Abstract
Peptic ulcer disease is a gastrointestinal disorder defined by mucosal damage and free oxygen radicals associated with peptic ulcer and gastritis. Cinnamon is a traditional herb used for many diseases and it has also effects as an antioxidant, anti-inflammatory, antispasmodic and anti-ulcerative. Our research is based on oxidative stress and effects of Oleum cinnamomi on stomach, liver and kidney disorders induced by ethanol. In our experiment, 2–3 month old male Sprague–Dawley rats were used. One hour before the mucosal damage induced by 70 % ethanol, O. cinnamomi (2.5 ml/kg) was added into the groups. Gastric pH, analysis of gastric mucus and ulcer index were calculated from samples obtained from the stomach. Superoxide dismutase (SOD), malondialdehyde and catalase (CAT) levels were determined in stomach, liver and kidney homogenates and erythrocyte hemolysate. Histopathological examination of stomach, liver and kidney were determined with H&E staining. The non-treated ulcerative group showed higher scores than the control group which was treated with O. cinnamomi, when ulcer scores, gastric mucus and pH level of stomach are compared. Increased lipid peroxidation levels were observed in the liver, kidney and erythrocyte hemolysate. SOD activity was decreased in liver whereas increased in stomach of ethanol treated ulcerative groups. CAT levels were increased in stomach and liver of ethanol treated rats. Histopathological findings showed that ethanol treatment cause multiply organ damage such as stomach, liver and kidney injury. O. cinnamomi treatment protected these tissues from ethanol-induced damage. Consequently, the current investigation shows that O. cinnamomi has protective effects on ethanol-induced oxidative and mucosal damage.
Keywords: Oleum cinnamomi, Ethanol, Oxidative stress, Rat, Ulcer, Ulcer index
Introduction
Peptic ulcer disease is a problem of the gastrointestinal tract characterized by mucosal damage secondary to pepsin and gastric acid secretion. It usually occurs in the stomach and proximal duodenum. Less generally, it occurs in the lower esophagus, distal duodenum, or jejunum, in hiatus hernias, or in ectopic gastric mucosa (Amar and Maysa 2010).
Oxygen free radicals are deleterious to the integrity of biological tissues and mediate their injury. The mechanism of damage involves lipid peroxidation, which destroys cell membranes with the release of intracellular components, such as lysosomal enzymes, leading to further tissue damage. The radicals also promote mucosal damage by causing degradation of the epithelial basement membrane components, complete alteration of the cell metabolism and DNA damage (Demir et al. 2003). The generation of the superoxide anion as a mechanism of damage is well established in different models of acute and chronic injury, but it has not been clarified whether this radical is involved in gastric mucosal damage (Demir et al. 2003).
Nowadays, drugs are expensive and have many side effects during treatment of any disorders. Therefore, the potential of the health promoting and disease preventing properties of plant-derived compounds has received increased attention from researchers in recent years (Amar and Maysa 2010).
Many herbs and spices have been shown to impart antioxidant effects in food; the active principles are phenolics. A wide variety of phenolic substances derived from herbs and spices possess potent antioxidant, anti-inflammatory, anti-mutagenic, anti-carcinogenic and anti-tumour activities, which contribute to their chemopreventive potential (Jayaprakasha et al. 2007).
The Lauraceae are an economically important family consisting mostly of trees or tree-like shrubs. The genus Cinnamomum comprises about 250 species, which are distributed in Asia and Australia. Cinnamomum zeylanicum, the source of cinnamon bark, leaf and their essential oils. Cinnamon is a widely used spice and has many applications in perfumery, flavoring and pharmaceutical industries (Jayaprakasha et al. 2007; Singh et al. 2007).
Cinnamon has chemopreventive, antispasmodic, sedative, hypothermic, choleretic, antibacterial, antifungal, antipyretic, antiviral, antiseptic, lipolytic, anesthetic, cytotoxic, anodyne, hypolipidemic and antiplatelet properties. It also stimulates the immune system that may be useful adjuncts in helping to reduce the risk of cardiovascular disease and cancer. Medicinally, it is used in the treatment of colic, colds, low vitality, poor appetite, rheumatism, kidney weakness and coldness, fevers, arthritic angina and palpitations. It is also used for the treatment of spasms, vomiting, infections and digestive or stomach complaints (Amar and Maysa 2010; Jayaprakasha et al. 2007; Singh et al. 2007).
Although the chemical constituents of essential oils of cinnamon bark have been studied, the potential anti ulcerative and antioxidant properties have yet not been studied. Hence, in the present study, attempt has been made to explore the possible antioxidant and anti-ulcerative properties of Oleum cinnamomi.
Materials and methods
Animals
In this study, 3 month old Spraque–Dawley breed male rats weighing 200–250 g were used. All experiments for animal testing were approved by the Eskisehir Osmangazi University School of Medicine Animal Use and Care Committee. The animals were housed in individual cages at room temperature and left for 1 week for acclimatization before the start of the experiment.
Treatment
Rats were intragastrically pretreated with 2.5 ml/kg BW Oleum cinnamomi (Defne&Doga Company®, Antalya, Turkey) 1 h before the ethanol administration (the groups are described in Table 1. Seven animals were used per group). Gastric mucosal damage was induced by intragastrically received 1 ml of 75 % ethanol. Doses of the given substrates are summarized in Table 1.
Table 1.
The substrates given to control and experimental groups
| Groups | Number of animals per group | Receiving substrates |
|---|---|---|
| 1. Control | 7 | Water |
| 2. Cinnamon oil control | 7 | Oleum cinnamomi (2.5 ml/kg, intragastric) + water |
| 3. Ulcerative control | 7 | Water + 75 % ethanol (1 ml, intragastric) |
| 4. Cinnamon oil + ulcer | 7 | Oleum cinnamomi (2.5 ml/kg, intragastric) + 75 % ethanol (1 ml, intragastric) |
Induction of gastric mucosal damage by ethanol
One milliliter of 75 % ethanol in water was given intragastrically to each animal using a gavage cannula. At the end of the experiment, samples were collected under ether anesthesia by using suitable techniques, 1 h after the administration of ethanol.
Determination of gastric acidity, gastric lesions and gastric mucus
The gastric contents were collected by washing with 1 ml of saline and subsequently centrifuged. The pH of supernatant was determined by pH meter (inoLab® pH 720, WTW Laboratory, Weilheim, Germany).
The stomach was cut along the greater curvature and the mucosa was washed with saline and imaging with scale bar. The total area of the stomach mucosa and the area of ulcerations are measured using ImageJ imaging software (http://rsbweb.nih.gov/ij/) for quantitative analysis.
Determination of gastric mucus was performed according to the procedure of using Alcian blue and evaluated spectrophotometrically. The mucus content (μg/g wet tissue) was calculated according to Kılıç et al. (2006).
Determination of biochemical parameters
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) levels were autoanalysed using standard assays with Crony Airore 200RA automated analyzer.
The preparations of hemolysate were performed according to the method described by Sun et al. (1988). After sacrificing the animals stomach, kidney and liver were quickly isolated and homogenized in potassium chloride (1 %) using an ultrasonic homogenizer. The homogenate was centrifuged at 4,000 rpm for 15 min at 4 °C in a refrigerated centrifuge. The supernatant so obtained was centrifuged was used to assay malondialdehyde (MDA), catalase (CAT), and superoxide dismutase (SOD) activity. SOD activity was assayed according to the method of Sun et al. (1988). The measurement of MDA levels by thiobarbituric acid reactivity is the most widely used method for assessing lipid peroxidation (Uchiyama and Mihara 1978). The optical density of the n-butanol layer was determined at 532 nm after centrifugation at 1,000g for 5 min and expressed as U/g wet tissue. CAT activity was measured spectrophotometrically as previously described by Goth (1991).
Histopathological evaluation
All tissue specimens were collected from rats. The fragments from tissues were fixed in 10 % neutral formalin solution, embedded in paraffin, and then stained with hematoxylin and eosin. The histological slides were investigated by light microscopy.
Statistical analysis
Parametric data were expressed as mean ± SD and were analyzed using ANOVA. Tukey’s test was used to test for significant differences (p < 0.05). Statistical comparisons for nonparametric data for the ulcerative lesions were carried out using Kruskal–Wallis nonparametric test and due to absence of ulcer area, control group were not included.
Result
Gastric lesions, gastric acidity and gastric mucus
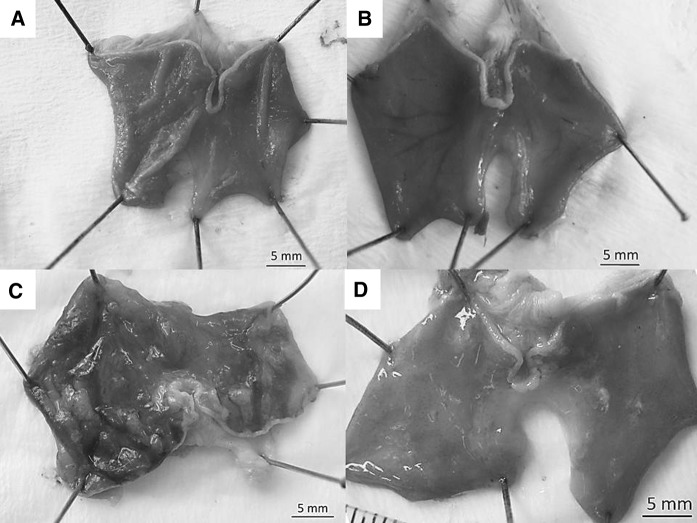
Intragastric administration of ethanol caused significant ulcerative lesions in the ethanol control group (Fig. 1c; Table 2) whereas stomach from the control and Oleum cinnamomi treated groups showed normal structure (Fig. 1a, b, respectively). There was significant (p < 0.001) reduction in ulcerative area in the Oleum cinnamomi treated ulcerative group (Fig. 1d; Table 2).
Fig. 1.
The stomach structure of experimental groups. a Control, b cinnamon oil treated control, c ulcerative control, d cinnamon oil treated ulcerative group
Table 2.
Ulcerative lesions (mm2)
| Groups* | n | Median (%25–%75) |
Multiple comparison | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| 1. Cinnamon oil control | 7 | 0.95 (0.71–1.08) | p < 0.001a | p = 0.043b | |
| 2. Ulcerative control | 7 | 17.5 (15.6–20.86) | p < 0.001a | p = 0.031c | |
| 3. Cinnamon oil + ulcer | 7 | 3.83 (1.86–4.99) | p = 0.043b | p = 0.031c | |
* Control group is not indicated due to lack of ulcerative lesions
aStatistical differences between group 2 and 3
bStatistical differences between group 1 and 3
cStatistical differences between group 2 and 3
Gastric mucus levels were significantly higher in the ulcerative control groups (p < 0.001), meanwhile Oleum cinnamomi treatment significantly decreased gastric mucus levels in ethanol induced ulcerative stomach (p < 0.001) (Table 3). Levels of gastric pH was significantly high in the ulcerative groups (groups 3 and 4) and Oleum cinnamomi treatment significantly decreased gastric pH levels (p < 0.001).
Table 3.
Gastric mucus and pH levels (mean ± SD)
| Groups | n | Gastiric mucus (Alcian blue μg/g tissue) |
Gastiric pH |
|---|---|---|---|
| 1. Control | 7 | 201 ± 59 | 4.3 ± 0.5 |
| 2. Cinnamon oil control | 7 | 248 ± 26 | 4.5 ± 0.8 |
| 3. Ulcerative control | 7 | 290 ± 61*** | 8.3 ± 0.5*** |
| 4. Cinnamon oil + ulcer | 7 | 136 ± 24+++ | 6.0 ± 0.7***,+++ |
*** p < 0.001 (compared to control); +++ p < 0.001 (significance of group 4 when compared to group 3)
Result of biochemical analysis
We found significantly increased AST, ALT and LDH levels in the ulcerative groups compared to the control (Table 4). Oleum cinnamomi treatment significantly decreased the AST and LDH levels (p < 0.001 and p < 0.01, respectively) in the ethanol treated ulcerative rats.
Table 4.
AST, ALT and LDH levels (mean ± SD)
| Groups | n | AST (U/l) |
ALT (U/l) |
LDH (U/l) |
|---|---|---|---|---|
| 1. Control | 7 | 131.14 ± 4.4 | 49.42 ± 1.8 | 208.71 ± 24.9 |
| 2. Cinnamon oil control | 7 | 130.42 ± 4.2 | 58.14 ± 1.6 | 238.14 ± 22.6 |
| 3. Ulcerative control | 7 | 256.57 ± 8.5*** | 69.85 ± 12.0*** | 548.28 ± 19.9*** |
| 4. Cinnamon oil + ulcer | 7 | 207.28 ± 1.7***,+++ | 63.71 ± 6.2** | 432.28 ± 10.4***,++ |
** p < 0.01; *** p < 0.001 (compared to control); +++ p < 0.001; ++ p < 0.01 (significance of group 4 when compared to group 3)
Antioxidant and free radical levels
Erythrocyte MDA, SOD and CAT levels are shown in Table 5. There were no significant differences in the SOD and CAT activities between control and ulcerative groups (p > 0.05). Erythrocyte MDA levels were significantly increased in the ulcerative control groups (p < 0.05).
Table 5.
SOD, MDA and CAT levels of erythrocyte hemolysate (mean ± SD)
| Groups | n | SOD (% inhibition) |
MDA U/g Hb |
CAT (kU/ml protein) |
|---|---|---|---|---|
| 1. Control | 7 | 81.44 ± 1.9 | 1.43 ± 0.1 | 0.55 ± .2 |
| 2. Cinnamon oil control | 7 | 86.59 ± 1.6 | 2.20 ± 1.3 | 0.62 ± 0.2 |
| 3. Ulcerative control | 7 | 80.53 ± 1.9 | 3.16 ± 1.3* | 0.83 ± 0.4 |
| 4. Cinnamon oil + ulcer | 7 | 87.71 ± 1.0 | 2.39 ± 1.0 | 0.34 ± 0.2 |
* p < 0.05 (compared to control)
SOD, MDA and CAT levels of stomach homogenates are shown in Table 5. Increased SOD and CAT levels were found in ulcerative control group (p < 0.01, p < 0.001). Also increased CAT levels were found in the Oleum cinnamomi treated ulcerative stomach (p < 0.001) when compared to control.
SOD, MDA and CAT levels of liver homogenates are shown in Table 6. Decreased SOD levels and increased MDA and CAT levels were found in ethanol treated rat liver (p < 0.05, p < 0.001, p < 0.01). When compared to group 3, MDA levels of Oleum cinnamomi treated ulcerative groups were decreased (p < 0.001) (Table 7).
Table 6.
SOD, MDA and CAT levels of the stomach tissue homogenate (mean ± SD)
| Groups | n | SOD (% inhibition) |
MDA U/g wet tissue |
CAT (kU/ml protein) |
|---|---|---|---|---|
| 1. Control | 7 | 77.62 ± 18.4 | 0.74 ± 0.30 | 52.1 ± 5.0 |
| 2. Cinnamon oil control | 7 | 91.16 ± 2.1 | 0.39 ± 0.03** | 80.8 ± 10.3 |
| 3. Ulcerative control | 7 | 92.69 ± 0.9** | 0.61 ± 0.11 | 260.0 ± 51.9*** |
| 4. Cinnamon oil + ulcer | 7 | 85.50 ± 5.4 | 0.87 ± 0.22 | 207.0 ± 57.7*** |
** p < 0.01; *** p < 0.001 (compared to control)
Table 7.
SOD, MDA and CAT levels of the liver tissue homogenate (mean ± SD)
| Groups | n | SOD (% inhibition) |
MDA U/g wet tissue |
CAT (kU/ml protein) |
|---|---|---|---|---|
| 1. Control | 7 | 81.94 ± 1.0 | 0.45 ± 0.08 | 51.0 ± 12.6 |
| 2. Cinnamon oil control | 7 | 78.25 ± 1.3 | 0.59 ± 0.15 | 62.4 ± 3.7 |
| 3. Ulcerative control | 7 | 77.36 ± 0.7* | 1.09 ± 0.29*** | 74.1 ± 8.9** |
| 4. Cinnamon oil + ulcer | 7 | 79.78 ± 1.0 | 0.45 ± 0.12+++ | 60.0 ± 18.5 |
* p < 0.05; ** p < 0.01; *** p < 0.001 (compared to control); +++ p < 0.001 (significance between Group 4 (treated ulcerative group) compared with group 3 (untreated ulcerative group))
Kidney SOD, MDA and CAT levels are presented in Table 8. SOD levels were increased in cinnamon treated groups (p < 0.01, p < 0.05). Ethanol administration increased the MDA levels in rat kidney (p < 0.01). There were no significant differences in the kidney CAT activities between control and ulcerative groups.
Table 8.
SOD, MDA and CAT levels of the kidney tissue homogenate (mean ± SD)
| Groups | n | SOD (% inhibition) | MDA U/g wet tissue | CAT (kU/ml protein) |
|---|---|---|---|---|
| Control | 7 | 84.56 ± 7.70 | 0.86 ± 0.40 | 137.28 ± 15.27 |
| Cinnamon oil control | 7 | 95.84 ± 1.15** | 1.16 ± 038 | 129.71 ± 8.73 |
| Ulcerative control | 7 | 90.57 ± 0.48 | 1.63 ± 0.49** | 122.28 ± 22.20 |
| Cinnamon oil + ulcer | 7 | 93.05 ± 4.75* | 1.31 ± 0.21 | 128.57 ± 9.27 |
* p < 0.05; ** p < 0.01 (compared to control)
Histopathological findings
Histological structure of stomach
All gland cells, gastric epithelial cells, parietal and the chief cells had normal histological structures in the stomach samples of control (Figs. 2, 3) and Oleum cinnamomi treated control groups (Figs. 4, 5).
Fig. 2.
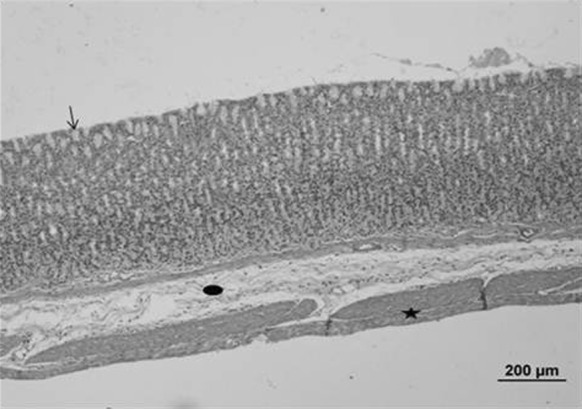
Stomach sample of the control group. Normal histological structures were seen. Gastric epithelial tissue (arrow) and all gland cells, connective (circle) and muscle (star) tissue (bar 200 µm, HE)
Fig. 3.
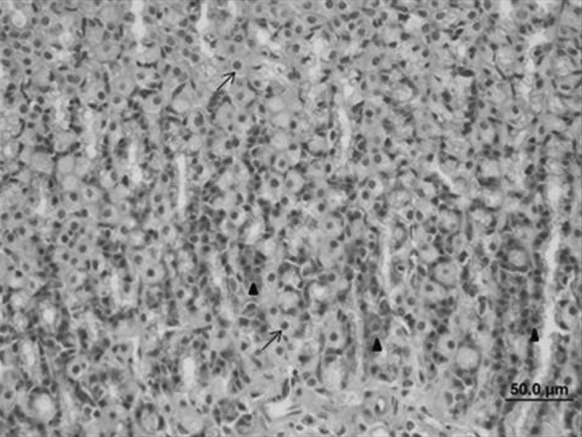
Stomach sample of the control group. Parietal (arrow) and the main-chief cells (triangle) had normal histological structures (bar 50.0 µm, HE)
Fig. 4.
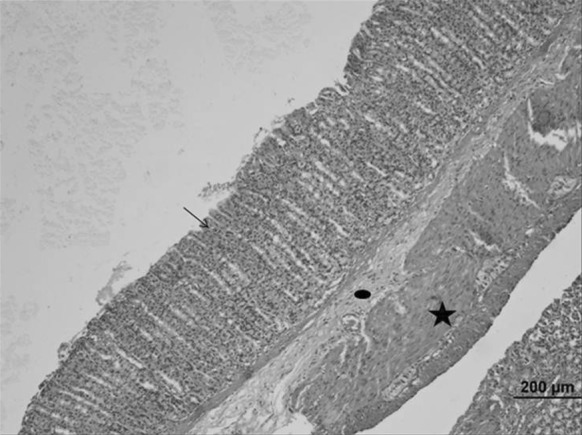
Stomach sample of the Oleum cinnamomi control group. Normal histological structures were seen: gastric epithelial tissue (arrow) and all gland cells, connective (circle) and muscle (star) tissue (bar 200 µm, HE)
Fig. 5.
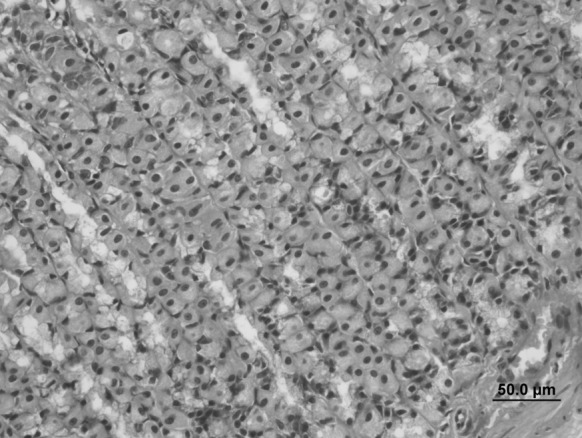
Stomach sample of the Oleum cinnamomi control group. Normal histological structures were seen (bar 50.0 µm, HE)
In the ulcerative control groups; gastric epithelial ulceration, intense cellular losses and connective tissue edema were observed. Ulceration of the stomach epithelium, cellular losses and in particular the parietal cells necrosis were determined (Figs. 6, 7).
Fig. 6.
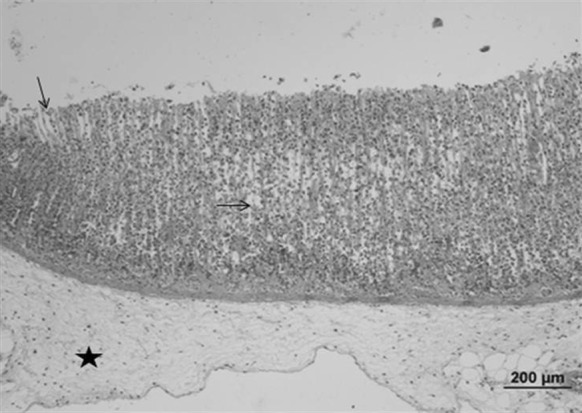
Stomach sample of the ulcerative control group: gastric epithelial ulceration and intense cell loss (arrow), connective tissue edema (star) (bar 200 µm, HE)
Fig. 7.
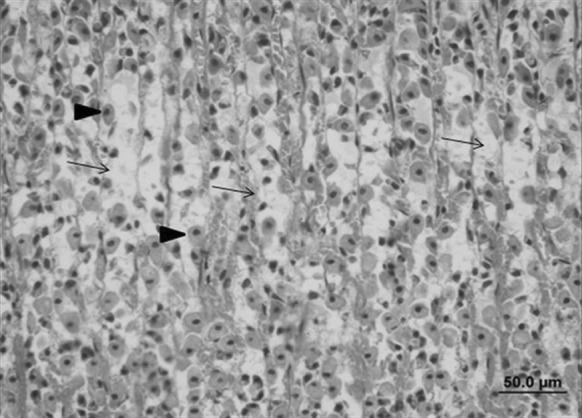
Stomach sample of the ulcerative control group: ulceration of the stomach epithelium, cellular losses (arrow) and, in particular, necrosis of parietal cells (triangle) (bar 50.0 µm, HE)
Decreased ulceration of gastric epithelium and connective tissue edema were observed in Oleum cinnamomi treated ulcerative groups. Also, decreased epithelial cell losses were detected (Figs. 8, 9).
Fig. 8.
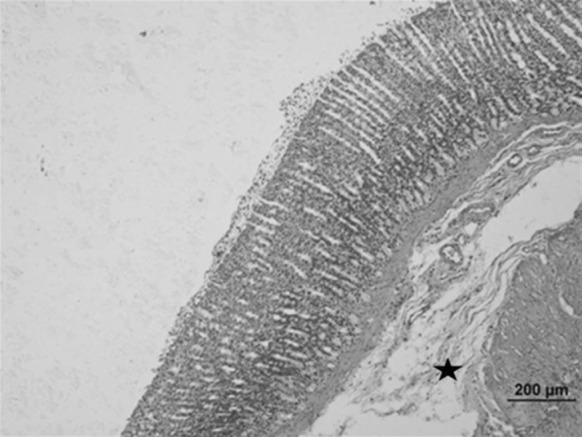
Stomach sample of the Oleum cinnamomi treated ulcerative group: decreased ulceration of gastric epithelium and decreased connective tissue edema (star) (bar 200 µm, HE)
Fig. 9.
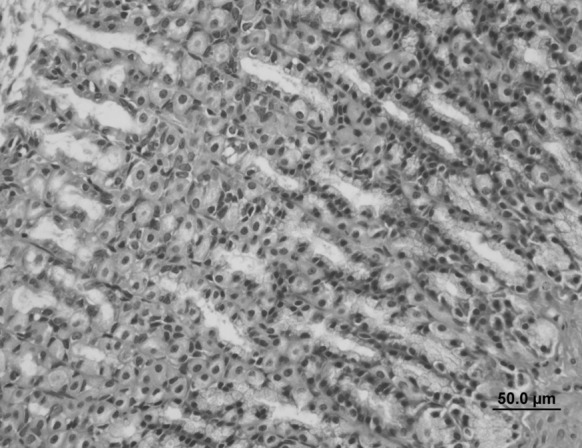
Stomach sample of the Oleum cinnamomi treated ulcerative group: decreased ulceration of gastric epithelium and decreased connective tissue edema (star) (bar 50.0 µm, HE)
Histological structure of liver
Livers of control and Oleum cinnamomi treated groups have normal histological structures (Fig. 10).
Fig. 10.
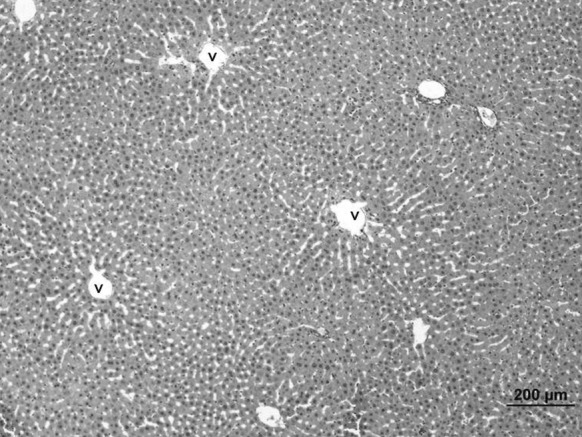
Liver sample of the control group. Normal histological structures were seen. v: vena sentralis (bar 200 µm, HE)
Hepatocytes injury, karyolysis, congestion in venules, sinusoidal dilatation and a partial bleeding were observed in the liver of ethanol induced ulcerative groups (Fig. 11).
Fig. 11.
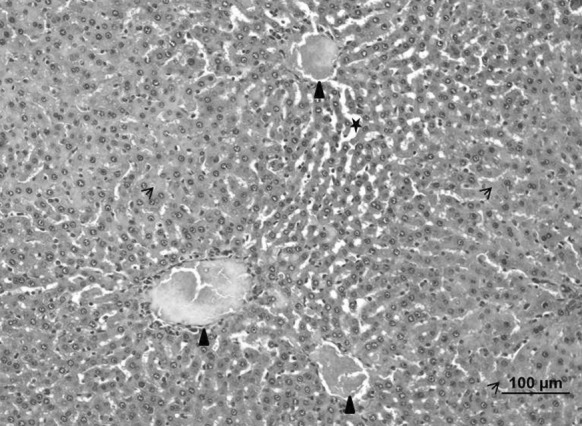
Liver of the ulcerative control group. Hepatocytes injury, karyolysis (arrow), congestion in venules (triangle) and sinusoidal dilatation (star) (bar 100 µm, HE)
When compared to the ulcerative group, nearly normal histological structures were detected in the liver of the Oleum cinnamomi treated ulcerative group whereas congestion in venules was observed in some areas (Fig. 12).
Fig. 12.
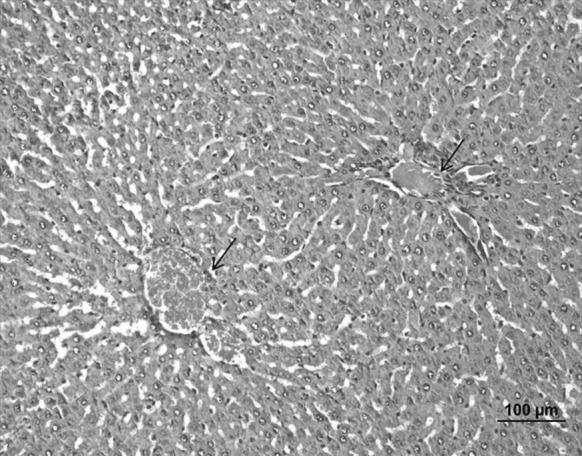
Liver sample of the Oleum cinnamomi treated ulcerative group. Congestion in venules (arrow) (bar 100 µm, HE)
Histological structure of kidney
Normal kidney structures were observed in control and Oleum cinnamomi treated control groups (Fig. 13).
Fig. 13.
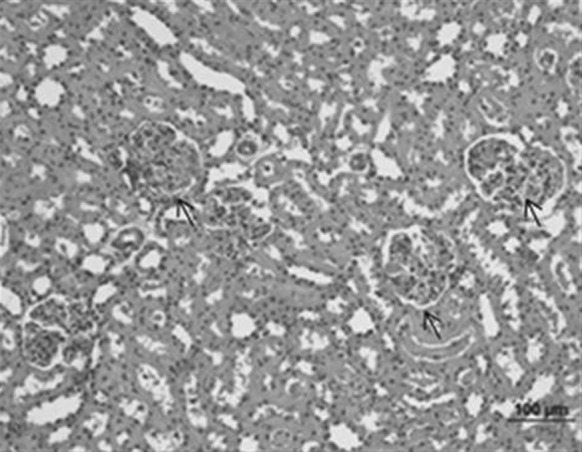
Kidney sample of the control group. Normal histological structures were seen (bar 100 µm, HE)
Epithelial cell injury and vacuolization, hemorrhages in interstitial area, necrosis of renal tubules and intravenous congestion were observed in kidneys of the acute injury group (ethanol control group) (Fig. 14).
Fig. 14.
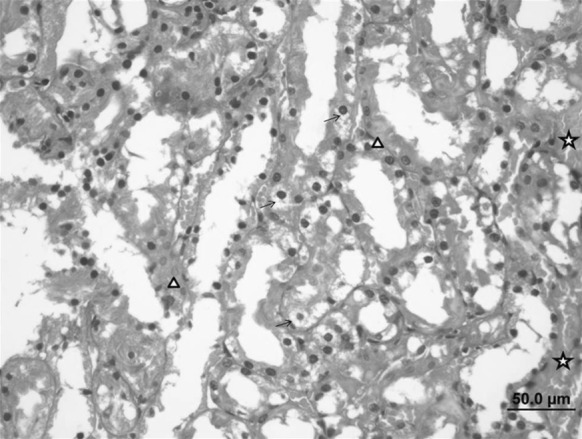
Kidney sample of the ulcerative control group. Tubular epithelial cell damage and vacuolization (arrow), necrotic cells (triangle) and interstitial hemorrhage (star) (bar 50.0 µm, HE)
Decreased renal tubular damage, normal glomerulus and Bowman’s capsule structure were observed in the kidney of the Oleum cinnamomi treated ethanol injury group whereas congestion in venules was seen in some areas (Fig. 15).
Fig. 15.
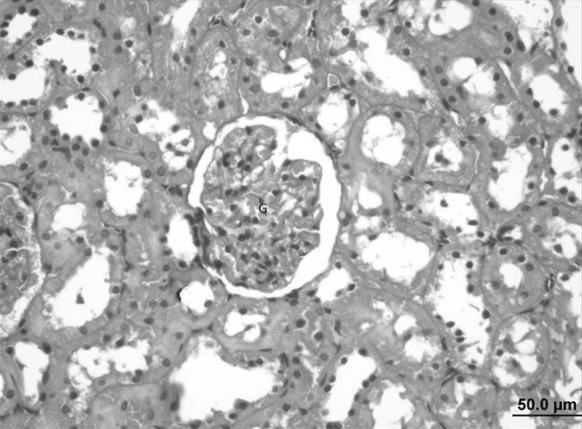
Kidney sample of the Oleum cinnamomi treated ulcerative group. Decreased renal tubular damage, normal glomerulus (G) and Bowman’s capsule structure (bar 50.0 µm, HE)
Discussion
Effects on ulcerative lesions
Ethanol is a well-known damaging agent to gastric mucosa for animals and clinical studies. At high concentrations, it causes marked mucosal hyperemia, necrosis, edema and mucosal or submucosal hemorrhage. The formation of lesions may be mediated by oxygen-derived free radicals (Chen et al. 2005).
Previous studies showed that cinnamon powder diet and treatment with cinnamon extract significantly protected animals against ulceration by stress, such as, ethanol, HCl and oral administration of aspirin (Amar and Maysa 2010; Tankam et al. 2013).
Similarly, in our study, ethanol caused significant ulcerative lesions and gastric lesions significantly decreased in Oleum cinnamomi treated group. Also, Oleum cinnamomi treatment decreased gastric mucus and gastric pH levels. This finding indicated that Oleum cinnamomi prevents the ulcerative lesions and has beneficial effects on gastric mucosa.
Effects on biochemical parameters
Alcohol is not digested like other foods. It avoids the normal digestive process and goes directly to the blood stream circulation. Alcohol consumption leads to the production of the highly reactive ethanol metabolite and acetaldehyde, which may affect intestinal tight junctions, increase paracellular permeability and the solubility of penetrating chemicals. Significant increase in AST, ALT and LDH levels were reported in ethanol intoxicated rats (Hamed 2011).
In the present study, administration of a single oral dose of ethanol increased the serum AST, ALT and LDH levels in the non-treated ulcerative groups whereas Oleum cinnamomi treatment decreased the AST, ALT and LDH levels. So, Oleum cinnamomi treatment prevented the disturbance of certain metabolic parameters by decreasing the effects of alcohol intoxication.
Effects on antioxidant and free radical levels
Malondialdehyde
Oxidative stress plays an important role in the pathogenesis of ethanol-induced liver injury (Saravanan et al. 2006). Ethanol leads to disturbances in the balance between pro-oxidant and antioxidant mechanism, hence oxidative stress occurs (Saravanan et al. 2006). Lipid peroxidation is a mediator of tissue damage (Sandhir and Gill 1999) and it is implicated in ethanol related toxicity (Brooks 1997). Alcohol leads to lipid peroxidation via generating reactive oxygen species (ROS). Walsh and Alexander (2000) indicated that oxygen free radical production in the alcoholic liver is major mechanism of tissue injury. ROS attack lipids and lipid peroxidation products are generated by this way. Malondialdehyde is the major product of lipid peroxidation (Brooks 1997). Kasdallah-Grissa et al. (2006) and Albano et al. (1996) indicated that ethanol treatment causes increase in MDA levels. Similarly, we observed increased MDA level in the liver, kidney and erythrocyte of the ulcerative control group associated with ethanol induced oxidative stress. In Oleum cinnamomi shows antioxidant activity thus Oleum cinnamomi treatment decreased the lipid peroxidation levels in Oleum cinnamomi treated ulcerative groups.
Superoxide dismutase
Superoxide dismutase, a common antioxidant enzyme, plays an important protective role by catalyzing the removal of superoxide radicals. It is delicate for toxic superoxide radicals and converts super oxide radicals to hydrogen peroxide, and hence hydrogen peroxide is degraded by catalase. Oxyradicals are potentially toxic molecules thus cellular efficacy of SOD enzyme reduction lead to increased lipid peroxidation (Balasubramaniyan et al. 2003; Sandhir and Gill 1999). Our finding indicates that SOD activity was decreased in the liver but increased in the stomach of the ethanol treated ulcerative groups. The reason of the decreased level of SOD could be due to excessive ROS generation (Scott et al. 2000). This decrease could be due to a feedback inhibition or oxidative inactivation of enzyme protein due to excess ROS generation.
Catalase
Catalase is present in peroxisomes and catalyzes the reaction between two hydrogen peroxide molecules. This reaction results in water and O2 production (Cederbaum et al. 2009). Sandhir and Gill (1999) reported that ethanol treatment causes liver damage and they found increased catalase levels in liver in ethanol treated rats. Similarly we found increased catalase levels in ethanol treated rat livers. Catalase is an important antioxidant enzyme responsible for the detoxification of H2O2 caused by ethanol intoxication (Kutlubay et al. 2008). In our study, catalase levels increased in response to increased ethanol-induced oxidative stress in stomach and liver of ethanol treated rats. A number of studies on the natural compounds have been demonstrated that cinnamon extracts show antioxidant and good free radical scavenging properties (Amar and Maysa 2010; Jayaprakasha et al. 2007; Singh et al. 2007). In the liver of the Oleum cinnamomi treated ulcerative group, catalase activities did not increased and thus Oleum cinnamomi treatment provided antioxidant support to the liver.
Effects on histological structure
Oral administration of ethanol causes the impairment of gastric defensive factors such as mucus and mucosa circulation and, in this way, it induces the necrotic lesions of the gastric mucosa (Alrashdi et al. 2012).
Gastric mucosal damage induced by ethanol is associated with a significant production of free oxygen radical. Also antioxidants play a significant role in the protection of gastric mucosa against various necrotic agents (Alrashdi et al. 2012; Srivastava et al. 2012).
Typical histological findings of ethanol induced ulcerations are linear hemorrhagic lesions, extensive submucosal edema, mucosal friability, inflammatory cells infiltration, and epithelial cell loss in the stomach (Alrashdi et al. 2012).
In our study we determined the gastric epithelial ulceration and intense cellular losses, necrosis of parietal cells, connective tissue edema caused by ethanol in the non-treated ulcerative group. Because of the antioxidant properties, Oleum cinnamomi treatment protected the gastric mucosa against ethanol toxicity and reduced the mucosal damage.
In various studies, the used intragastric treatment of ethanol showed that oxidative stress plays a major role in the mechanisms of hepatic damage (Dey and Cederbaum 2006; Kurose et al. 1997). Acute and chronic ethanol administration has been shown to enhance lipid peroxidation, lower cellular antioxidant levels and decrease in hepatic antioxidant defense (Dey and Cederbaum 2006). Increased lipid peroxidation destroys cell membranes with the release of intracellular components, such as lysosomal enzymes, leading to further tissue damage (Demir et al. 2003). Ethanol treatment induced hepatic damage, necrosis, sinusoidal dilatation and cytoplasmic vacuolations (Kutlubay et al. 2008). It was demonstrated that reduction and prevention of lipid peroxidation reduced or prevented liver damage induced by ethanol (Dey and Cederbaum 2006).
In the present study, we determined the hepatic injury, karyolysis, congestion in venules and sinusoidal dilatation in the ethanol treated groups. In the liver of Oleum cinnamomi treated ethanol group nearly normal histological structures were observed. Owing to the antioxidant properties, Oleum cinnamomi decreased the lipid peroxidation in liver and protected the liver from ethanol induced injury.
Ethanol induced reactive oxygen species play a role in renal toxicity. It has been shown that ethanol induced the kidney injury and caused vacuolations in tubule cells, and pathological changes in cell nuclei of tubular epithelium (Kutlubay et al. 2008).
In the present histological examination, necrosis of renal tubules, tubular damage and intravenous congestion were seen in the ethanol treated control group. In the Oleum cinnamomi treated injury group, the kidney histology had nearly normal structure. Oleum cinnamomi treatment decreased the kidney damage caused by ethanol.
Consequently, the current investigation shows that Oleum cinnamomi has protective effects on ethanol induced oxidative and tissue damage.
References
- Albano E, Clot P, Morimoto M, Tomasi A, Ingelman-Sundberg M, French SW. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology. 1996;23:155–163. doi: 10.1002/hep.510230121. [DOI] [PubMed] [Google Scholar]
- Alrashdi AS, Salama SM, Alkiyumi SS, Abdulla MA, Hadi AH, Abdelwahab SI, Taha MM, Hussiani J, Asykin N (2012) Mechanisms of gastroprotective effects of ethanolic leaf extract of Jasminum sambac against HCl/ethanol-induced gastric mucosal injury in rats. Evid Based Complement Alternat Med. doi:10.1155/2012/786426 [DOI] [PMC free article] [PubMed]
- Amar AR, Maysa ME. Anti-ulcer effects of cinnamon and chamomile aqueous extracts in rat models. J Am Sci. 2010;6:209–216. [Google Scholar]
- Balasubramaniyan V, Kalaivani Sailaja J, Nalini N. Role of leptin on alcohol-induced oxidative stress in Swiss mice. Pharmacol Res. 2003;47:211–216. doi: 10.1016/S1043-6618(02)00317-1. [DOI] [PubMed] [Google Scholar]
- Brooks PJ. DNA damage, DNA repair, and alcohol toxicity—a review. Alcohol Clin Exp Res. 1997;21:1073–1082. [PubMed] [Google Scholar]
- Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- Chen SH, Liang YC, Chao JC, Tsai LH, Chang CC, Wang CC, Pan S (2005) Protective effects of Ginkgo biloba extract on the ethanol-induced gastric ulcer in rats. World J Gastroenterol 28;11:3746–3750 [DOI] [PMC free article] [PubMed]
- Demir S, Yilmaz M, Köseoglu M, Akalin N, Aslan D, Aydin A. Role of free radicals in peptic ulcer and gastritis. Turkish J Gastroenterol. 2003;14:39–43. [PubMed] [Google Scholar]
- Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–152. doi: 10.1016/0009-8981(91)90067-M. [DOI] [PubMed] [Google Scholar]
- Hamed MA. Metabolic profile of rats after one hour of intoxication with a single oral dose of ethanol. J Pharmacol Toxicol. 2011;6:158–165. doi: 10.3923/jpt.2011.158.165. [DOI] [Google Scholar]
- Jayaprakasha GK, Negi PS, Jena BS, Jagan Mohan Rao L. Antioxidant and antimutagenic activities of Cinnamomum zeylanicum fruit extracts. J Food Compos Anal. 2007;20:330–336. doi: 10.1016/j.jfca.2006.07.006. [DOI] [Google Scholar]
- Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, Gharbi N, Kamoun A, El-Fazaa S. Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol. 2006;41:236–239. doi: 10.1093/alcalc/agh256. [DOI] [PubMed] [Google Scholar]
- Kılıç FS, Sırmagül B, Batu Ö, Erol K. Dose-dependent effects of verapamil on ethanol-induced gastric lesions in rats. J Health Sci. 2006;52:781–786. doi: 10.1248/jhs.52.781. [DOI] [Google Scholar]
- Kurose I, Higuchi H, Miura S, Saito H, Watanabe N, Hokari R, Hirokawa M, Takaishi M, Zeki S, Nakamura T, Ebinuma H, Kato S, Ishii H. Oxidative stress-mediated apoptosis of hepatocytes exposed to acute ethanol intoxication. Hepatology. 1997;25:368–378. doi: 10.1002/hep.510250219. [DOI] [PubMed] [Google Scholar]
- Kutlubay R, Oğuz EO, Turgut G, Kocamaz E. Karaciğer ve böbrek üzerine etanolün toksisitesi ve L-NAME’in koruyucu etkisi. SDÜ Tıp Fak Derg. 2008;15:11–17. [Google Scholar]
- Sandhir R, Gill KD. Hepatoprotective effects of Liv-52 on ethanol induced liver damage in rats. Indian J Exp Biol. 1999;37:762–766. [PubMed] [Google Scholar]
- Saravanan R, Viswanathan P, Pugalendi KV (2006) Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci 11;78:713–718 [DOI] [PubMed]
- Scott RB, Reddy KS, Husain K, Schlorff EC, Rybak LP, Somani SM. Dose response of ethanol on antioxidant defense system of liver, lung, and kidney in rat. Pathophysiology. 2000;7:25–32. doi: 10.1016/S0928-4680(99)00034-6. [DOI] [PubMed] [Google Scholar]
- Singh G, Maurya S, DeLampasona MP, Catalan CA. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem Toxicol. 2007;45:1650–1661. doi: 10.1016/j.fct.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Srivastava V, Mohan G, Viswanathswamy AHM. Protection of ethanol induced ulcers by sodium cromoglycate in albino rats. Ind J Pharm Edu Res. 2012;46:32–36. [Google Scholar]
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;3413:497–500. [PubMed] [Google Scholar]
- Tankam JM, Sawada Y, Ito M (2013) Regular ingestion of cinnamomi cortex pulveratus offers gastroprotective activity in mice. J Nat Med 67:289–295 [DOI] [PubMed]
- Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:279–286. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- Walsh K, Alexander G. Alcoholic liver disease. Postgrad Med J. 2000;76:280–286. doi: 10.1136/pmj.76.895.280. [DOI] [PMC free article] [PubMed] [Google Scholar]