Abstract
Cisplatin (CP) is a chemotherapeutic drug used in treatment of malignancies. However, its clinical utility is limited by nephrotoxicity. The purpose of the present study was to investigate the protective role of grape seed proanthocyanidin extract (GSPE) (100 mg/kg/day) or fish oil (FO) (5 ml/kg/day) against cisplatin induced nephrotoxicity in terms of biochemical parameters, oxidative stress and DNA damage. CP nephrotoxiciy is manifested by increased levels of serum creatinine, urea and uric acid, accompanied by their decrease in urine. Na, K and Ca levels were altered in both serum and urine. In addition, cisplatin caused a decrease in renal GSH, SH-group, SOD, GST, and Na–K–ATPase levels. However the levels of MDA, H2O2 and NO were increased. Also, we assessed the renal genotoxic potential of cisplatin as manifested by an increase in the tail length of DNA, tail intensity (DNA %) and tail moment. On the other hand, administration of GSPE or FO pre-cisplatin treatment ameliorated the current changes in most of the above tested parameters, particularly oxidative stress, endogenous antioxidant defense system and DNA damage indicating their curative effect. Thus, it can be concluded that the consumption of GSPE or FO might be useful for preventing nephrotoxicity caused by cisplatin treatment.
Keywords: Cisplatin, GSPE, FO, Nephrotoxiciy, Antioxidants, DNA damage
Introduction
The use of chemotherapeutic agents for the treatment of cancer has opened new prospective for improvement of the quality of life of cancer patients. However, besides its success, many anticancer drugs have been shown to be teratogenic and carcinogenic in experimental systems (Cherry et al. 2004). Cisplatin (cis diamminedichloroplatinum II, CP) is a major antineoplastic chemotherapy drug for the treatment of various forms of cancers such as ovarian, testicular, bladder, head and neck, and uterine cervix carcinomas (Taguchi et al. 2005; Pabla and Dong, 2008). However the clinical use of cisplatin is limited because of its unwanted side effects such as nephrotoxicity (Arany and Safirstein 2003; Pabla and Dong 2008), neurotoxicity (Barabas et al. 2008), ototoxicity (Rybak et al. 2009) and hepatotoxicity (Liao et al. 2008). Thus, there is a continuous search for agents that provide nephroprotection against CP and other platinum drugs.
Grape seed extracts are industrial derivatives from whole grape seeds that have a high concentration of vitamin E, flavonoids, linoleic acid, and oligomeric proanthocyanidins (OPCs). Typically, the commercial opportunity of extracting grape seed constituents has been done for chemicals known as polyphenols, including oligomeric proanthocyanidins recognized as antioxidants, which are naturally occurring polyphenolic compounds widely available in fruits, vegetables, nuts, seeds, flowers and bark (Zhang et al. 2005). GSPE has been shown to serve as one of the most potent free radical scavengers and provide significantly greater protection against damage of oxidative stress than vitamins C, E and beta-carotene (Hassan et al, 2013). Furthermore proanthocyanidins have been reported to exert antibacterial, antiviral, anticarcinogenic, antimutagenic, anti-inflammatory, anti-allergic, and vasodilatory actions (Fine 2000; Hassan and Al-Rawi 2013).
Furthermore, a number of investigations have also demonstrated that diet supplemented with fish oil (FO) enriched in ω-3 fatty acids has profound beneficial health effects against various pathologies (Simopoulos 1991) including cardiovascular diseases, respiratory diseases, diabetes, depression, cancers, inflammatory and immune renal disorders (Thakkar et al. 2000). Thus, it was thought worthwhile to investigate in detail the role of the supplementation of GSPE or fish oil in amelioration of cisplatin induced nephrotoxicity in an experimental rat model.
Materials and methods
Chemicals and drugs
Cisplatin (CP) was purchased from Mayne Pharmaceuticals (Warwickshire, UK), Fish oil (FO) [Menhaden, Sigma Chemical Company, St. Louis, MO, USA] and a dried powdered grape seed proanthocyanidin extract (GSPE) commercially known as Noxy life was obtained from Pharco Pharmaceuticals (Mansoura, Egypt).
Experimental animals
Adult male Wistar rats, weighing 150–180 g, were kept under a photoperiod of 12 h light: 12 h darkness schedule with lights-on from 06.00 to 18.00 h. They were housed in stainless cages in a windowless room with automatically regulated temperature (22–25 °C). The animals received standard chow and water ad libitum.
Experimental design
After 2 weeks of acclimatization, the animals were divided at random into six groups (eight animals each group): group I served as control, group II was orally treated with GSPE (100 mg/kg BW/day) dissolved in water for 6 weeks (Yamakoshi et al. 2002). Group III was orally treated with fish oil (5 ml/Kg BW/day) for 6 weeks (El-Daly 1996). Group IV received a single intraperitoneal dose of cisplatin (7.5 mg/kg BW) (Yilmaz et al. (2004); Atessahin et al. 2005). Group V received GSPE (100 mg/kg BW/day) dissolved in water orally for 6 weeks in addition to cisplatin (7.5 mg/kg BW) as a single intraperitoneal dose after the 5th week of the experiment. Group VI was orally treated with fish oil (5 ml/Kg BW/day) for 6 weeks in addition to cisplatin (7.5 mg/kg BW) as a single intraperitoneal dose after the 5th week of the experiment. All the animals were sacrificed after 1 week of cisplatin administration.
Sample collection and tissue preparations:
Urine samples were collected for 24 h in standard metabolic cages a day before the sacrifice of rats. After that, animals were sacrificed and blood was withdrawn from left jugular vein and put into chilled non-heparinized tubes, which were centrifuged at 860 g for 10 min for separation of serum. The sera were frozen at −20 °C for future measurements. Then the animals were dissected and the kidneys were removed and decapsulated. One of the two kidneys was stored at −80 °C for the Comet assay. The other was minced and homogenized (10 % w/v), separately, in ice-cold sodium, potassium phosphate buffer (0.01 M, pH 7.4) containing 1.15 % KCl in a Potter– Elvehjem type homogenizer. The homogenates were centrifuged at 10,000g for 20 min for biochemical parameters analysis.
Biochemical parameters
Serum creatinine, urea, uric acid, sodium, potassium, calcium levels were measured using kits purchased from Diamond Diagnostics Company (Gizza, Egypt). Na–K–ATPase activity was measured by the method of Bonting et al. (1961). Superoxide dismutase (SOD) activity was assayed according to Misra and Fridovich (1972), Glutathione-S-transferase activity was assayed spectrophotometrically using 1-chloro-2-4-dinitrobenzene (CDNB) and glutathione as described by Habig et al. (1974). Reduced glutathione (GSH) content was assayed according to the method of Beutler et al. (1963), SH- group was measured by the method of Ellman (1959). The amount of malondialdehyde (MDA) was measured by the thiobarbituric acid assay (Ohkawa et al. 1982). Hydrogen peroxide was determined using the colorimetric kit purchased from Biodiagnostic (Gizza, Eygpt) according to the technique used by Aebi (1984). The nitric oxide level was estimated according to the method of Montgomery and Dymock (1961), using the nitric acid kit obtained from Biodiagnostic company for laboratory services, Egypt.
Single-cell gel electrophoresis (comet essay):
The kidneys were removed, decapsulated and immediately stored at −80 °C for Comet assay. The specimens were homogenized in chilled homogenization buffer, pH 7.5 containing 75 mM NaCl and 24 mM Na2EDTA, pH 13, to obtain a 10 % tissue solution. A potter-type homogenizer was used and samples were kept on ice during and after homogenization. Six microliters of kidney homogenate were suspended in 0.5 % low melting agarose and sandwiched between a layer of 0.6 % normal-melting agarose and a top layer of 0.5 % low melting agarose on fully frosted slides. The slides were kept on ice during the polymerization of each gel layer. After the solidification of the 0.6 % agarose layer, the slides were immersed in a lysis solution (1 % sodium surcosinate, 2.5 m NaCl, 100 mM Na2EDTA, 10 mm Tris-HCl, 1 % TritonX-100 and 10 % DMSO) at 4 °C. After 1 h, the slides were placed in electrophoresis buffer (0.3 M NaOH, 1 mM Na2EDTA, pH 13) for 10 minutes at 0 °C to allow DNA to unwind. Electrophoresis was performed for 10 min at 300 mA and 1 V/cm. The slides were neutralized with Tris–HCl buffer, pH 7.5, and stained with 20 µg/ml ethidium-bromide. Each slide was analyzed using a Leitz Orthoplan epifluorescence microscope (Wetzlar, Germany). One hundred cells were analyzed on each slide using the comet assay II automatic digital analysis system. Perspective tail length (µm) is the distance of DNA migration from the center of the body of the nuclear core and is used to evaluate the DNA damage. The tail moment is defined as the product of the tail length and the fraction of total DNA in the tail (tail moment = tail length × % of DNA in the tail). Both tail length and tail intensity are measured automatically by image analysis software (Sasaki et al. 1997; Robbiano et al. 2004).
Single-cell gel electrophoresis assay, also known as the “comet” assay, is a fairly recent, rapid, simple, and reliable biochemical technique for evaluating DNA damage in mammalian cells. Perspective tail length (μm) is the distance of DNA migration from the center of the body of the nuclear core and is used to evaluate the of DNA damage. The tail moment is defined as the product of the tail length and the fraction of total DNA in the tail (Tail moment = tail length × % of DNA in the tail). Both tail length and tail intensity are measured automatically by image analysis software (Sasaki et al. 1997; Robbiano et al. 2004).
Statistical analysis
All data were statistically analyzed by one way ANOVA (analysis of variance) test and post comparison was carried out with Waller–Duncan k ratio (Waller and Duncan 1969) using SPSS program (Statistical Package for Social Science) version 11. The results are presented as mean ± SE. The values of p ≤ 0.05 were considered statistically significant based on least significant difference (LSD) probability.
Results
Cisplatin treatment to rats resulted in a significant increase in serum creatinine, urea and uric acid compared with control rat group, but excretion of them in urine were decreased (Tables 1, 2). Also, cisplatin affects reabsorption of some electrolytes which manifested itself by decreasing serum sodium and calcium and increasing serum potasium, while their levels increased in urine. Pretreatment with GSPE or FO significantly reduced CP-induced high levels in creatinine, urea and uric acid in serum and increased their excretion in urine. The levels of sodium and calcium were significantly increased in serum and potassium was decreased in the groups pretreated with GSPE and FO as compared to the groups treated with cisplatin, but their levels significantly decreased in urine.
Table 1.
Serum biochemical parameters in control and different treated rat groups
| Animal groups | Control | GSPE | Fish oil | Cisplatin | GSPE + Cisplatin | Fish oil + Cisplatin | |
|---|---|---|---|---|---|---|---|
| Creatinine (mg/dl) | Mean ± SE | 1.08 ± 0.043a | 1.08 ± 0.051a | 1.21 ± 0.078a | 6.26 ± 0.44b | 3.96 ± 0.27d | 4.54 ± 0.40d |
| Urea (mg/dl) | Mean ± SE | 63.45 ± 2.28a | 60.36 ± 3.04a | 62.25 ± 2.81a | 123.35 ± 2.73b | 109.13 ± 3.30d | 103.83 ± 3.06d |
| Uric acid (mg/dl) | Mean ± SE | 1.50 ± 0.15a | 1.71 ± 0.19a | 1.24 ± 0.08a | 3.02 ± 0.27b | 1.88 ± 0.12a | 1.99 ± 0.29a |
| Na (mmol/L) | Mean ± SE | 147.13 ± 2.73a | 168.61 ± 6.77b | 162.44 ± 7.85a,b | 84.72 ± 3.36c | 120.80 ± 6.30d | 120.64 ± 8.32d |
| K (mmol/L) | Mean ± SE | 6.44 ± 0.31a | 6.59 ± 0.35a | 5.95 ± 0.28a | 4.33 ± 0.41b | 6.05 ± 0.12a | 4.65 ± 0.25b |
| Ca (mg/dl) | Mean ± SE | 5.01 ± 0.14a | 5.25 ± 0.36a | 4.72 ± 0.17a | 3.35 ± 0.29b | 4.16 ± 0.21c | 4.01 ± 0.35d |
GSPE Grape seed proanthcyanidin extract
Data are expressed as mean ± SE of 6 rats. Within each row, means with different superscript (a, b, c, d, e) were significantly different at p < 0.05, whereas means superscripts with the same letters mean that there is no significant difference at (p > 0.05)
Table 2.
Urine biochemical parameters in control and different treated rat groups
| Animal groups | Control | GSPE | Fish oil | Cisplatin | GSPE + Cisplatin | Fish oil + Cisplatin | |
|---|---|---|---|---|---|---|---|
| Creatinine (mg/dl) | Mean ± SE | 38.24 ± 1.89a | 39.49 ± 1.92a,b | 44.95 ± 3.08b | 16.72 ± 1.65c | 27.13 ± 1.74d | 33.66 ± 1.08d |
| Urea (g/dl) | Mean ± SE | 1.87 ± 0.09a | 2.72 ± 0.30b | 2.80 ± 0.23b | 0.31 ± 0.04c | 1.63 ± 0.08a | 0.97 ± 0.07d |
| Uric acid (mg/dl) | Mean ± SE | 124.61 ± 3.33a | 126.06 ± 5.36a | 114.73 ± 5.36a | 34.24 ± 2.93b | 56.81 ± 2.59c | 58.13 ± 2.67c |
| Na (mmol/L) | Mean ± SE | 65.29 ± 2.82a | 58.20 ± 2.92a | 44.57 ± 2.00b | 151.41 ± 3.75c | 105.41 ± 3.75d | 92.32 ± 8.32e |
| K (mmol/L) | Mean ± SE | 85.65 ± 4.86a | 97.90 ± 2.83a | 98.48 ± 6.40a | 139.55 ± 4.15b | 115.46 ± 3.36d | 115.91 ± 5.51d |
| Ca (mg/dl) | Mean ± SE | 0.79 ± 0.11a | 0.65 ± 0.19a | 0.84 ± 0.13a | 2.77 ± 0.14b | 2.10 ± 0.13d | 1.94 ± 0.17d |
| crcl (ml/min) | Mean ± SE | 0.24 ± 0.01a | 0.29 ± 0.026b | 0.27 ± 0.022a | 0.01 ± 0.003c | 0.07 ± 0.013d | 0.06 ± 0.016c |
GSPE Grape seed proanthcyanidin extract
Data are expressed as mean ± SE of 6 rats. Within each row, means with different superscript (a, b, c, d, e) were significantly different at p < 0.05, whereas means superscripts with the same letters mean that there is no significant difference (p > 0.05)
As shown in Table 3 the levels of kidney GSH and SH group were significantly decreased in rats treated with cisplatin when compared with the control rats. Also, the activities of SOD, GST and Na–K ATPase were significantly decreased in the rats treated with cisplatin. In contrast, administration of GSPE or FO significantly increased renal GSH and SH group levels and SOD, GST and Na–K ATPase activities as compared to the cisplatin treated rats. Also, cisplatin caused oxidative stress through a significant increase in kidney MDA, H2O2 and NO as compared to control rats. However, pretreatment with GSPE or FO significantly decreased their levels in kidney compared to the cisplatin group.
Table 3.
Renal antioxidant biomarkers and oxidative stress in control and different treated rat groups
| Animal groups | Control | GSPE | Fish oil | Cisplatin | GSPE + Cisplatin | Fish oil + Cisplatin | |
|---|---|---|---|---|---|---|---|
| GSH (mg/g wet tissue) | Mean ± SE | 1.09 ± 0.06a | 1.02 ± 0.03a | 0.99 ± 0.04a | 0.78 ± 0.01b | 0.87 ± 0.03b | 0.87 ± 0.02b |
| SH (mM/g wet tissue) | Mean ± SE | 11.83 ± 0.52a | 12.99 ± 0.49a | 11.45 ± 0.67a | 8.59 ± 0.20b | 10.60 ± 0.55c | 9.93 ± 0.51b |
| SOD (U/g wet tissue) | Mean ± SE | 164.81 ± 2.37a | 160.45 ± 1.79a | 168.03 ± 8.50a | 105.59 ± 2.91b | 128.03 ± 3.06d | 110.91b ± 5.56b |
| GST (µmol/min/g tissue) | Mean ± SE | 5.55 ± 0.27a | 5.79 ± 0.23a | 5.54 ± 0.21a | 3.98 ± 0.16b | 4.48 ± 0.13b,d | 4.78 ± 0.10d |
| Na–K ATPase (µmol/Pi/min/gm wet tissue) | Mean ± SE | 59.94 ± 2.31a | 59.84 ± 2.60a | 60.02 ± 2.49a | 27.09 ± 1.82b | 41.07 ± 2.65c | 33.37 ± 1.87b |
| MDA (nmol/g wet tissue) | Mean ± SE | 59.8 ± 3.17a | 53.74 ± 4.14a | 59.30 ± 3.02a | 198.49 ± 7.08b | 121.70 ± 6.10c | 139.08 ± 5.40d |
| H2O2 (mM/g wet tissue) | Mean ± SE | 3.26 ± 0.25a | 3.21 ± 0.21a | 4.04 ± 0.27a | 14.83 ± 0.44b | 9.21 ± 0.42d | 7.74 ± 0.30c |
| NO (mmol/g wet tissue) | Mean ± SE | 0.84 ± 0.01a | 0.77 ± 0.02a | 0.81 ± 0.04a | 3.48 ± 0.15b | 2.07 ± 0.21d | 2.12 ± 0.37c |
GSPE Grape seed proanthcyanidin extract
Data are expressed as mean ± SE of 6 rats. Within each row, means with different superscript (a, b, c, d, e) were significantly different at p < 0.05, whereas means superscripts with the same letters mean that there is no significant difference (p > 0.05)
Concerning the renal genotoxic potential of cisplatin using the comet essay there was a significant increase in the tail length of DNA, tail intensity (DNA %) and tail moment in the cisplatin treated rats compared to the control. On the other hand pretreatment with GSPE or FO significantly decreased DNA tail length, intensity and moment as compared to the cisplatin treated rats (Table 4 and Fig. 1).
Table 4.
Renal DNA tail length, tail intensity (% of total genomic DNA found in the tail of the comets), and tail moment (tail length × tail intensity/100) measured by the comet essay in control and different treated rat groups
| Animal groups | Control | GSPE | Fish oil | Cisplatin | GSPE + Cisplatin | Fish oil + Cisplatin | |
|---|---|---|---|---|---|---|---|
| Tail length (µm) | Mean ± SE | 2.43 ± 0.02a | 2.47 ± 0.02a | 2.46 ± 0.01a | 5.00 ± 0.07b | 3.66 ± 0.04c | 3.94 ± 0.01d |
| Tail intensity (%) | Mean ± SE | 2.37 ± 0.03a | 2.40 ± 0.02a | 2.49 ± 0.01b | 4.89 ± 0.01c | 3.72 ± 0.05d | 3.58 ± 0.02e |
| Tail moment (UNIT) | Mean ± SE | 0.058 ± 0.0008a | 0.059 ± 0.0007a | 0.062 ± 0.001a | 0.244 ± 0.004b | 0.135 ± 0.001c | 0.141 ± 0.001d |
Data are expressed as mean ± SE of 6 rats. Within each row, means with different superscript (a, b, c or d) were significantly different at p < 0.05, whereas means superscripts with the same letters mean that there is no significant difference (p > 0.05). Grape seed proanthcyanidin extract
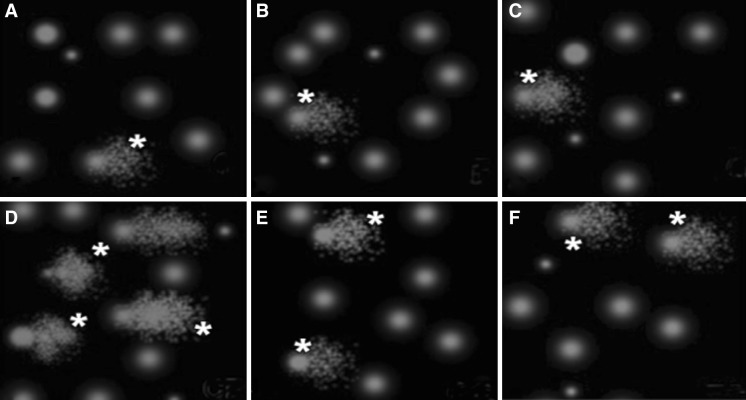
Fig. 1.
Image from the comet essay on kidney cells: a Control group, b GSPE group and c FO group [No DNA damage], d Cisplatin group [Significant damage and movement of DNA (DNA tail significantly increased)], e GSPE + CP group and f FO + CP group [There is a significant decrease in DNA tail and movement]. * indicates DNA tail
Discussion
In the present study, the observed nephrotoxicity due to cisplatin treatment was manifested by marked increases in serum creatinine, urea and uric acid accompanied by a decrease in urine creatinine, urea, uric acid and creatinine clearance. This may be due to the decrease in the glomerular filtration rate or may be secondary due to the increase of the reactive oxygen species (Noori and Mahboob 2010) which induce mesangial cells contraction, altering the filteration surface area and modifying the ultrafiltration coefficient factors that thereby decrease the glomerular filtration rate (Aydogan et al. 2008). These results are in accordance with previous studies (Fouad et al. 2008).
Also, the destruction of proximal and distal tubules observed in the cisplatin treated mice preceded the renal homodynamics, suppressed the reabsorption, increased vascular resistance and caused the elevation in BUN and creatinine levels (Daugaard and Abildgaard 1989). The current results also indicated that administration of cisplatin decreased the activity Na–K ATPase and induced disturbances in the electrolytes which are manifested by a decrease in the Na and Ca and K levels in the serum and an increase in their levels in urine. Daugaard and Abildgaard (1989) recorded that cisplatin causes decreased mitochondrial functions, decreased ATPase activity, altered cell cation content, and altered solute transport. Also, Arany and Safirstein (2003) reported that cispaltin treatment decreased sodium reabsorption in the proximal tubule and decreased sodium and water reabsorption in the distal tubule. These disturbances may be due to impairment in proximal and distal reabsorption and increased vascular resistance after cisplatin administration (Daugaard and Abildgaard 1989).
Hence, cisplatin treatment results in impaired tubular reabsorption and decreased urinary concentration. Moreover, the disruptive action of ROS induced by cisplatin may cause alterations in the membrane structure and function, including fluidity, permeability, and activity of enzymes, channels, and receptors. It has also been shown that ROS could inhibit Na+, K+- ATPase and Ca2+-ATPase of in vitro membrane ion transport systems (Lehotsky et al. 1999). However, pre-administration of GSPE or FO improved the kidney function through decreasing the concentration of serum creatinine, urea, uric acid and creatinine clearance, and improved the reabsorption of electrolytes such as Na, K and Ca and increasing the concentration of Na–K–ATPase (Priyamvada et al. 2008). This improvement in kidney function may be due to the antioxidant properties of proanthocyanidins and FO enriched in ω-3 fatty acids which enhanced the resistance to free radical attack generated by cisplatin (Guendez et al. 2005).
It was shown that ROS generated during normal cellular processes are immediately detoxified by endogenous antioxidants like GSH, catalase, GR, GRx, GST etc., but excessive ROS accumulation by cisplatin causes an antioxidant status imbalance leading to lipid peroxidation and GSH depletion (Kim et al. 2006). Also, the increased reactive oxygen species that attack the cell membrane lipids leads to increased tissue lipid peroxides as manifested by increased MDA level and overaccumulation of lipid peroxides in tissue causes overconsumption and depletion of GSH and inhibition of antioxidant enzymes (Noori and Mahboob 2010). The present study showed that administration of cisplatin decreased the activity of antioxidant enzymes, such as SOD and GST, depletion of, both, GSH and SH group and enhancement of MDA production in renal tissue, and increase of kidney H2O2 and NO. This may be due to increased activity of NADPH oxidase, xanthine oxidase and adenosine deaminase that leads to decline in the activity of the antioxidant enzymes (catalase, SOD and GPx), depletion of, both, the GSH and protein thiols and enhancement of MDA production in renal tissue (Ali et al. 2007; Chirino et al. 2008). A significant decline in antioxidant enzymes activity and increase in free radicals in experimental models as well as in subjects is typical during the regimens of commonly used chemotherapy, and this is particularly related to cisplatin treatment (Partibha et al. (2006). Ajith et al. (2007) also, reported a significant increase in kidney malondialdehyde (MDA) and a decrease in the activity of antioxidant enzymes due to cisplatin treatment. Similarly, Yuce et al. (2007) have also reported an increase in MDA and decrease in the activity of antioxidant enzymes upon similar cisplatin treatments of rats. This impairment may be due to accumulation of cisplatin in the human kidney cells (Stewart et al. 1982), resulting in the enhanced production of ROS and the decrease in the antioxidant enzymes (Weijl et al. 1997).
Furthermore, GSH may be decreased due to its consumption in the detoxification of toxicants including chemotherapeutic drugs, metabolism of nutrients and regulation of various pathways to maintain cellular homeostasis (Wu et al. 2005). Thiols such as the sulfur of GSH will bind to the platinum molecule, replacing one of the chloride ions and preventing binding to other cellular nucleophiles (Berners-Price and Kuchel 1990). Increased intracellular GSH concentrations correlate with decreased platinum–DNA binding in freshly isolated peripheral blood mononuclear cells (Sadowitz et al. 2002). Different enzymatic and non-enzymatic reactions could be involved in GSH mediated scavenging of free radicals and other oxygen species. Therefore, lipid peroxidation due to cisplatin administration is a consequence of GSH depletion and impaired antioxidant enzyme activity. These observations support the evidence that part of the mechanism of nephrotoxicity in cisplatin-treated rats is related to depletion of the antioxidant system. The decrease in SOD activity could cause the initiation and propagation of lipid peroxidation in the cisplatin treated rats (Ajith et al. 2007). Cisplatin has been demonstrated to cause loss of copper and zinc in the kidneys (Badary et al. 2004). The reduction in the activity of the antiperoxidative enzymes (SOD and catalase) may be due to the increased generation of ROS such as superoxide and hydrogen peroxide, which in turn leads to the inhibition of the activity of these enzymes (Karthikeyan et al. 2007a, b).
Moreover, cisplatin is known to accumulate in mitochondria of renal epithelial cell; it is the primary target for cisplatin-induced oxidative stress resulting in loss of mitochondrial protein-SH, inhibition of calcium uptake and a reduction in the mitochondrial membrane potential (Saad et al. 2004). The reduction observed in the activitiy of GST accompanied with CP-induced injury might be due to decreased availability of its substrate, reduced glutathione (Karthikeyan et al. 2007a, b). GST catalyses the conjugation of reduced glutathione via the sulfhydryl group, to electrophilic centers on a wide variety of substrates. This activity is useful in the detoxification of endogenous compounds such as peroxidised lipids, as well as the metabolism of xenobiotics (Valavanidisa et al. 2006). In addition to that the increased content of MDA may result from an increase of hydroxyl radicals (.OH) which intiate lipid peroxidation in tissues (Celik and Tuluce 2006), and MDA is a major oxidation product of peroxidized polyunsaturated fatty acids and increased MDA content is an important product of lipid peroxidation. Glutathione S-transferase (GST) catalyses the conjugation of reduced glutathione via the sulfhydryl group, to electrophilic centers on a wide variety of substrates. This activity is useful in the detoxification of endogenous compounds such as peroxidised lipids, as well as the metabolism of xenobiotics (Valavanidisa et al. 2006). Excess NO reacts with superoxide anion to generate peroxynitrite radical that causes further cell damage by oxidizing and nitrating cellular macromolecules. Also, excess NO depletes intracellular GSH increasing the susceptibility to oxidative stress (Jung et al. 2009). NO reacts with free oxygen radicals and this lead to the formation of the most harmful peroxynitrite anion and this anion leads to lipid peroxidation, cellular damage and apoptosis (Groeneveld et al. 1996).
On the other hand, the administration of proanthocynidin (PAC) exhibited a clear protective action against the deleterious effects resulting from the administration of cisplatin on the antioxidant status. This observation is in harmony with Karthikeyan et al. (2007a, b); Yousef et al. (2009). The last author investigated that combined treatment of rats with proanthocyanidin and cisplatin increased the level of GSH and SOD activity in the liver compared to rats treated with cisplatin only.
This improvement in the antioxidant status may be due to its phenolic components (Cetin et al. 2008) which act as antioxidants not only because they are hydrogen and electron donators but also because they stabilize radical intermediators, thus preventing oxidation (Sun et al. 1999). In addition, procyanidin B1 has been assumed to be one of the most important radical scavenging in grape seed extracts (Guendez et al. 2005). Moreover, Hassan and Abdel-Aziz (2010) found that black berry juice (1.6 g/kg bw) increased GSH level, TAC and SOD activity in rats treated with sodium fluoride. The reason was probably due to the protection of sulfhydryl groups in glutathione from oxidative damages via the free radicals quenching action of the di-OH (catechol) structure in the B ring of proanthocyanidin (Ishige et al. 2001).
Furthermore, the improvement in SOD activity may be due to the potential quenching of free radicals by proanthocyanidin (PAC) through the formation of resonance stabilized phenoxyl radicals, which significantly decrease the superoxide radical level (Rice-Evans et al. 1996). Also, grape seed proanthcyanidin extract can clear off free radicals and protect the over oxidative stress caused by free radicals (Spranger et al. 2008), and showed a generalized anti-peroxidative effect, which is effective against H2O2 (Roychowdhury et al. 2001).
Moreover, proanthocyanidin has been demonstrated to inhibit oxidative stress through modulation of metabolic functions, enhancement of detoxification pathways, and/or prevention of the interaction of xenobiotics with biological molecules (Bagchi et al. 2000; Hassan et al. 2013). In conclusion, the antioxidant function of PAC may work by increasing the activity of antioxidant enzymes of the body (Shan et al. 2010).
Also, the oral administration of fish oil (FO) to rats prevented cisplatin induced oxidative stress and suppression of antioxidant enzyme activity. This in accordance with Priyamvada et al. (2008) who reported that the feeding of FO diet to GM treated rats prevented GM-induced augmentation of lipid peroxidation (LPO) and suppression of antioxidant enzyme activity.
The protection against cisplatin effect by FO can be attributed to its intrinsic biochemical and natural antioxidant properties. Thus, it appears that FO enriched in ω-3 fatty acids enhanced resistance to free radical attack generated by cisplatin administration similarly as demonstrated in lupus nephritis and other pathologies (Chandrasekar and Fernandes 1994). Dietary FO supplementation has also been shown to strengthen antioxidant defense mechanisms in the plasma of normal rats (Erdogan et al. 2004). Recently, dietary FO has been shown to protect against acetaminophen (paracetamol)-induced hepatotoxicity (Speck and Lauterburgh 1991), ethanol-induced gastric mucosal injury in rats (Leung 1992), and a number of inflammatory diseases such as lupus nephritis (Chandrasekar and Fernandes 1994). Preliminary reports also showed partial protection by dietary FO ω-3 fatty acids against cyclosporine/GM-induced nephrotoxicity (Thakkar et al. 2000; Ali and Bashir 1994); however, the mechanism involved was not studied in detail. Our results thus support the rationale that ω-3 fatty acids enriched FO may be an effective dietary supplementation in the management of cisplatin nephrotoxicity and other pathologies in which antioxidant defense mechanisms are impaired. Moreover, the data including those from the comet assay showed that administration of cisplatin induced DNA damage through the increase in the migration of DNA (comet tail) compared with the control rats. The reported DNA damage due to CDDP treatment is in agreement with De Martinis and Bianchi (2001) and Satoh et al. (2003).
Cisplatin binds to DNA to form covalent platinum DNA adducts and also acts as a DNA alkylator. In addition, cisplatin generates reactive oxygen species (ROS), which are known as one of the pathogenic intermediates triggering DNA damage following chemotherapy. Through these mechanisms, cisplatin triggers cellular responses involving multiple pathways, including DNA repair, transcription inhibition, cell cycle arrest, cellular transport system impairment, ATPase activity reduction and mitochondrial damage (Yin et al. 2007). The degradation of cellular DNA by endonucleases is an important component of renal tubular epithelial cell death induced by ischemia or nephrotoxins (Basnakian et al. 2005). This observation also holds true for cisplatin nephrotoxicity, in which necrotic cell death is encountered with higher doses whereas lower concentrations induce apoptosis (Razzaque 2007). Grape seed proanthocynidin extract administration prior to cisplatin decreased DNA damage in kidney cells due to scavenging free radicals and inhibition of oxidative tissue damage, DNA fragmentation, and subsequent apoptosis than all the antioxidant vitamins (Bagchi et al. 1997). This beneficial effect of GSPE or FO may be also due to scavenging activity especially for peroxyl and superoxide radicals (Ricardo da Silva et al. 1991; Ariga 2004). Furthermore, GSPE is bioavailable, and its significant potential to prevent CDDP induced acute renal failure may be attributed to the attenuation of renal tubular damage and enhancement of the regenerative response of the damaged tubular cells (Ray et al. 1999).
In conclusion, the present data indicated that grape seed proanthocyanidin extract or fish oil may be effective to maximize the clinical use of cisplatin in the treatment of various malignancies without nephrotoxicity and other side effects. This may occur through intracellular pathways, involving suppression of oxidative stress and modulation of endogenous antioxidant defense mechanism However, the treatment by grape seed proanthocyanidin extract seems more effective than fish oil.
List of abbreviations
- CP
Cisplatin
- GSPE
Grape seed proanthocyanidin extract
- FO
Fish oil
- DNA
Deoxyribonucleic acid
- SH
Sulfhydryl group
- SOD
Superoxide dismutase
- GST
Glutathione-S-transferase
- ATPase
Adenosine Triphosphatase
- MDA
Malondialdehyde
- H2O2
Hydrogen peroxide
- NO
Nitric oxide
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ajith TA, Nivitha V, Usha S. Zingiber officinale Rosco alone and in combination with α- tocopherol protect the kidney against cisplatin-induced acute renal failure. Food Chem Toxic. 2007;45:921–927. doi: 10.1016/j.fct.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Ali BH, Bashir AA. Effect of fish oil treatment on gentamicin nephrotoxicity in rats. Anal Nutr Metab. 1994;38:336–339. doi: 10.1159/000177831. [DOI] [PubMed] [Google Scholar]
- Ali BH, Al Moundhri MS, Tag Eldin MT, Nemmar A, Tanira MO. The ameliorative effect of cysteine prodrug L-2-oxothiazolidine-4-carboxylic acid on cisplatin-induced nephrotoxicity in rats. Fundam Clin Pharmacol. 2007;21:547–553. doi: 10.1111/j.1472-8206.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460–464. doi: 10.1016/S0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- Ariga T. The antioxidant function, preventive action on disease and utilization of proanthocyanidins. BioFactors. 2004;21:197–201. doi: 10.1002/biof.552210140. [DOI] [PubMed] [Google Scholar]
- Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicol. 2005;212:116–123. doi: 10.1016/j.tox.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Aydogan S, Yapislar H, Artis S, Aydogan B. Impaired erythrocytes deformability in H2O2-induced oxidative stress: protective effect of L-carnosine. Clin Hemorheol Microcirc. 2008;39:93–98. [PubMed] [Google Scholar]
- Badary OA, Abdel-Maksoud S, Ahmed WA, Owieda GH. Naringenin attenuates cisplatin nephrotoxicity in rats. Life Science. 2004;76:2125–2135. doi: 10.1016/j.lfs.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, Kuszynski CA, Joshi SS, Pruess HG. Free radicals and grape seed pranthocynidin extract: importance in human health and disease prevention. Toxicol. 2000;148:187–197. doi: 10.1016/S0300-483X(00)00210-9. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Gargb A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95:179–189. [PubMed] [Google Scholar]
- Barabas K, Milner R, Lurie D, Adin C. Cisplatin: a review of toxicities and therapeutic applications. Vet Comp Oncol. 2008;6:1–18. doi: 10.1111/j.1476-5829.2007.00142.x. [DOI] [PubMed] [Google Scholar]
- Basnakian AG, Apostolov EO, Yin X, Napirei M, Mannherz HG, Shah SV. Cisplatin nephrotoxicity is mediated by deoxyribonuclease I. J Am Soc Nephrol. 2005;16:697–702. doi: 10.1681/ASN.2004060494. [DOI] [PubMed] [Google Scholar]
- Berners-Price SJ, Kuchel PW. Reaction of cis- and trans-[PtCl2(NH3)2] with reduced glutathione inside human red blood cells, studied by 1H and 15N-[1H] DEPT NMR. J Lab Clin Med. 1990;61(38):327–345. doi: 10.1016/0162-0134(90)80006-j. [DOI] [PubMed] [Google Scholar]
- Beutler E, Duron O, Kelly BM. An improved method for the detection of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Bonting SL, Simon KA, Hawkins NM. Studies on sodium potassium—activated adenosine triphosphatase. Arch Biochem Biohpys. 1961;95:416–421. doi: 10.1016/0003-9861(61)90170-9. [DOI] [PubMed] [Google Scholar]
- Celik I, Tuluce Y. Effects of indoleacetic acid and kinetin on lipid peroxidation and antioxidant defense in various tissues of rats. Pestic Biochem Phys. 2006;84:49–54. doi: 10.1016/j.pestbp.2005.05.004. [DOI] [Google Scholar]
- Cetin A, Kaynar L, Kocyigit I, Hacioglu SK, Saraymen R, Ozturk A, Orhan O, Sagdic O. The effect of grape seed extract on radiation induced oxidative stress in the rat liver. Turk J Gasrtoenterol. 2008;19:92–98. [PubMed] [Google Scholar]
- Chandrasekar B, Fernandes G. Decreased pro-inflammatory cytokines and increased antioxidant enzyme gene expression by ω-3 lipids in murine lupus nephritis. Biochem Biophys Res Commun. 1994;200:893–898. doi: 10.1006/bbrc.1994.1534. [DOI] [PubMed] [Google Scholar]
- Cherry SM, Hunt PA, Hassold TJ. Cisplatin disrupts mammalian spermatogenesis, but does not recombination or chromosome segregation. Mutat Res. 2004;564:115–128. doi: 10.1016/j.mrgentox.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Chirino YI, Sanchez-Gonzalez DJ, Martinez CM, Cruz C, Pedraza Chaverri J. Protective effects of apocynin against cisplatin-induced oxidative stress and nephrotoxicity. Toxicol. 2008;245:18–23. doi: 10.1016/j.tox.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Daugaard G, Abildgaard U. Cisplatin nephrotoxicity: a review. Cancer Chemother Pharmacol. 1989;25:1–9. doi: 10.1007/BF00694330. [DOI] [PubMed] [Google Scholar]
- De Martinis BS, Bianchi M. Effect of vitamin C supplementation against cisplatin–induced toxicity and oxidative DNA damage in rats. Pharmacol Res. 2001;44:317–320. doi: 10.1006/phrs.2001.0860. [DOI] [PubMed] [Google Scholar]
- El-Daly ES. Effect of methimazole and fish oil treatment on gentamicin nephrotoxicity. J Islamic Acad Sci. 1996;9:37–48. [PubMed] [Google Scholar]
- Ellman GL. Tissue sulphydryl group. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Erdogan H, Fadillioglu E, Ozgocmen S, Sogut S, Ozyurt B, Akyol O, Ardicoglu O. Effect of fish oil supplementation on plasma oxidant/antioxidant status in rats. Prostaglandin Leukot Essent Fatty Acids. 2004;71:149–152. doi: 10.1016/j.plefa.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Fine AM. Oligomeric proanthocyanidin complexes: history, structure, and phytopharmaceutical applications. Alter Med Rev. 2000;5:144–151. [PubMed] [Google Scholar]
- Fouad AA, Morsy MA, Gomaa W. Protective effect of carnosine against cisplatin-induced nephrotoxicity in mice. Environ Toxicol Pharmacol. 2008;24:292–297. doi: 10.1016/j.etap.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Groeneveld PH, Kwappenberg KM, Langermans JA, Nibbering PH, Curtis L. Nitric oxide production correlates with renal insufficiency and multiple organ dysfunction syndrome in severe sepsis. Int Care Med. 1996;22:1197–1202. doi: 10.1007/BF01709336. [DOI] [PubMed] [Google Scholar]
- Guendez R, Kallithraka S, Makris DP, Kefalas P. Determination of low molecular weight polyphenolics constituents in grape (vitis vinifera) seed extracts: correlation with antiradial activity. Food Chem. 2005;89:1–9. doi: 10.1016/j.foodchem.2004.02.010. [DOI] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathion-S-Transferase the first enzyme step in mercapturic acid formation. J Biol Chem. 1974;1:7139–7150. [Google Scholar]
- Hassan HA, Abdel-Aziz AF. Evaluation of free radical-scavenging and anti-oxidant properties of black berry against fluoride toxicity in rats. Food Chem Toxicol. 2010;48:1999–2004. doi: 10.1016/j.fct.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Hassan HA, Al-Rawi MM (2013) Grape seeds proanthocyanidin extract as a hepatic-reno-protective agent against gibberellic acid induced oxidative stress and cellular alterations. Cytotechnology. doi:10.1007/s10616-012-9506-6 [DOI] [PMC free article] [PubMed]
- Hassan HA, Isa AM, El-Kholy WM, Nour SE (2013) Testicular disorders induced by plant growth regulators: cellular protection with proanthocyanidins grape seeds extract. Cytotechnology. doi:10.1007/s10616-012-9525-3 [DOI] [PMC free article] [PubMed]
- Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 2001;30:433–446. doi: 10.1016/S0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- Jung M, Hotter G, Vinas JL, Sola A. Cisplatin upregulates mitochondrial nitric oxide synthase and peroxynitrite formation to promote renal injury. Toxicol Appl Pharmacol. 2009;234:236–246. doi: 10.1016/j.taap.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Karthikeyan K, Sarala Bai BR, Niranjali Devaraj S. Cardioprotective effect of grape seed proanthocyanidins on isoproterenol induced myocardial injury in rats. Int J Cardiol. 2007;115:326–333. doi: 10.1016/j.ijcard.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Karthikeyan K, Sarala Bai BR, Niranjali Devaraj S. Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats. Int J Cardiol. 2007;115:326–333. doi: 10.1016/j.ijcard.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Kim YH, Kim YW, Oh YJ, Back NI, Chung SA, Chung HG, Jeong TS, Choi MS, Lee KT. Protective effect of the ethanol extract of the roots of Brassica rapa on cisplatin-induced nephrotoxicity in LLC-PK 1 cells and rats. Biol Pharm Bull. 2006;29:2436–2441. doi: 10.1248/bpb.29.2436. [DOI] [PubMed] [Google Scholar]
- Lehotsky J, Kaplan P, Racay P, Matejovicova M, Drgova A, Mezesova V. Membrane ion transport systems during oxidative stress in rodent brain: protective effect of stobadine and other antioxidants. Life Sci. 1999;65:1951–1958. doi: 10.1016/S0024-3205(99)00454-3. [DOI] [PubMed] [Google Scholar]
- Leung FW. Fish oil protects against ethanol-induced gastric mucosal injury in rats. Dig Dis Sci. 1992;37:636–637. doi: 10.1007/BF01307595. [DOI] [PubMed] [Google Scholar]
- Liao Y, Lu X, Lu C, Li G, Jin Y, Tang H. Selection of agents for prevention of cisplatin-induced hepatotoxicity. Pharmacol Res. 2008;57:125–131. doi: 10.1016/j.phrs.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Montgomery C, Dymock F. The determination of nitrite in water. Analyst. 1961;86:141–146. doi: 10.1039/an9618600141. [DOI] [Google Scholar]
- Noori S, Mahboob T. Antioxidant effect of carnosine pretreatment on cisplatininduced renal oxidative stress in rats. Ind J Clin Biochem. 2010;25:86–91. doi: 10.1007/s12291-010-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Wakatsuki A, Kaneda C. Assay for lipid peroxides in animals tissues by thiobarbituric acid reaction. Anal Biochem. 1982;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- Partibha R, Sameer R, Rataboli PV, Bhiwgade DA, Dhume CY. Enzymatic studies of cisplatin-induced oxidative stress in hepatic tissue of rats. Eur J Pharmacol. 2006;532:290–293. doi: 10.1016/j.ejphar.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Priyamvada SM, Priyadarshini NA, Arivarasu N, Farooq S, Khan SA, Khan Md, Khan W, Yusufi ANK. Studies on the protective effect of dietary fish oil on gentamicin- induced nephrotoxicity and oxidative damage in rat kidney. Prostaglandins Leukot Essent Fat Acids. 2008;78:369–381. doi: 10.1016/j.plefa.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Ray SD, Kumar MA, Bagchi D. In vivo abrogation of acetaminophen induced hepatic genomic DNA fragmentation and apoptotic cell death by a novel grape seed proanthocyanidin extract. Arch Biochem Biophys. 1999;369:42–58. doi: 10.1006/abbi.1999.1333. [DOI] [PubMed] [Google Scholar]
- Razzaque MS. Cisplatin nephropathy: is cytotoxicity avoidable? Nephrol Dial Transplant. 2007;22:2112–2116. doi: 10.1093/ndt/gfm378. [DOI] [PubMed] [Google Scholar]
- Ricardo da Silva JM, Darmon N, Fenandez Y, Mitjavila S. Oxygen free radical scavenger capacity in aqueous models of different procyanidins from grape seeds. J Agric Food Chem. 1991;39:1549–1552. doi: 10.1021/jf00009a002. [DOI] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganda G. Structure antioxidant activity relationships of flavonoids and phenolic acids. Free Rad Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Robbiano L, Baroni D, Carrozzino R, Mereto E, Brambilla G. DNA damage and micronuclei induced in rat and human kidney cells by six chemicals carcinogenic to the rat kidney. Toxicol. 2004;204:187–195. doi: 10.1016/j.tox.2004.06.057. [DOI] [PubMed] [Google Scholar]
- Roychowdhury S, Wolf G, Keilhoff G, Bagchi D, Horn T. Protection of primary glial cells by grape seed proanthocyanidin extract against nitrosative/oxidative stress. Nitric oxide: Biol Chem. 2001;5:137–149. doi: 10.1006/niox.2001.0335. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Mukherjea D, Jajoo S, Ramkumar V. Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J Exp Med. 2009;219:177–186. doi: 10.1620/tjem.219.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad SY, Najjar TA, Alashari M. Role of nonselective adenosine receptor blockade and phosphodiesterase inhibition in cisplatin-induced nephrogonadal toxicity in rats. Clin Exp Pharmacol Physiol. 2004;31:862–867. doi: 10.1111/j.1440-1681.2004.04127.x. [DOI] [PubMed] [Google Scholar]
- Sadowitz PD, Hubbard BA, Dabrowiak JC, Goodisman J, Tacka KA, Aktas MK, Cunningham MJ, Dubowy RL, Souid A-K. Kinetics of cisplatin binding to cellular DNA and modulations by thiol-blocking agents and thiol drugs. Drug Metab Dispos. 2002;30:183–190. doi: 10.1124/dmd.30.2.183. [DOI] [PubMed] [Google Scholar]
- Sasaki YF, Nishidate E, Izumiyama F, Matsusaka N, Tsuda S. Simple detection of chemical mutagens by the alkaline single-cell gel electrophoresis (Comet) assay in multiple mouse organs. Mutat Res. 1997;391:215–231. doi: 10.1016/S1383-5718(97)00073-9. [DOI] [PubMed] [Google Scholar]
- Satoh M, Kashihara N, Fujimoto S, Horike H, Tokura T, Namikoshi T, Sasaki T, Makino H. A novel free radical scavenger, edarabone, protects against cisplatin-induced acute renal damage in vitro and in vivo. J Pharmacol Exp Ther. 2003;305:1183–1190. doi: 10.1124/jpet.102.047522. [DOI] [PubMed] [Google Scholar]
- Shan Y, Ye X, Xin H. Effect of the grape seed proanthocynidin extract on the free radical and energy metabolism indicators during the movement. Sci Res Essay. 2010;5:148–153. [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- Speck RF, Lauterburgh BH. Fish oil protects mice against acetaminophen hepatotoxocity in vivo. Hepatol. 1991;13:557–561. doi: 10.1016/0270-9139(91)90311-i. [DOI] [PubMed] [Google Scholar]
- Spranger I, Sun B, Mateus AM, Freitas V, Ricardo-da-Silva JM. Chemical characterization and antioxidant activity of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008;108:519–532. doi: 10.1016/j.foodchem.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Stewart DJ, Benjamin RS, Luna M. Human tissue distribution of platinum after cis-diaminedichloroplatinum. Cancer Chemother Pharmacol. 1982;10:51–54. doi: 10.1007/BF00257239. [DOI] [PubMed] [Google Scholar]
- Sun BS, Belchior GP, Ricardo-da-silva JM, Spranger MI. Isolation and purification of dimeric and trimeric procyanidins from grape seeds. J A Chromatography. 1999;841:115–121. doi: 10.1016/S0021-9673(99)00281-2. [DOI] [Google Scholar]
- Taguchi T, Nazneen A, Abid MR, Razzaque MS. Cisplatin-associated nephrotoxicity and pathological events. Contrib Nephrol. 2005;148:107–121. doi: 10.1159/000086055. [DOI] [PubMed] [Google Scholar]
- Thakkar RR, Wang OL, Zerouga M, Stillwell W, Haq A, Kissling R. Docosahexaenoic acid reverses cyclosporin-A induced changes in membrane structure and function. Biochim Biophys Acta. 2000;1474:183–195. doi: 10.1016/S0304-4165(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Valavanidisa A, Vlahogiannia T, Dassenakisb M, Scoullosb M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf. 2006;64:178–189. doi: 10.1016/j.ecoenv.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Waller RA, Duncan DB. Bayes rule for the symmetric multiple comparison problems. J Am Stat Assoc. 1969;64:1484–1503. [Google Scholar]
- Weijl NI, Cleton FJ, Osanto S (1997) Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev 23:209–240 [DOI] [PubMed]
- Wu YJ, Muldoon LL, Neuwelt EA. The chemoprotective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signaling pathway. J Pharmacol Exp Ther. 2005;312:424–431. doi: 10.1124/jpet.104.075119. [DOI] [PubMed] [Google Scholar]
- Yamakoshi J, Saito M, Kataoka S, Kikuchi M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem Toxicol. 2002;40:599–607. doi: 10.1016/S0278-6915(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Yilmaz HR, Iraz M, Sogut S, Ozyurt ZY, Akyol O, Gergerlioglu S. The effects of erdostiene on the activity of some metabolic enzymes during cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2004;50:287–290. doi: 10.1016/j.phrs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Yin X, Apostolov EO, Shah SV, Wang X, Bogdanov KV, Buzder T, Stewart AG, Basnakian AG. Cisplatin-induced nephrotoxicity is mediated by DNase I and endonuclease G in mice. J Am Soc Nephrol. 2007;16:697–702. [Google Scholar]
- Yousef MI, Saad AA, El- Shennawy LK. Protective effect of grape seed proanthocynidins extract against oxidative stress induced by cisplatin in rats. Food Chem Toxicol. 2009;47:1176–1183. doi: 10.1016/j.fct.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Yuce A, Atessahin A, Ceribası AO, Aksakal M. Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin Pharmacol Toxicol. 2007;101:345–349. doi: 10.1111/j.1742-7843.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Li WG, Wu YJ, Bai DC, Liu NF. Proanthocyanidin from grape seeds enhances doxorubicin-induced antitumor effect and reverses drug resistance in doxorubicin-resistant K562/DOX cells. Can J Physiol Phamacol. 2005;83:309–318. doi: 10.1139/y05-018. [DOI] [PubMed] [Google Scholar]