley et al. 2009). It has become quite important in phytotherapy due to its healing effects against dermatological diseases (Bedi and Shenefelt 2002; Leach 2008; Fronza et al. 2009). The plant has been reported to contain mainly carotenoids, flavonoids, phenolic acids, and triterpenes (Wojciak-Kosior et al. 2003; Kishimoto et al. 2005). The aim of the current study was to evaluate the protective role of thyme and calendula extract singly or in combination against AF-induced genotoxicity, DNA damage and oxidative stress in rats.
Materials and methods
Chemicals and kits
Glutathione peroxidase (GPx) and superoxide dismutase (SOD) kits were purchased from Randox (Antrim, UK). Malondialdehyde (MDA) kit was purchased from Oxis Research™ Co. (Foster City, CA, USA). Alpha fetoprotein (AFP) kit was purchased from Monobind Inc. (Lake Forest, CA, USA). Interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNF-α) kits were purchased from Orgenium (Helsinki, Finland). All other chemicals were of the highest analytical grade available.
Preparation of aflatoxin-contaminated diet
The AFs were produced via fermentation of mycotoxins free rice with A. parasiticus NRRL 2999 as described (Shotwell et al. 1966) and modified by Demet et al. (1995). The fermented rice was autoclaved, dried and ground to a powder and the AFs content was measured by HPLC (Hustchins and Hagler 1983). The AFs within the rice powder consisted of 79.4 % B1, 14.3 % B2, 5.2 % G1 and 1.1 % G2 based on total AFs in the rice powder. The rice powder was incorporated into the basal diet of rats to provide the desired level of 2.5 mg of AFs/kg diet. The diet containing AFs was analysed and the presence of parent AFs was confirmed and determined as mentioned above. The safety measures recommended by WHO (1998) were taken when handling the AF-contaminated diet.
Plant materials
Thyme (T. vulgaris) and calendula (Calendula officinalis) were purchased from the local market. The plants were identified by the Department of Medicinal Plants, National Research Center and vouchers were kept in the herbarium of NRC.
Preparation of thyme and calendula extracts
Dried and ground flowers of calendula and leaves of thyme (50 g) were subjected to extraction with 400 ml of ethanol (95 %) for 48 h. The extracts were filtered and concentrated under reduced pressure of nitrogen and completely evaporated in a vacuum oven at a temperature not exceeding 40 °C until constant weights were obtained.
Determination of total phenolic contents of the extracts
The concentration of phenolics in the extracts was determined using the method of Jayaprakasha and Rao (2000). In brief, 5 mg of each extracts was dissolved in a 10 ml mixture of acetone and water (6:4 v/v). Samples (0.2 ml) were mixed with 1 ml of tenfold diluted Folin-Ciocalteu reagent and 0.8 ml of sodium carbonate solution (7.5 %). The absorbance was measured at 765 nm using UV-160 IPC UV–visible spectrophotometer (Shimadzu, Tokyo, Japan) after 30 min at room temperature. Estimation of phenolic compounds as catechin equivalents (CE) was carried out using a standard curve of catechin (Jayaprakasha et al. 2003).
Experimental animals
Three-months old male Sprague–Dawley rats (100–120 g, purchased from the animal house colony, Giza, Egypt) were maintained on standard lab diet (protein: 160.4; fat: 36.3; fiber: 41 g/kg and metabolizable energy of 12.08 MJ) purchased from Meladco Feed Co. (Aubor City, Cairo, Egypt). Animals were housed in a room free from any source of chemical contamination, artificially illuminated and thermally controlled at the Animal House Lab., National Research Center (Dokki, Cairo, Egypt). After an acclimatization period of 1 week, the animals were divided into eight groups (10 rats/group) and housed in filter-top polycarbonate cages. All animals have received humane care in compliance with the guidelines of the Animal Care and Use Committee of the National Research Center (Dokki, Cairo, Egypt).
Experimental design
Animals within different treatment groups were treated daily for 6 weeks as follows: group (1), untreated control; group (2), animals fed AFs-contaminated diet (2.5 mg/kg diet) for 5 weeks; group (3), animals treated orally with thyme extract (0.5 g/kg b.w) for 6 weeks; group (4), animals treated orally with calendula extract (0.5 g/kg b.w) for 6 weeks; group (5) animals treated orally with thyme and calendula extracts for 6 weeks; group (6), animals pretreated orally with thyme extract for 1 week before and during AFs treatment for further 5 weeks; group (7), animals pretreated orally with calendula extract for 1 week before and during AFs treatment for further 5 weeks and group (8), animals pretreated orally with calendula and thyme extracts for 1 week before and during AFs treatment for further 5 weeks. At the end of the treatment period, blood samples were collected from the retro-orbital venous plexus from each animal under ether anesthesia. Blood samples were left to clot and the sera were separated using cooling centrifugation at 3,000 rpm for 15 min and stored at −20 °C until analysis. The sera were used for the determination of AFP, TNF-α and IL-1β according to the instructions of the analytical kits.
After the collection of blood samples, all animals were killed by cervical dislocation and samples of the livers were weighed (approximately 0.05–0.1 g) and homogenized in phosphate buffer (pH 7.4) to give 20 % (w/v) homogenate. This homogenate was centrifuged at 1,700 rpm at 4 °C for 10 min and the supernatant was stored at −70 °C until analysis. This supernatant was used for the assessment of GPx, MDA and SOD according to the instructions of the kits. Other samples of liver and bone marrow of each animal were dissected for molecular and cytogenetic studies.
Micronucleus assay
Femur bone marrow was used for analysis of micronucleus formation by scoring micronucleated polychromatic erythrocytes (Mn-PCEs) and normochromatic erythrocytes (Mn-NCEs) as described by Heddle (1973). The Mn-NCEs were prepared according to the method described by Salamone et al. (1980). A total of 2,000 erythrocytes was analyzed per rat and the micronucleus frequency in both cell types was determined. The ratio of polychromatic erythrocytes (PCEs) to normochromatic erythrocytes (NCEs) was calculated to determine the cytotoxicity.
DNA fragmentation
Deoxyribose sugar derived from DNA gives a bluish color in presence of perchloric acid and diphenylamine reagent. The latter could be measured spectrophotometrically at 575 nm. DNA fragmentation in liver tissue was carried out according to the method described by Sambrook et al. (1989) and the modifications described by Xu et al. (1996). In brief, 10–20 mg of liver tissue were grinded in 400 ml hypotonic lysis buffer (10 mM Tris base, 1 mM EDTA and 0.2 % Triton X-10) and the cell lysate was centrifuged at 11,000 rpm for 15 min at 4 °C. The supernatant containing small DNA fragments were separated; one-half the volume was used for gel electrophoresis and the other half, together with the pellet containing large pieces of DNA were used for quantification of fragmented DNA by the Diphenyl amine. Extracted DNA was reconstituted in 12 ml of Tris-EDTA buffer and 3 ml loading buffer. The samples were incubated at 37 °C for 20 min then electrophoresed on 1 % agarose gels containing 0.71 mg/ml ethidium bromides. At the end of the runs, gels were examined using UV transillumination. The percentage of DNA fragmentation was calculated.
Agarose gel electrophoresis
A gel was prepared with 2 % agarose containing 0.1 % ethidium bromide and was electrophoresed using the submarine gel electrophoresis machine. The DNA was visualized and photographed with illumination under UV light.
Gene expressions
RNA extraction
Immediately after the animals were sacrificed, samples of liver tissues were taken and frozen in liquid nitrogen and stored at −80 °C prior to extraction. Total RNA was extracted from 50 to 100 μg of each sample by the standard TRIzol extraction method (Invitrogen, Paisley, UK). The solution of extracted RNA was recovered in 100 μl molecular biology grade water. The total RNA samples were pretreated using DNA-free™ DNase to remove any possible genomic DNA contamination. These steps were performed according to manufacturer’s protocol (Ambion, Austin, TX, USA).
Reverse transcription
The complete Poly(A) + RNA isolated from the liver samples was reverse transcribed into cDNA in a total volume of 20 μl using 1 μl oligo (dT) primer. The composition of the reaction mixture, termed as master mix (MM), consisted of 50 mM MgCl2, 200 U/μl reverse transcriptase (RNase H-free), 10× reverse transcription (RT) buffer (50 mM KCl; 10 mM Tris–HCl; pH 8.3), 10 mM of each dNTP and 50 μM of oligo (dT) primer. The reaction was carried out at 25 °C for 10 min, followed by 1 h at 42 °C and finished with a denaturation step at 99 °C for 5 min. The reaction tubes containing RT preparations were flash cooled in an ice chamber until being used for cDNA amplification through polymerase chain reaction (PCR) (Brun et al. 2006; Abdel-Aziem et al. 2011b).
Polymerase chain reaction (PCR)
The first strand cDNA from different liver samples was used as templates for PCR with a pair of specific primers. The sequences of specific primers and product sizes are listed in Table 1. The reaction mixture for semi-quantitative-PCR in a total volume of 20 μl consisted of 10 mM dNTP’s, 50 mM MgCl2, 1 U/μl Taq polymerase, 10× PCR buffer (50 mM KCl; 20 mM Tris–HCl; pH 8.3) and autoclaved water. The PCR cycling parameters were one cycle at 95 °C for 4 min, 50 cycles at 94 °C for 30 s, 55–60 °C for 30 s, 72 °C for 60 s and a final cycle at 72 °C for 7 min. The PCR products were then loaded onto 2.0 % agarose gel with PCR products derived from β-actin of the different rat samples (Kronmiller et al. 1995; Hassan et al. 2012).
Table 1.
Sequences of primers used for amplification
| Gene | Sense and antisense | PCR product (bp) | References |
|---|---|---|---|
| P53 | Sense 5′-GGAGGTTGTGAGGCGCTGC-3′ Antisense 5′-CACGCACCTCAAAGCTGTTC-3′ |
773 | Bai and Meng (2005) |
| Bcl-2 | Sense 5′-TTGTGGCCTTCTTTGAGTTCG-3′ Antisense 5′-TACTGCTTTAGTGAACCTTTT-3′ |
332 | Agrawal et al. (1999) |
| Bax | Sense 5′-ACCAGCTCTGAGCAGATCATG-3′ Antisense 5′-GGGATTGATCAGACACGTAAG-3′ |
626 | Zhang et al. (2006) |
| β-Actin | Sense 5′-CGTGACATCAAAGAGAAGCTGTGC-3′ Antisense 5′-CTCAGGAGGAGCAATGATCTTGAT-3′ |
376 | Baek et al. (2007) |
Statistical analysis
All data were statistically analyzed with the General Linear Model Procedure of the Statistical Analysis System (SAS 1982). The significance of the differences among treatment groups was determined with the Waller–Duncan k-ratio (Waller and Duncan 1969). All statements of significance were based on a probability of P ≤ 0.05.
Results
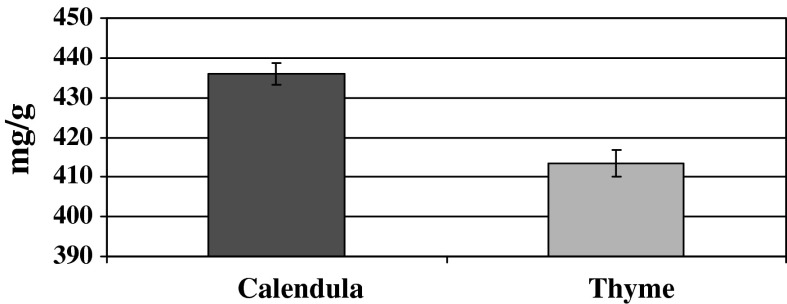
Total phenolic contents of the ethanolic extract of both thyme and calendula extract are presented in Fig. 1. These results revealed that the total phenolic content of the ethanolic extract of calendula was higher than that of thyme extract.
Fig. 1.
Total phenolic content of the ethanolic extracts of calendula and thyme
The results presented in Table 2 revealed that animals treated with AFs showed a significant increase in the tumor marker AFP. However, no significant difference was observed in serum AFP in the animals treated with thyme and/or calendula extracts. Animals fed AFs-contaminated diet and treated with thyme or calendula extract showed a significant improvement in the level of serum AFP although it was still higher than in the control group. On the other hand, animals fed AFs-contaminated diet and having received the combined treatment of thyme plus calendula were comparable to the control (Table 2). The effect of different treatments on serum inflammatory cytokines (Table 2) revealed that serum TNF-α and IL-1β were significantly increased in the group fed AFs-contaminated diet. However, animals treated with thyme or calendula extracts were comparable to the control group. The combined treatment of thyme and calendula extracts resulted in a significant decrease in the level of these inflammatory cytokines. On the other hand, animals fed AFs-contaminated diet and treated with thyme or calendula extract showed a significant improvement in these parameters. This improvement was more pronounced in the group treated with calendula extract. Moreover, animals fed AFs-contaminated diet and treated with two extracts were comparable to the control group regarding to the level of TNF-α and IL-1β.
Table 2.
Effect of thyme and calendula extracts on serum AFP and inflammatory cytokines
| Treatment | AFP (ng/ml) | TNF-α (pg/ml) | IL-1β (pg/ml) |
|---|---|---|---|
| Control | 1.63 ± 0.1a | 61.37 ± 2.80a | 0.63 ± 0.64a |
| AFs | 3.87 ± 0.17b | 123.13 ± 2.61b | 1.93 ± 0.09b |
| TE | 1.54 ± 0.05a | 61.07 ± 2.07a | 0.65 ± 0.04a |
| CE | 1.74 ± 0.09a | 58.37 ± 1.92a | 0.63 ± 0.04a |
| TE + CE | 1.46 ± 0.09a | 52.5 ± 2.18c | 0.63 ± 0.07a |
| AFs + TE | 2.23 ± 0.11c | 84.88 ± 2.28d | 0.75 ± 0.05c |
| AFs + CE | 2.46 ± 0.39c | 75.99 ± 2.90e | 0.58 ± 0.04d |
| AFs + TE + CE | 1.88 ± 0.1a | 65.67 ± 4.98a | 0.60 ± 0.05a |
Within each column, means superscript with different letters are significantly different (P ≤ 0.05)
AFs aflatoxins; TE thyme extract; CE calendula extract
The results of MDA in liver tissue (Table 3) showed a significant increase in the animals fed AFs-contaminated diet. Both thyme and calendula extracts alone or in combination succeeded to decrease MDA in liver tissue. Moreover, calendula alone was more effective than thyme and the combined treatment was more effective than the single extracts. The activity of hepatic GPx and SOD (Table 3) showed a significant decrease in the group fed AFs-contaminated diet. The extracts singly or in combination resulted in a significant increase in these antioxidant enzymes activities. This increase was pronounced in the group treated with calendula extract and was more pronounced in the group treated with both thyme and calendula extracts.
Table 3.
Effects of thyme and calendula on lipid peroxidation, GPx and SOD in liver of rats fed AF-contaminated diet
| Treatment | MDA (nmol/g) | GPx (Unit/mg protein) | SOD (U/mg protein) |
|---|---|---|---|
| Control | 47.4 ± 3.0a | 33.25 ± 2.34a | 288.73 ± 7.47a |
| AFs | 135.1 ± 3.4b | 14.22 ± 1.2b | 166.42 ± 4.27b |
| TE | 36.2 ± 2.3c | 44.16 ± 2.23c | 298.48 ± 5.73c |
| CE | 28.4 ± 1.2d | 55.15 ± 2.35d | 305.49 ± 3.83d |
| TE + CE | 25.5 ± 3.0c | 64.75 ± 1.19e | 325.14 ± 4.33e |
| AFs + TE | 75.1 ± 2.75e | 30.05 ± 2.41a | 234.36 ± 4.06f |
| AFs + CE | 63.7 ± 2.6f | 35.61 ± 1.43a | 281.57 ± 5.04a |
| AFs1 + TE + CE | 61.6 ± 3.3 g | 42.53 ± 1.13c | 297.60 ± 5.57c |
Within each column, means superscript with different letters are significantly different (P ≤ 0.05)
The results of the current study indicated that animals fed AFs-contaminated diet showed severe bone-marrow toxicity as indicated by the significant increase in total number of PCEs compared to the control group (Table 4). Animals treated with thyme alone or in combination with calendula were comparable to the control group; however, those treated with calendula alone showed a significant decrease in Mn-PCEs compared to the control group. Animals fed AFs-contaminated diet and treated with thyme or calendula extracts showed a significant increase in the number of PCEs and a reduced number of Mn-NCEs although they were still significantly different from the control group. On the other hand, animals fed AFs-contaminated diet and treated with thyme plus calendula were comparable with the control group regarding the number of PCEs and the normalized PCE/NCE ratio (Table 4).
Table 4.
Effects of thyme and/or calendula on micronucleus formation in rat bone-marrow erythrocytes
| Treatment | Mn-NCEs/2000 | Mn-PCEs/2000 | PCEs (%) | NCEs (%) | Ratio PCE:NCE |
|---|---|---|---|---|---|
| Control | 3.0 ± 0.49a | 3.2 ± 0.37a | 53.4 ± 1.9a | 50.6 ± 1.8a | 1.01 ± 0.1a |
| AFs | 37.0 ± 1.14b | 29.6 ± 2.38b | 30.2 ± 1.7b | 67.8 ± 1.7b | 0.48 ± 0.4b |
| TE | 4.2 ± 0.32c | 4.0 ± 0.32c | 45.0 ± 1.58c | 55.0 ± 1.6c | 0.91 ± 0.5a |
| CE | 3.5 ± 0.37c | 3.2 ± 0.24a | 55.0 ± 1.3a | 50.0 ± 1.3a | 1.0 ± 0.6a |
| TE + CE | 2.9 ± 0.25a | 3.0 ± 0.37a | 50.6 ± 0.81a | 47.6 ± 0.9d | 1.2 ± 0.3a |
| AFs + TE | 19.4 ± 0.74d | 17.6 ± 0.68d | 40.8 ± 0.73d | 59.2 ± 0.74e | 0.68 ± 0.2c |
| AFs + CE | 17.6 ± 1.29e | 14.6 ± 0.87e | 47.0 ± 0.9c | 53.0 ± 0.9c | 0.88 ± 0.3d |
| AFs + TE + CE | 12.4 ± 0.25e | 12.6 ± 0.51e | 51.2 ± 1.2a | 45.4 ± 1.2d | 1.07 ± 0.5a |
Within each column, means superscript with different letters are significantly different (P ≤ 0.05)
The results presented in Table 5 indicate that the percentage of DNA fragmentation in the liver of animals fed AFs-contaminated diet was significantly increased compared to the control group. Animals treated with calendula alone or plus thyme showed an insignificant increase in the percentage of DNA fragmentation. However, those treated with thyme alone showed a significant decrease in DNA fragmentation in liver tissue. On the other hand, animals fed AFs-contaminated diet and having received thyme or calendula alone or in combination showed a significant reduction in the percentage of DNA fragmentation relative to the control values although these treatments did not normalize it. DNA fragmentation in response to exposure to AF was also detected by gel electrophoresis as a DNA ladder representing a series of fragments that are multiples of 180–200 bp.
Table 5.
Effects of thyme and/or calendula extracts on DNA fragmentation in liver tissues of rats fed AF-contaminated diet
| Treatment | DNA fragmentation (%) | Change |
|---|---|---|
| Control | 9.0 | – |
| AFs | 43 | +34 |
| TE | 12.3 | +3.2 |
| CE | 9.7 | +0.7 |
| TE + CE | 13.6 | +4.6 |
| AFs + TE | 25.2 | +16.2 |
| AFs + CE | 20.6 | +11.6 |
| AFs + TE + CE | 19.5 | +10.5 |
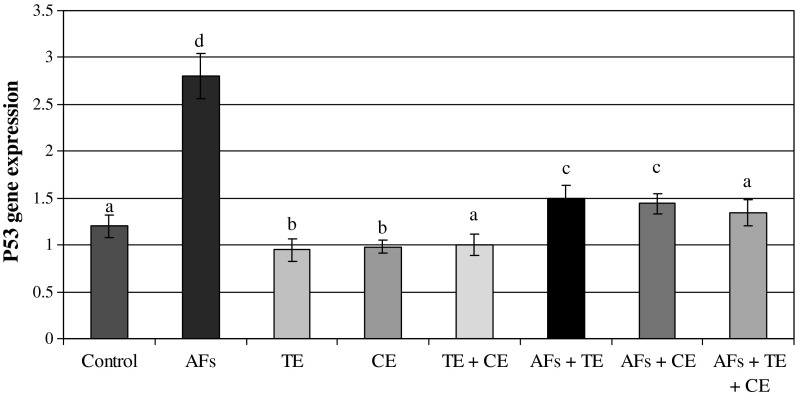
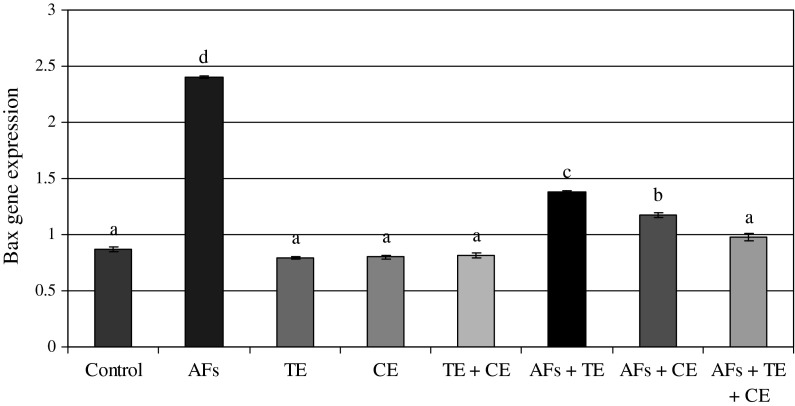
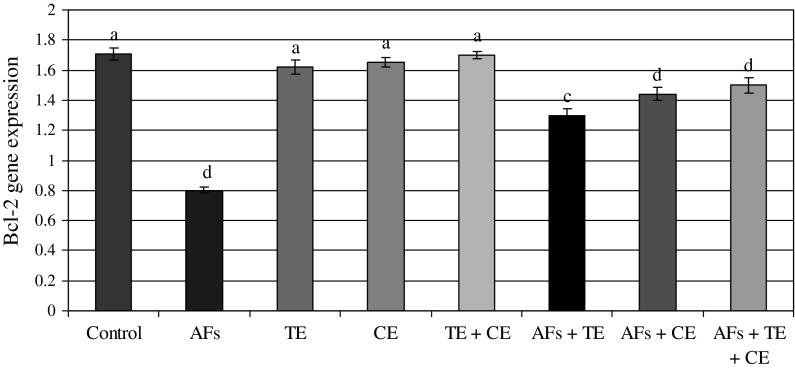
The data presented in Figs. 2 and 5 show the ratio of optical density of p53/β-actin expression in the liver of the controls and the treated animals. These results indicated that p53 expression was significantly increased in AFs-treated animals compared to the controls. However, those fed AFs-contaminated diet and treated with thyme and/or calendula showed a significant decrease in p53 expression. Moreover, thyme and/or calendula themselves did not induce any significant effect on the expression of p53. Meanwhile, the ratio between bax/β-actin indicated an over expression in bax compared to the ratio between control Bcl-2/β-actin (Figs. 3, 5) which increased in the animals treated AFs (2.4) compared to the control group (0.87). On the other hand, the ratio of Bcl-2/β-actin (Figs. 4, 5) was decreased in animals fed AFs-contaminated diet (0.8) compared to control Bcl-2/β-actin ratio (1.17). Treatment with thyme succeeded to reduce the ratio of expression of mRNA bax from 2.4 in the AFs-treated group to 1.38 in the group treated with AFs plus thyme extract. While the treatment with calendula resulted in a further reduction in the expression ratio of mRNA bax to reach 1.17 compared to the AFs alone treated group. A higher reduction in the ratio of bax/β-actin was observed in the group fed AFs-contaminated diet and treated with thyme plus calendula. Moreover, treatment with thyme increased the expression of mRNA Bcl-2/β-actin ratio from 0.8 in AFs-treated group to reach 1.3 in the group treated with AFs plus thyme. However, treatment with calendula extract increased the ratio to 1.44. Animals fed AFs-contaminated diet and treated with thyme plus calendula showed a further increase in the ratio of Bcl-2/β-actin.
Fig. 2.
The ratio between p53/β actin in rat treated with AFs alone or in combination with thyme extract (TE) and/or calendula extract (CE). Values represent mean ± SE for each group. Column superscripts with different letter are significantly different (P ≤ 0.05)
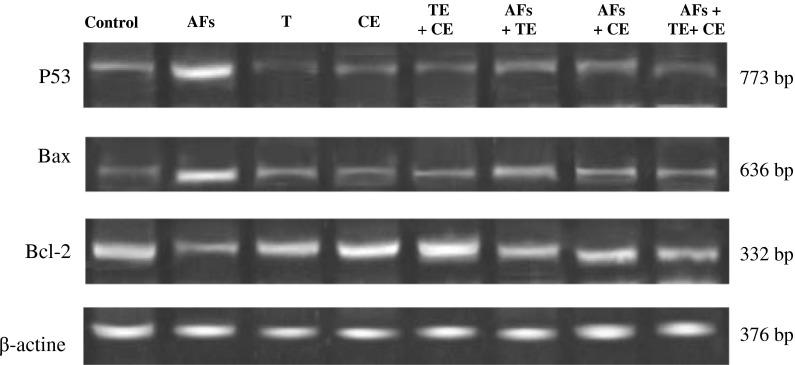
Fig. 5.
Effects of thyme extracts (TE) and/or calendula extracts (CE) on transcript product of hepatic genes (P53, Bax and Bcl-2) in rats treated with AFs. Agarose gel electrophoresis of P53, Bax, Bcl-2 and β-actin RT-PCR products of different groups are presented: Group I: Control, Group II: AFs, Group III: TE, Group IV: CE, Group V: TE + CE, Group VI: AFs + TE, Group VII: AFs + CE and Group VIII: AFs + TE + CE
Fig. 3.
The ratio between bax/β-actin in rats treated with AFs alone or in combination with thyme extract (TE) and/or calendula extract (CE). Values represent mean ± SE for each group. Column superscripts with different letter are significantly different (P ≤ 0.05)
Fig. 4.
The ratio between Bcl-2/β-actin in rat treated with AFs alone or in combination with thyme extract (TE) and/or calendula extract (CE). Values represent mean ± SE for each group. Column superscripts with different letter are significantly different (P ≤ 0.05)
Discussion
Nowadays there is an increased interest in natural products which may counteract the detrimental effects of environmental toxic compounds and prevent multiple diseases in humans. In this concern, different types of natural products have been re-evaluated and recognized as valuable sources of nutraceuticals. According to several reports, calendula and/or thyme extracts contain important nutrients and exhibit antioxidant functions. Moreover, it is well known that the extraction of active ingredient compounds from plant material depends on the type of solvent used in the extraction procedure (Majhenic et al. 2007). The results of the present study revealed that the total phenolic compounds of the ethanolic extract of both plants are high. These results are in agreement with those reported recently by Rababah et al. (2010).
In the present work, we evaluated the protective effect of calendula and/or thyme extracts in rats by monitoring their effects on oxidative stress, genotoxicity and cell death pathway induced by AFs. The selected dose of AFs, thyme were based on our previous work (Abdel-Wahhab and Aly 2003; Hamzawy et al. 2012, respectively) however; the dose of calendula extracts was literature based (Gladine et al. 2007). Exposure to AFs is one of the major risk factors in the etiology of human hepatocellular carcinoma and AFB1 is a potent hepatocarcinogen when given sub-chronically at a low level. AFB1 generates ROS and causes oxidative DNA damage which may play a major role in its carcinogenicity (Yang et al. 2000; Abdel-Wahhab and Aly 2003; Abdel-Aziz et al. 2005). Alpha-fetoprotein (AFP) is considered a specific biomarker for the development of liver cancer. The significant elevation of AFP reported herein in AFs-treated rats may be due to induction of expression of mRNAs of liver AFP (Yang et al. 2000). Moreover, AFB1-induced hepatocarcinogenesis is associated with defective DNA-damage response by passing via p53 activation (Yuzugullu et al. 2011) and modulation of insulin-like growth factor 2 (IGF-2) dependent signal axis (Ubagai et al. 2010). Similar to the current observation, AFB1 administration resulted in the elevation of serum AFP levels in both ducks (Sell et al. 1998) and rats (Yang et al. 2000; Abdel-Wahhab et al. 2006).
Tumor necrosis factor-alpha and interleukin-1 alpha (IL-1α) are produced by macrophages and they play an important role in tumor progression (Moon et al. 1999; Abdel-Wahhab et al. 2006) and TNF-α is an essential factor in tumor promotion (Suganuma et al. 2000). Furthermore, interleukin-1 polymorphisms are important mediators in the inflammatory process (Rollinson et al. 2003). TNF-α is one of the major inflammatory mediators secreted by activated macrophages and is involved in many crucial events for the initiation of both acute and chronic inflammation, such as regulating the production of several cytokines, up-regulation of the expression of adhesion molecule and activation of leukocyte-specific chemotactic cytokines (Zhou et al. 2009). Moreover, it plays a causal role in the development of liver injury (Barton et al. 2001), modulating mycotoxin-induced hepatotoxicity (Voss et al. 2006) and in mediating the proliferation and differentiation of immune cells and the development of immune response (Cao et al. 2008). In the current study, the ingestion of AFs-contaminated diet significantly increased TNF-α and IL-1α suggesting that AFs preferentially affect macrophage functions. In particular, it decouples the close correlations usually observed between transcriptional and translational controls of IL-1α and TNF-α production by these cells (Hopkins 2003).
The hepatic antioxidants represent the major defense against toxic liver injury, and they act anti-apoptotic. The oxidative damage caused by AF is considered to be the main mechanism leading to the subsequent hepatotoxicity (Preetha et al. 2006). Moreover, AFB1 may disturb the integrity of cell membranes through stimulating phospholipid A2 to initiate lipid peroxidation in cells (Rastogi et al. 2005). In the current study, animals fed AFs-contaminated diet suffer from oxidative stress as indicated by the significant increment of lipid peroxidation (MDA) and the significant reduction of enzymatic antioxidant such as SOD and GPx. These results are in agreement with previous reports which suggested that oxidative stress may be due to direct effect of AFs themselves or by their metabolites and the generation of free radicals during the formation of these metabolites (Abdel Wahhab et al. 2010; Abdel Wahhab and Aly 2003, 2005; Kanbur et al. 2011; Abdel-Aziem et al. 2011a). Moreover, the reduction of protein synthesis in AFs-treated animals may affect certain metal ions (i.e. iron and copper), which play an important role in free radical production and liberation. In normal state, these metal ions are bound to transfer proteins, such as ceruloplasmin and transferrin (Vladimirov 1998; Gitto et al. 2009). Consequently, the activities of the enzymes SOD, CAT and GSH-Px, which constitute the enzymatic antioxidant defense system of the cell, undergo direct changes, either in the form of increase or decrease, depending on the particular tissue.
The results also indicated that rats received AFs showed a high percentage of Mn-PCEs in bone marrow cells. It is acknowledged that an increase in this frequency is associated with an increased overall risk of cancer (Abdel-Aziem et al. 2011b; Hassan et al. 2012). Similarly, micronucleus damage and an increase in Mn-PCEs by AFB1 were previously reported in mice (Madrigal-Santillán et al. 2006) and support the earlier findings which indicate that AFs are potent mutagens (Abdel-Wahhab et al. 2006). It is well known that AFs are activated by the hepatic cytochrome P450 enzyme system to produce a highly reactive intermediate, AFB1-8, 9-epoxide, which subsequently binds to nucleophilic sites in DNA and the major adduct 8, 9-dihydro-8-(N7guanyl)-9-hydroxy-AFB1 (AFB1 N7-Gua) is formed (Sharma and Farmer 2004). The formation of AFB1-DNA adducts is regarded as a critical step in the initiation of AFB1-induced hepatocarcinogenesis (Preston and Williams 2005; Abdel-Wahhab et al. 2006). These genotoxic endpoints are well known markers of genotoxicity and any reduction in the frequency of these genotoxic endpoints gives an indication of the antigenotoxicity of a particular compound (Albertini et al. 2000). In the current study, treatment with AFs induced oxidative damage in rats and DNA fragmentation in liver. There is a tendency for AFs especially AFB1 to convert into the epoxide and produce DNA adducts resulting in the formation of DNA strand breaks and mutations (Eaton and Gallagher 1994). Indeed, the current results showed that treatment with AFs induced a significant DNA fragmentation in liver cells compared to the control animals since no specific DNA fragments were observed in the control group.
The current results also showed that AFs induced higher expressions of p53 and bax pro-apoptotic proteins in liver tissues. The same treatment induced a down-regulation of the antiapoptotic protein Bcl2. Similar to our results, Ranchal et al. (2009) reported that AFs induced DNA damage, reduced p27 expression and increased cell death in cultured hepatocytes. The involvement of AFs in DNA damage reported herein and its correlation with biomarkers of cellular oxidative stress and apoptosis induction were also evaluated. Oxidative stress can be considered as an apoptosis inducer and many agents that induce apoptosis are either oxidants or stimulators of cellular oxidative metabolism (Chandra et al. 2000). This is the case of AFs which induced oxidative stress and apoptotic cell death. Biological responses mediated by expression of bax gene mRNA are dependent on receptor-associated signaling complex proteins. It is well documented that the increased bax/β-actin ratio indicates that apoptosis is favored in a variety of cells (Horn et al. 2000). Susceptibility toward bax-mediated apoptosis depends on expression of the bax receptor, intact bax-signaling pathways and the absence of apoptosis-inhibiting molecules that interfere with bax signal transduction pathways. In the current study, mRNA FAS expression in liver was increased in AFs-treated rats. During AFs-induced liver injury, damaged hepatocytes may be eliminated by up regulating their constitutive expression of bax. The lethal signal to remove injured cells is potentially delivered by infiltrating or resident immune cells that bear bax (Messmer et al. 2001).
It is now accepted that the oxidative damage caused by AF is considered to be the main mechanism leading to the subsequent hepatotoxicity (Preetha et al. 2006). In a previous work, we reported that the main components of thyme oil are carvarcrol, thymol, β-phellandrene, linalool, humuline, α-phellandrene and Myrcene. However, α and β-pinene, Myrcene, α-thyjone, tricyclene, 1, 8-cineole, and β-sabinene were found in lower concentrations (El-Nekeety et al. 2011). Consequently, the possible antioxidant effect of thyme may be attributed mainly to carvacol and thymol (Alam et al. 1999; Hassan and Barakat 2008; Aristatile et al. 2009; El-Nekeety et al. 2011). On the other hand, calendula contains carotenoids, flavonoids, phenolic acids and triterpenes (Kishimoto et al. 2005; Ercetin et al. 2012). The higher content of flavonoids, quinones, volatile oils and carotenoids confirmed its free radical scavenging and antioxidant activity (Cordova et al. 2002; Muley et al. 2009).
In the current study, administration of either thyme and/or calendula extracts succeeded to counteract the oxidative stress resulted from AFs. The antioxidant compounds in thyme and calendula extracts exhibited potent antioxidant activity comparable to the known antioxidants, BHT and α-tocopherol (Lee and Shibamoto 2002). Considering the abundance of these aromatic compounds in natural plants, the total activity may be comparable to those of known antioxidants. Furthermore, ingestion of these aromatic compounds may help to prevent oxidative damage such as lipid peroxidation which is associated with cancer, premature aging, atherosclerosis and diabetes (Lee et al. 2005; Verma and Nair 2001). In the same concern, Hassan and Barakat (2008) and El-Nekeety et al. (2011) reported that thyme has a protective effect attributed to its content of polyphenol compound. Generally the protective role of both herbs can be attributed to the antioxidant effect and their free radical scavenger properties.
The current results revealed that both thyme and calendula extracts succeeded to restore AFP and IL-1β to normal levels and reduced TNF-α in rats treated with AFs. These results suggest that thyme extract possess an anti-inflammatory activity due to thymol content which was found to inhibit human elastase activity (Dal Sassoa et al. 2006). Moreover, thyme is known to inhibit a large number of inflammatory cytokines such as TNF-α, enterotoxins A and B and alpha-hemolysin production in Staphylococcus aureus isolate (Qiu et al. 2010). In addition, caravacol has been described as promoter for liver regeneration and inhibitor in TNF-α and IL-6 level in rats undergoing partial hepatectomy (Uyanoglu et al. 2008). Furthermore, carvarcrol in thyme decreases TNF-α and IL-1β levels in intoxicated rats through the suppression of cycloxygensae-2 (COX-2) mRNA and protein causing repression of inflammation (Tsai et al. 2011). Another mechanism by which thyme extract may possess anti-inflammatory response was suggested as thyme is able to modulate transcription factors such as NF-κB that play critical roles in inflammation, immunity, cell proliferation, differentiation, and survival in both in vitro and in vivo (Paur et al. 2010). On the other hand, calendula extract induced its chemoprotective properties through its ability to stimulate T-lymphocytes, B-lymphocytes and a subset of CD4+ T cells (Barajas-Farias et al. 2006; Jimenez-Medina et al. 2006) beside its crucial role in the prevention of DNA damage (Frankič et al. 2009).
The protective effects of both thyme and calendula extracts against the genotoxicity of AFs were further studied. Rats treated by thyme and/or calendula extracts showed a significant reduction in the percentage of Mn-NCEs in bone marrow cells and prevent DNA fragmentation induced by AFs. Indeed, as indicated earlier, treatment with AFs induced a significant DNA fragmentation in liver cells of treated animals. Administration of thyme and/or calendula extracts to AFs-treated animals showed a significant restoration of DNA integrity. These results were in agreement with those reported by Frankič et al. (2009) who noticed that calendula extract decreased DNA fragmentation in young growing pigs. On the other hand, Hassan and Barakat (2008) indicated that thyme treatment protected testicular DNA and reduced the percentage of DNA fragmentation. The protection, afforded by either thyme and/or calendula extract against AFs-induced genotoxicity is likely due to their ability to inhibit oxidative processes induced by the mycotoxin. This protective effect could be the result of direct free radical scavenger properties (Lee et al. 2005; Hassan and Barakat 2008).
It is well known that phenolic compounds, which are powerful antioxidants, found in both herbs could also react with membrane phospholipid bilayers to break the chain reaction initiated by ROS (Lee and Shibamoto 2002). However, it can not be excluded that these extracts acts as antigenotoxic complexes which enhance the DNA repair system or DNA synthesis (Nogueira et al. 2006; El-Nekeety et al. 2011). As described for other polyphenols such as flavonoids, they inhibit microsomal activation or protect DNA strands from the electrophilic metabolite of the mutagen compounds. These compounds may inhibit several metabolic intermediates and ROS formed during the process of microsomal enzyme activation which are capable of breaking DNA strands (Muley et al. 2009).
The modulator effect of either thyme or calendula extracts on AFs toxicity reported herein was attributed to some alterations in the cell death pathway. It is well documented that P53 and Bax/Bcl-2 ratios play an important role in determining whether cells will undergo apoptosis. The current results showed that treatment with AFs induced higher expressions of p53 and bax pro-apoptotic proteins in liver tissues. AFs treatment also induced a down-regulation of the antiapoptotic protein Bcl2. Similar to these results, Ranchal et al. (2009) reported that AFs-induced DNA damage reduced p27 expression and increased cell death in cultured hepatocytes. Meanwhile, treatments with the extracts plus AFs induce an anti-apoptotic effect via inhibition of p53 and bax genes expression which indicated the modulation of the p53 dependent apoptotic pathway to restrict AFs toxicity. In general, the protective effects of the extracts are mainly due to the radical scavenging properties and the enhancement of antioxidant capacity (Chandra et al. 2000).
Conclusion
It could be concluded that thyme and calendula extracts are hepatoprotective against AFs and enhanced the activities of liver function, as evidenced by the decrease in MDA, micronulueus PCEs and DNA fragmentation. They showed a potential protection against AFs-induced genotoxicity and decreased the expressions of pro-apoptotic proteins p53 and bax. The mode of action of theses extracts might be through the prevention and/or scavenging of ROS. Therefore, these plants have anti-genotoxic properties due to their higher content of total phenolic compounds and should be considered as an accessible source of natural antioxidants.
Acknowledgments
This work was supported by the National Research Centre (Dokki, Cairo, Egypt) project # S90402.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Abdel-Aziem SH, Hassan AM, Abdel-Wahhab MA. Dietary supplementation with whey protein and ginseng extract counteracts oxidative stress and DNA damage in rats fed an aflatoxin-contaminated diet. Mutat Res. 2011;723:65–71. doi: 10.1016/j.mrgentox.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Abdel-Aziem SH, Hassan AM, Salman AS, Waly AI, Abdel-Wahhab MA. Genetic alterations and gene expression profile in male Balb/c mice treated with carbon tetrachloride with or without carboxymethyl chitosan. J Am Sci. 2011;7:1065–1076. [Google Scholar]
- Abdel-Aziz TA, Aziz MA, Fouad HH, Rashed LA, Salama H, Abd-Alla S, Abdel-Wahhab MA, Ahmed T. Interferon-alpha gene therapy prevents aflatoxin and carbon tetrachloride promoted hepatic carcinogenesis in rats. Int J Mol Med. 2005;15:21–26. [PubMed] [Google Scholar]
- Abdel-Wahhab MA, Aly SE. Antioxidants and radical scavenging properties of vegetable extracts in rats fed aflatoxin-contaminated diet. J Agric Food Chem. 2003;51:2409–2414. doi: 10.1021/jf0209185. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahhab MA, Aly SE. Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during a flatoxicosis. J Appl Toxicol. 2005;25:218–223. doi: 10.1002/jat.1057. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahhab MA, Ahmed HH, Hagazi MM. Prevention of aflatoxin B1-initiated hepatotoxicity in rat by marine algae extracts. J Appl Toxicol. 2006;26:229–238. doi: 10.1002/jat.1127. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahhab MA, Hassan NS, El-Kady AA, Khadrawy AY, El-Nekeety AA, Mohamed SR, Sharaf HA, Mannaa FA. Red ginseng extract protects against aflatoxin B1 and fumonisins-induced hepatic pre-cancerous lesions in rats. Food Chem Toxicol. 2010;48:733–742. doi: 10.1016/j.fct.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Agrawal AA, Laforsch C, Tollrian R (1999) Transgenerational induction of defences in animals and plants. Nature 401:60–63
- Alam K, Nagi MN, Badary OA, Al-Shabanah OA, Al-Rikabi AC, Al-Bekairi AM. The protective action of thymol against carbon tetrachloride hepatotoxicity in mice. Pharmacol Res. 1999;40:159–163. doi: 10.1006/phrs.1999.0472. [DOI] [PubMed] [Google Scholar]
- Albertini RJ, Ardell SK, Judice SA, Jacobson S, Allegretta M. Hypoxanthine–guanine phosphoribosyltransferase reporter gene mutation for analysis of in vivo clonal amplification in patients with HTLV type 1-associated Myelopathy/Tropical spastic paraparesis. AIDS Res Hum Retroviruses. 2000;16:1747–1752. doi: 10.1089/08892220050193254. [DOI] [PubMed] [Google Scholar]
- Aristatile B, Al Numair KS, Veeramani C, Pugalendi KV. Effect of carvacrol on hepatic marker enzymes and antioxidant status in d-galactosamine induced hepatotoxicity in rats. Fundam Clin Pharmacol. 2009;23:757–765. doi: 10.1111/j.1472-8206.2009.00721.x. [DOI] [PubMed] [Google Scholar]
- Baek D, Davis C, Ewing B, Gordon D, Green P (2007) Characterization and predictive discovery of evolutionarily conserved mammalian alternative promoters. Genome Res 17:145–155 [DOI] [PMC free article] [PubMed]
- Bai J, Meng Z. Effects of sulfur dioxide on apoptosis-related gene expressions in lungs from rats. Regul Toxicol Pharmacol. 2005;43:272–279. doi: 10.1016/j.yrtph.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Barajas-Farias LM, Pérez-Carreón JI, Arce-Popoca E, Fattel-Fazenda S, Alemán-Lazarini L, Hernández-García S, Salcido-Neyoy M, Cruz-Jiménez FG, Camacho J, Villa-Treviño S. A dual and opposite effect of Calendula officinalis flower extract: chemoprotector and promoter in a rat hepatocarcinogenesis model. Planta Med. 2006;72:217–221. doi: 10.1055/s-2005-916196. [DOI] [PubMed] [Google Scholar]
- Baranauskiene R, Venskutonis PR, Viskelis P, Dambrauskiene E. Influence of nitrogen fertilizers on the yield and composition of thyme (Thymus vulgaris) J Agric Food Chem. 2003;51:7751–7758. doi: 10.1021/jf0303316. [DOI] [PubMed] [Google Scholar]
- Barton CC, Barton EX, Ganey PE, Kunkel SL, Roth RA. Bacterial lipopolysaccharide enhances aflatoxin B1 hepatotoxicity in rats by a mechanism that depends on tumor necrosis factor. Hepatology. 2001;33:66–73. doi: 10.1053/jhep.2001.20643. [DOI] [PubMed] [Google Scholar]
- Bedard LL, Massey TE. Aflatoxin B1-induced DNA damage and its repair. Cancer Lett. 2006;241:174–183. doi: 10.1016/j.canlet.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Bedi MK, Shenefelt PD. Herbal therapy in dermatology. Arch Dermatol. 2002;138:232–242. doi: 10.1001/archderm.138.2.232. [DOI] [PubMed] [Google Scholar]
- Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D, Youdim MB, Riederer P. Redox imbalance. Cell Tissue Res. 2004;318:201–213. doi: 10.1007/s00441-004-0976-5. [DOI] [PubMed] [Google Scholar]
- Brun ME, Gasca S, Girard C, Bouton K, DeMassy B, De Sario A. Characterization and expression analysis during embryo development of the mouse ortholog of MLL3. Gene. 2006;371:25–33. doi: 10.1016/j.gene.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Cao J, Jiang LP, Zhang XM, Yao XF, Geng CY, Xue XX, Zhong LF. Boric acid inhibits LPS-induced TNF-alpha formation through a thiol-dependent mechanism in THP-1 cells. J Trace Elem Med Biol. 2008;22:189–195. doi: 10.1016/j.jtemb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29:323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- Cordova CAS, Siqueira IR, Netto CA, Yunes RA, Volpato AM, Valdir Filho C, Curi-Pedrosa R, Creczynski-Pasa TB. Protective properties of butanolic extract of the Calendula officinalis L. (marigold) against lipid peroxidation of rat liver microsomes and action as free radical scavenger. Red Report. 2002;7:95–102. doi: 10.1179/135100002125000325. [DOI] [PubMed] [Google Scholar]
- Dal Sassoa PCBM, Bianchib MCT, Marabinia LBL. Anti-inflammatory activity of thymol: inhibitory effect on the release of human neutrophil elastase. Pharmacology. 2006;77:130–136. doi: 10.1159/000093790. [DOI] [PubMed] [Google Scholar]
- Demet O, Oguz H, Celik I, Adiguzei H. Production of aflatoxin on wheat, corn, rice and peanut. J Vet Sci. 1995;11:135–140. [Google Scholar]
- Eaton DL, Gallagher EP. Mechanisms of aflatoxin carcinogenesis. Annu Rev Pharmacol Toxicol. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- El-Nekeety AA, Mohamed SR, Hathout AS, Hassan NS, Aly SA, Abdel-Wahhab MA. Antioxidant properties of Thymus vulgaris oil against aflatoxin-induce oxidative stress in male rats. Toxicon. 2011;57:984–991. doi: 10.1016/j.toxicon.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Ercetin T, Senol FS, Erdogan Orhan I, Toker G. Comparative assessment of antioxidant and cholinesterase inhibitory properties of the marigold extracts from Calendula arvensis L. Ind Crop Prod. 2012;36:203–208. [Google Scholar]
- Fink-Gremmels J. Mycotoxins: their implications for human and animal health. Vet Q. 1999;21:115–120. doi: 10.1080/01652176.1999.9695005. [DOI] [PubMed] [Google Scholar]
- Frankič T, Salobir K, Salobir J. The comparison of in vivo antigenotoxic and antioxidative capacity of two propylene glycol extracts of Calendula officinalis (marigold) and vitamin E in young growing pigs. J Anim Physiol Anim Nutr. 2009;93:688–694. doi: 10.1111/j.1439-0396.2008.00855.x. [DOI] [PubMed] [Google Scholar]
- Fronza M, Heinzmann B, Hamburger M, Laufer S, Merfort I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J Ethnopharmacol. 2009;126:463–467. doi: 10.1016/j.jep.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Gallagher EP, Kunze KL, Stapleton PL, Eaton DL. The kinetics of aflatoxin B1 oxidation by human cDNA-expressed and human liver microsomal cytochromes P450 1A2 and 3A4. Toxicol Appl Pharmacol. 1996;141:595–606. doi: 10.1006/taap.1996.0326. [DOI] [PubMed] [Google Scholar]
- Gitto E, Pellegrino S, Gitto P, Barberi I, Reiter RJ. Oxidative stress of the newborn in the pre and postnatal period and the clinical utility of melatonin. J Pineal Res. 2009;46:128–139. doi: 10.1111/j.1600-079X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Gladine C, Morand C, Rock E, Gruffat D, Bauchart D, Durand D. The antioxidative effect of plant extracts rich in polyphenols differs between liver and muscle tissues in rats fed n-3PUFA rich diets. Anim Feed Sci Technol. 2007;139:257–272. [Google Scholar]
- Hamzawy MA, El-Denshary ES, Hassan NS, Mannaa FA, Abdel-Wahhab MA. Antioxidant and hepatorenoprotective effect of Thymus vulgaris extract in rats during aflatoxicosis. Global J Pharmacol. 2012;6:106–117. [Google Scholar]
- Hassan AM, Barakat IAH. Assessment of oxidative stress induced by nickel chloride and antioxidant effects of Basil (Ocimum basilicum L.) and Thyme (Tymus vulgaris L.) J Gen Eng Biotechnol. 2008;6:29–38. [Google Scholar]
- Hassan AM, Abdel-Aziem SH, Abdel-Wahhab MA. Modulation of DNA damage and alteration of gene expression during aflatoxicosis via dietary supplementation of Spirulina (Arthrospira) and whey protein concentrate. Ecotoxicol Environ Saf. 2012;79:294–300. doi: 10.1016/j.ecoenv.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Heddle JA. A rapid in vivo test for chromosomal damage. Mutat Res. 1973;18:187–190. doi: 10.1016/0027-5107(73)90035-3. [DOI] [PubMed] [Google Scholar]
- Hopkins SJ. The pathophysiological role of cytokines. Legal Med. 2003;5:S45–S57. doi: 10.1016/s1344-6223(02)00088-3. [DOI] [PubMed] [Google Scholar]
- Horn TL, Bhattacharjee A, Schook LB, Rutherford MS. Altered hepatic mRNA expression of apoptotic genes during dimethylnitrosamine exposure. Toxicol Sci. 2000;57:240–249. doi: 10.1093/toxsci/57.2.240. [DOI] [PubMed] [Google Scholar]
- Hustchins JE, Jr Hagler WM. Rapid liquid chromatographic determination of aflatoxins in heavily contaminated corn. J Assoc Off Anal Chem. 1983;66:1458–1465. [PubMed] [Google Scholar]
- Javanmardi J, Khalighi A, Kashi A, Bais HP, Vivanco JM. Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J Agric Food Chem. 2002;50:5878–5883. doi: 10.1021/jf020487q. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha GK, Rao LJ. Phenolic constituents from the lichen Parmotrema stuppeum (Nyl.) Hale and their antioxidant activity. Z Naturforsch C. 2000;55:1018–1022. doi: 10.1515/znc-2000-11-1227. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha GK, Selvi T, Sakariah KK. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res Int. 2003;36:117–122. [Google Scholar]
- Jimenez-Medina E, Garcia-Lora A, Paco L, Algarra I, Collado A, Garrido F. A new extract of the plant Calendula officinalis produces a dual in vitro effect: cytotoxic anti-tumor activity and lymphocyte activation. BMC Cancer. 2006;6:119. doi: 10.1186/1471-2407-6-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse R, Dumont J, Fautre A, Robin M, Guillouzo A. Identification of early target genes of aflatoxin B1 in human hepatocytes, inter-individual variability and comparison with other genotoxic compounds. Toxicol Appl Pharmacol. 2012;258:176–187. doi: 10.1016/j.taap.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Kanbur M, Eraslan G, SarIca ZS, Aslan O. The effects of evening primrose oil on lipid peroxidation induced by subacute aflatoxin exposure in mice. Food Chem Toxicol. 2011;49:1960–1964. doi: 10.1016/j.fct.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Maoka T, Sumitomo K, Ohmiya A. Analysis of carotenoid composition in petals of Calendula (Calendula officinalis L.) Biosci Biotechnol Biochem. 2005;69:2122–2128. doi: 10.1271/bbb.69.2122. [DOI] [PubMed] [Google Scholar]
- Kronmiller JE, Nguyen T, Berndt W, Wickson A. Spatial and temporal distribution of Sonic hedgehog mRNA in the embryonic mouse mandible by reverse transcription/polymerase chain reaction and in situ hybridization analysis. Arch Oral Biol. 1995;40:831–838. doi: 10.1016/0003-9969(95)00053-r. [DOI] [PubMed] [Google Scholar]
- Leach MJ. Calendula officinalis and wound healing: a systematic review. Wounds. 2008;20:236–243. [PubMed] [Google Scholar]
- Lee KG, Shibamoto T. Determination of antioxidant potential of volatile extracts isolated from various herbs spices. J Agric Food Chem. 2002;50:4947–4952. doi: 10.1021/jf0255681. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Umano K, Shibamoto T, Lee KG. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005;91:131–137. [Google Scholar]
- Madrigal-Santillán E, Madrigal-Bujaidar R, Márquez-Márquez A, Reyes Antigenotoxic effect of Saccharomyces cerevisiae on the damage produced in mice fed with aflatoxin B1 contaminated corn. Food Chem Toxicol. 2006;44:2058–2063. doi: 10.1016/j.fct.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Majhenic L, Skerget M, Knez Z. Antioxidant and antimicrobial activity of guarana seed extracts. Food Chem. 2007;104:1258–1268. [Google Scholar]
- Messmer UK, Pereda-Fernandez C, Manderscheid M, Pfeilschifter J. Dexamethasone inhibits TNF-alpha-induced apoptosis and IAP protein downregulation in MCF-7 cells. Brit J pharmacol. 2001;133:467–476. doi: 10.1038/sj.bjp.0704093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EY, Rhee DK, Pyo S. Involvement of NO, H2O2 and TNF-α in the reduced antitumor activity of murine peritoneal macrophages by aflatoxin B1. Cancer Lett. 1999;136:167–1676. doi: 10.1016/s0304-3835(98)00320-6. [DOI] [PubMed] [Google Scholar]
- Muley BP, Khadabadi SS, Banarase NB. Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae): a review. Trop J Pharm Res. 2009;8:455–465. [Google Scholar]
- Nogueira ME, Passoni MH, Biso FI, Longo Mdo C, Cardoso CR, Santos LC, Varanda EA. Investigation of genotoxic and antigenotoxic activities of Melampodium divaricatum in Salmonella typhimurium. Toxicol In Vitro. 2006;20:361–366. doi: 10.1016/j.tiv.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Paur I, Balstad TR, Kolberg M, Pedersen MK, Austenaa LM, Jacobs DR, Blomhoff R. Extract of oregano, coffee, thyme, clove, and walnuts inhibits NF-B in monocytes and in transgenic reporter mice. Cancer Prev Res. 2010;3:653–663. doi: 10.1158/1940-6207.CAPR-09-0089. [DOI] [PubMed] [Google Scholar]
- Preetha SP, Kanniappan M, Selvakumar E, Nagaraj M, Varalakshmi P. Lupeol ameliorates aflatoxin B1-induced peroxidative hepatic damage in rats. Comp Biochem Physiol Comp Pharmacol. 2006;143:333–339. doi: 10.1016/j.cbpc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Preston RJ, Williams GM. DNA-reactive carcinogens: mode of action and human cancer hazard. Crit Rev Toxicol. 2005;35:673–683. doi: 10.1080/10408440591007278. [DOI] [PubMed] [Google Scholar]
- Qiu J, Zhang X, Luo M, Li H, Dong J, Wang J, Leng B, Wang X, Feng H, Ren W. Subinhibitory concentrations of Perilla oil affect the expression of secreted virulence factor genes in Staphylococcus aureus. PLoS One. 2010;6:e16160. doi: 10.1371/journal.pone.0016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rababah TM, Banat F, Rababah A, Ereifej K, Yang W. Optimization of extraction conditions of total phenolics, antioxidant activities, and anthocyanin of oregano, thyme, terebinth, and pomegranate. J Food Sci. 2010;75:C626–C632. doi: 10.1111/j.1750-3841.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- Ranchal I, Gonzalez R, Bello RI, Ferrin G, Hidalgo AB, Linares CI, Aguilar Melero P, Gonzalez Rubio S, Barrera P, Marchal T, Nakayama KI, de la malta M, Muntane J. The reduction of cell death and proliferation by P27 (Kip1) minimizes DNA damage in an experimental model of genotoxicity. Int J Cancer. 2009;125:2270–2280. doi: 10.1002/ijc.24621. [DOI] [PubMed] [Google Scholar]
- Rastogi S, Dogra RK, Khanna SK, Das M. Skin tumorigenic potential of aflatoxin B1 in mice. Food Chem Toxicol. 2005;44:670–677. doi: 10.1016/j.fct.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Rejsková A, Brom J, Pokorny J, Korecko J. Temperature distribution in light-coloured flowers and inflorescences of early spring temperate species measured by infrared camera. Flora Morphol Distr Func Ecol Plants. 2010;205:282–289. [Google Scholar]
- Rollinson S, Levene AP, Mensah FK, Roddam PL, Allan JM, Diss TC, Roman E, Jack A, MacLennan K, Dixon MF, Morgan GJ. Gastric marginal zone lymphoma is associated with polymorphisms in genes involved in inflammatory response and antioxidative capacity. Blood. 2003;102:1007–1011. doi: 10.1182/blood-2002-12-3803. [DOI] [PubMed] [Google Scholar]
- Salamone M, Heddle J, Stuart E, Katz M. Towards an improved micronucleus test. Mutat Res. 1980;346:69–75. doi: 10.1016/0165-1161(80)90193-4. [DOI] [PubMed] [Google Scholar]
- Sambrook L, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Cold Spring Harbour: Cold Spring Harbor Laboratory Press; 1989. pp. 16–19. [Google Scholar]
- SAS user’s guide: statistics. Cary, NC, USA: SAS Institute Inc.; 1982. [Google Scholar]
- Sell S, Xu KL, Huff WE, Kabena LF, Harvery RB, Dunsford HA. Aflatoxin exposure produces serum alphafetoprotein elevations and marked oval cell proliferation in young male Pekin ducklings. Pathology. 1998;30:34–39. doi: 10.1080/00313029800169645. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Farmer PB. Biological relevance of adduct detection to the chemoprevention of cancer. Clin Cancer Res. 2004;10:4901–4912. doi: 10.1158/1078-0432.CCR-04-0098. [DOI] [PubMed] [Google Scholar]
- Shotwell OL, Hesseltine CV, Stubblefield RD, Sorenson WG. Production of aflatoxin on rice. Appl Microbiol. 1966;14:425–429. doi: 10.1128/am.14.3.425-428.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn DH, Kim YC, Oh SH, Park EJ, Li X, Lee BH. Hepatoprotective and free radical scavenging effects of Nelumbo nucifera. Phytomedicine. 2003;10:165–169. doi: 10.1078/094471103321659889. [DOI] [PubMed] [Google Scholar]
- Suganuma M, Sueoka E, Sueoka N, Okabe S, Fujiki H. Mechanisms of cancer prevention by tea polyphenols based on inhibition of TNF-alpha expression. Biofactors. 2000;13:67–72. doi: 10.1002/biof.5520130112. [DOI] [PubMed] [Google Scholar]
- Towner RA, Qian SY, Kadiiska MB, Mason RP. In vivo identification of aflatoxin-induced free radicals in rat bile. Free Radic Biol Med. 2003;35:1330–1340. doi: 10.1016/j.freeradbiomed.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Tsai ML, Lin CC, Lin WC, Yang CH. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils from five selected herbs. Biosci Biotechnol Biochem. 2011;75:1977–1983. doi: 10.1271/bbb.110377. [DOI] [PubMed] [Google Scholar]
- Ubagai T, Kikuchi T, Fukusato T, Ono Y. Aflatoxin B1 modulates the insulin-like growth factor-2 dependent signaling axis. Toxicol In Vitro. 2010;24:783–789. doi: 10.1016/j.tiv.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Uyanoglu M, Canbek M, Aral E, Husnu Can Baser K. Effects of carvacrol upon the liver of rats undergoing partial hepatectomy. Phytomedicine. 2008;15:226–229. doi: 10.1016/j.phymed.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Verma RJ, Nair A. Ameliorative effect of vitamin E on aflatoxin-induced lipid peroxidation in the testis of mice. Asian J Androl. 2001;3:217–221. [PubMed] [Google Scholar]
- Vladimirov IA (1998) Free radicals and antioxidants. Vestn Ross Akad Med Nauk 7:43–51 [PubMed]
- Voss KA, Riley R, Dunn C, Christopher CJ. The role of tumor necrosis factor and the peroxisome proliferator-activated receptor in modulating the effects of fumonisin in mouse liver. Toxicology. 2006;222:165–174. doi: 10.1016/j.tox.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Waller RA, Duncan DB. A Bayes rule for the symmetric multiple comparison problems. J Am Stat Assoc. 1969;64:1484–1503. [Google Scholar]
- Forty-ninth report of the joint FAO/WHO expert committee on food additives. WHO technical report series. Geneva: WHO; 1998. [Google Scholar]
- Wojciak-Kosior M, Matysik G, Soczewinski E. Investigations of phenolic acids occurring in plant components of Naran N by HPLC and HPTLC densitometric methods. Herba Pol. 2003;49:194–201. [Google Scholar]
- Xu R, Zhao W, Xu J, Shao B, Qin G. Studies on bioactive saponins from Chinese medicinal plants. Adv Exp Med Biol. 1996;404:371–382. doi: 10.1007/978-1-4899-1367-8_30. [DOI] [PubMed] [Google Scholar]
- Yang CF, Liu J, Wasser S, Shen HM, Tan CEL, Ong CN. Inhibition of ebselen on aflatoxin B1-induced hepatocarcinogenesis in Fischer 344 rats. Carcinogenesis. 2000;21:2237–2243. doi: 10.1093/carcin/21.12.2237. [DOI] [PubMed] [Google Scholar]
- Yuzugullu GO, Yuzugullu H, Yilmaz M, Ozturk M. Aflatoxin genotoxicity is associated with a defective DNA damage response bypassing p53 activation. Liver Int. 2011;31:561–571. doi: 10.1111/j.1478-3231.2011.02474.x. [DOI] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD (2006) Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA 103:9357–9362 [DOI] [PMC free article] [PubMed]
- Zhou HP, Lutterodt H, Cheng ZH, Yu LL. Anti-inflammatory and anti proliferative activities of trifolirhizin, a flavonoid from Sophora flavescens roots. J Agric Food Chem. 2009;57:4580–4585. doi: 10.1021/jf900340b. [DOI] [PMC free article] [PubMed] [Google Scholar]