Abstract
Both all-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) have proven to be very effective in obtaining high clinical complete remission (CR) rates in acute promyelocytic leukemia (APL), but they had not been used jointly in an integrated treatment protocol for remission induction or maintenance among newly diagnosed APL patients. In this study, 61 newly diagnosed APL subjects were randomized into three treatment groups, namely by ATRA, As2O3, and the combination of the two drugs. CR was determined by hematological analysis, tumor burden was examined with real-time quantitative RT-PCR of the PML-RARα (promyelocytic leukemia-retinoic acid receptor α) fusion transcripts, and side effects were evaluated by means of clinical examinations. Mechanisms possibly involved were also investigated with cellular and molecular biology methods. Although CR rates in three groups were all high (≥90%), the time to achieve CR differed significantly, with that of the combination group being the shortest one. Earlier recovery of platelet count was also found in this group. The disease burden as reflected by fold change of PML-RARα transcripts at CR decreased more significantly in combined therapy as compared with ATRA or As2O3 mono-therapy (P < 0.01). This difference persisted after consolidation (P < 0.05). Importantly, all 20 cases in the combination group remained in CR whereas 7 of 37 cases treated with mono-therapy relapsed (P < 0.05) after a follow-up of 8–30 months (median: 18 months). Synergism of ATRA and As2O3 on apoptosis and degradation of PML-RARα oncoprotein might provide a plausible explanation for superior efficacy of combinative therapy in clinic. In conclusion, the ATRA/As2O3 combination for remission/maintenance therapy of APL brings much better results than either of the two drugs used alone in terms of the quality of CR and the status of the disease-free survival.
Acute promyelocytic leukemia (APL) accounts for 10–15% of acute myeloid leukemia in which the maturation of granulocytic cells was blocked at the promyelocytic stage. It is also characterized by the t(15;17)(q22;q21) chromosome translocation generating the PML-RARα (promyelocytic leukemia-retinoic acid receptor α) fusion gene, of which the leukemogenic role has been demonstrated by the transgenic mouse models (1). Although conventional chemotherapy such as anthracyclines and cytosine arabinoside (ara-C) succeeded in two-thirds of APL patients in obtaining complete remission, high frequency of early death mainly due to exacerbation of bleeding syndrome and low 5-year disease-free survival (DFS) rates dwarf them to new drugs (2). Our group in the Shanghai Institute of Hematology (SIH) has long been interested in differentiation therapy of human cancers, as inspired by the Chinese philosophy that it is better to transform a bad element instead of simply getting rid of it. After the discovery in the 1970s to early 1980s showing that some leukemic cells could undergo phenotypic reversion under differentiation inducers (3, 4), we started to screen a large number of compounds and identified, in 1984, all-trans retinoic acid (ATRA) as a strong differentiation inducer for cell line and fresh cells of promyelocytic leukemia. Since 1986, ATRA has been applied to the treatment of APL at SIH, establishing a successful model of differentiation therapy (5–7). Unlike cytotoxic chemotherapy, ATRA induces terminal differentiation of leukemic cells along the granulocytic lineage (5). Several prospective randomized trials demonstrated that ATRA-based differentiation therapy achieved clinical complete remission (CR) rates as high as 92–95% with rapid improvement of hemorrhage (8–11). Moreover, after ATRA-induced CR, chemotherapy consolidation and maintenance together with ATRA can reach a 5-year DFS rate of ≈60–70% (8–11). However, one-third of the patients who have already obtained CR ultimately relapse within 5 years (8–11), indicating the necessity to further improve the currently used ATRA/chemotherapy combination in APL treatment. Recently, arsenic compounds, mostly arsenic trioxide (As2O3), ancient drugs used in the traditional medicine in both eastern and western worlds, have been rediscovered in China as effective therapy not only in primary APL patients, but also in relapsed ones who obtained CR with ATRA/chemotherapy (11–16). Results from our group and others showed, nevertheless, that the overall DFS of relapsed patients who used As2O3 to reinduce CR was not satisfactory (14). Hence, how to take advantage of the three types of drugs (ATRA, As2O3, and cytotoxic chemotherapy) to make APL a really curable disease remained a question to hematologists and oncologists.
Although some reports on the joint effects of ATRA and As2O3 were controversial, mostly on some differentiation parameters obtained from in vitro culture systems (17), several lines of evidence support a strong synergistic effect between ATRA and As2O3 (18–28), raising the feasibility of their combination in clinical application for newly diagnosed APL. First, little cross-resistance between ATRA and As2O3 was observed, suggesting the existence of distinct but convergent mechanisms of action (11, 18). Second, data from APL animal models and some isolated case studies tend to support a better prognosis when the two drugs were used in combination. For example, synergistic effects on prolonging survival or eliminating leukemia were revealed in vivo in a transplantable ascites mouse model as well as a transgenic mouse model (18–20). Thirdly, molecular investigations have provided theoretical support to this possibility in that both ATRA and As2O3 induce modulation and/or degradation of the PML-RARα oncoprotein, with different target sites through distinct pathways (24–29).
Because ATRA with chemotherapy have already borne an extremely high CR rate, we hypothesized that a potential clinical benefit in combining ATRA and As2O3 could be reflected either in a higher quality of the CR or in a better DFS. In view of the fact that chemotherapy plays an essential role in eradicating the leukemia clone in APL even after the introduction of ATRA or As2O3, we rejected the idea that chemotherapy could be deleted from the protocol design when testing the ATRA/As2O3 combination but instead believed a better result could be achieved only in the full play of the potential of a triad therapy. In addition, we assumed that an objective and precise evaluation of the response to therapy should be possible in APL, owing to the presence of PML-RARα as a specific molecular marker and the availability of a quantitative assay of this marker.
In the present work, we carried out a perspective clinical trial to compare the effects of the ATRA/As2O3 combination with those of ATRA or As2O3 mono-therapy in remission induction and maintenance therapy. Particular attention was given to the clinical efficacy and prognosis evaluated with both clinical and molecular parameters, as well as side effects, among different groups.
Methods
Patients. From April 2001 to February 2003, 61 successive cases of newly diagnosed APL without prior exposure to any anti-leukemic therapy were included, with informed consent, in the current study. The diagnosis of APL was established according to clinical presentation and morphological criteria of the French-American-British (FAB) classification, and was confirmed by cytogenetic assay for t(15;17)(q22;q21) and RT-PCR analysis for PML-RARα transcripts with reference to the more recently proposed World Health Organization classification of malignant hemopathies (30). The subjects were randomized into three groups for induction therapy: group 1 (n = 20) with ATRA, group 2 (n = 20) with As2O3, and group 3 (n = 21) with the combination of ATRA and As2O3. The clinical characteristics of these subjects are shown in Table 1(General data), and no statistically significant differences were found in terms of sex and age distribution or hematological data among different groups. Bone marrow (BM) aspirates for morphological, cytogenetic and molecular analysis were performed at several monitoring points: (i) before any anti-leukemic therapy (pretreatment); (ii) during induction and when clinical and hematological remission was achieved (at CR); (iii) after consolidation and maintenance therapy. Mononuclear cells were isolated from BM samples by Ficoll density centrifugation and were either used immediately or frozen and stored in liquid nitrogen.
Table 1. Clinical data of the patients.
| Treatment options (group)
|
|||
|---|---|---|---|
| ATRA (1) | As2O3 (2) | ATRA/As2O3 (3) | |
| General data | |||
| Cases | 20 | 20 | 21 |
| Sex (M/F) | 12/8 | 9/11 | 12/9 |
| Median age (range, years) | 30.5 (14-74) | 39.5 (15-69) | 34 (14-62) |
| Median WBC (range, × 109/liter) | 3.0 (1.2-49.4) | 2.7 (0.9-40) | 2.1 (0.5-52.6) |
| <2 | 6 | 8 | 7 |
| 2-10 | 10 | 8 | 8 |
| >10 | 4 | 4 | 6 |
| Median hemoglobin (range, g/liter) | 77 (43-123) | 76 (34-105) | 81 (53-120) |
| Median platelet (range, × 109/liter) | 23 (4-76) | 27 (12-72) | 30 (6-73) |
| Median % of blasts and promyelocytes in BM (range) | 86 (31-94) | 87.1 (38-95.5) | 88.5 (70.5-96.5) |
| Treatment outcome | |||
| CR (%) | 19 (95%) | 18 (90%) | 20 (95.2%) |
| Median days to CR (range) | 40.5 (25-65) | 31 (28-38)* | 25.5 (18-35)†‡ |
| Accumulated dosage, mg | |||
| Median(range) of ATRA | 1,230 (750-1,950) | 810 (578-1,200) | |
| Median (range) of As2O3 | 290 (235-380) | 210 (100-300)§ | |
| Chemotherapy administration (%) | 11 (57.9%) | 12 (66.7%) | 14 (70%) |
| Side effects | |||
| Skin reaction | 4 (21%) | 0 | 2 (10%) |
| Dysrhythmia | 0 | 0 | 0 |
| Gastrointestinal reaction | 0 | 1 (5.6%) | 1 (5%) |
| Dryness of mouth | 11 (57.9%) | 2 (11.1%) | 12 (60%) |
| Headache | 4 (21%) | 1 (5.6%) | 2 (10%) |
| Hyperleukocytosis | 10 (52.6%) | 12 (66.7%) | 14 (70%) |
| Liver dysfunction | |||
| Grade 1 | 4 | 7 | 8 |
| Grade 2 | 0 | 2 | 3 |
| Grade 3 | 1 | 2 | 2 |
| Grade 4 | 0 | 0 | 0 |
, P = 0.0233 vs. group 1; †, P = 0.0003 vs. group 1; ‡, P = 0.002 vs. group 2; §, P = 0.046 vs. group 2. The frequencies of side effects were calculated in patients who achieved CR.
Induction Therapy. ATRA was manufactured by the Shanghai 6th Pharmaceutical Company and encapsulated at the pharmacy of Rui Jin Hospital (Shanghai, China). The conventional protocol for APL remission induction with ATRA initiated at SIH in 1986 was oral administration of the drug at 45 mg/m2 per day for 6–8 weeks. Although very effective in inducing CR, this relatively high pharmacological concentration of ATRA was sometimes associated with significant side effects, particularly a clinical entity designated retinoic acid syndrome (RAS) (31). Since the late 1990s, ATRA has been used at an oral dose of 25 mg/m2 per day as a routine treatment for APL patients at SIH, after a study showing that lower dose ATRA had the same therapeutic effect as the conventional dosage but with fewer side effects, including RAS (32). In this study, ATRA was also used with a dosage of 25 mg/m2 per day until CR was achieved. As2O3 was provided by the Yida Pharmaceutical Company of the Harbin Medical University (Harbin, China) and was administered intravenously at a dose of 0.16 mg/kg per day until CR. Subjects in group 3 were given ATRA and As2O3 concurrently with the same doses as mentioned above.
Supportive Care. Serial examinations of whole peripheral blood cell counts (every other day), BM morphological analysis (every 7 to 10 days), and renal and hepatic function tests (every week) were performed during remission induction treatment. Patients were given hydroxyurea (daily doses of 20–40 mg/kg) or IA regimen (idarubicin: 6 mg/m2 per day for 3 days, a dose adapted to the clinical tolerance of most Chinese patients; Ara-C: 100 mg/m2 per day for 3–5 days) when their peripheral white blood cell (WBC) counts were over 10 × 109/liter. Coagulation and fibrinolysis parameters such as fibrinogen, DD dimers, fibrin degradation product (FDP), prothrombin time, and activated partial thromboplastin time (APTT), were monitored to direct the use of low-dose heparin, platelet transfusion, and fresh plasma when necessary. Symptomatic therapy was performed for mild to moderate general side effects of ATRA and/or As2O3. However, hepatic toxicity was given particular caution with the following principle: As2O3 should be decreased to half dose (0.08 mg/kg per day), and symptomatic therapy should be added for those with grade 0–1 liver dysfunction and ought to be withdrawn immediately in the case of grade 2–4, according to Miller et al. (33).
Definition of Clinical Outcomes. Achievement of CR demanded that clinical evidence of APL be absent, untransfused Hb be >100 g/liter, neutrophils be >1.5 × 109/liter, platelets be >100 × 109/liter, and BM morphology reveal normocellularity, with <5% promyelocytes and absence of Auer rod-containing leukemic cells. DFS was defined as the time from CR to relapse, death from any causes, or censoring of the data on the patients.
Consolidation and Maintenance Therapy. After achieving CR, all patients were given three courses of consolidation therapy. Each course included three consecutive regimens, i.e., DA regimen (daunorubicin, 45 mg/m2 per day for 3 days; Ara-C, 100 mg/m2 per day for 7 days), Ara-C “pulse” regimen (Ara-C, 1.5–2.5 g/m2 per day for 3 days), and HA regimen (homoharringtonine, 2–3mg/m2 per day for 3 days; Ara-C, 100 mg/m2 per day for 7 days). Maintenance treatments were different among the three groups, i.e., group 1 (ATRA, 25 mg/m2 per day for 30 days; then 6-mercaptopurine, 100 mg/day for 30 days or 15 mg of methotrexate once a week for 4 weeks), group 2 (As2O3,0.16mg/kg per day for 30 days; then 6-mercaptopurine, 100 mg/day for 30 days or 15 mg of methotrexate once a week for 4 weeks), and group 3 (ATRA 25 mg/m2 per day for 30 days; then As2O3 0.16 mg/kg per day for 30 days; then 6-mercaptopurine, 100 mg/day for 30 days or 15 mg of methotrexate once a week for 4 weeks). The above regimens for maintenance treatments should be applied for five cycles.
Reagents. ATRA and As2O3 for experimental purposes were purchased from Sigma. ATRA was dissolved in PBS, ethanol, and dimethyl sulfoxide as stock solution with different concentrations (10 mM, 1 mM, and 50 μg/ml). As2O3 was dissolved in 1 M NaOH and further diluted to 5 mM in PBS as stock solution. Specific rabbit polyclonal anti-PML antibody was used for Western blot analysis. Anti-PML N-terminal antibody for immunofluorescence analysis was kindly provided by T. Naoe (Nagoya University School of Medicine Branch Hospital, Nagoya, Japan). Horseradish peroxidase-labeled second antibody was purchased from Santa Cruz Biotechnology. CD11b monoclonal antibody and the idiotype control antibody were supplied by Beckman Coulter Company (Sarveille, France). SuperScript II reverse transcriptase and RNase inhibitor used in reverse transcription were purchased from Invitrogen. Taqman PCR core reagent kit used in real-time quantitative RT-PCR and human total RNA for GAPDH standard curve construction were purchased from Perkin–Elmer.
Cell Culture and Treatment. Primary APL cells and NB4 cells (kindly gifted by M. Lanotte, Paris, France) were maintained in RPMI medium 1640 supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, 1 mM l-glutamine, and 10% FBS and were incubated at 37°C in humidified air with 5% CO2. Cells in logarithmic growth were seeded at 5 × 105 cells per ml and were treated with 0.1 μM ATRA and 0.25 μM As2O3 individually or concurrently, each of which being done in triplicate and repeated at least three times. The percentage of viable cells was determined by trypan blue exclusion.
Cytogenetic Analysis. Metaphase chromosomes were prepared and R-banded on unstimulated BM cells after 24 h of culture. Karyotypes were classified according to the International System for Human Cytogenetic Nomenclature (34, 35).
Morphological Study, Nitroblue Tetrazolium (NBT) Analysis, and Flow Cytometry. Cultured cells were centrifuged onto slides and stained with Wright's Giemsa together with peripheral blood smear or BM smear. NBT reduction was performed as described (36). Granulocytic differentiation antigen CD11b was examined by fluorescence-activated cell sorter (FACS) with the standard method.
Immunofluorescence Analysis. Cytospinned slides were fixed with acetone at 4°C for 10 min. The slides were used at once or stored at –80°C for future use. Before use, the slides were first dipped into 1× PBS for 15 min and then stained with fluorescent-labeled anti-PML antibody as described (36).
In Situ Terminal Deoxynucleotidyltransferase Labeling. The labeling reaction was performed on cytospinned slides of cultured cells or BM smears by cell death detecting kit (Roche) according to the manufacturer's recommendation. Positive apoptotic cells were calculated under light microscope observation.
Real-Time Quantitative RT-PCR. Total RNA was extracted from APL samples with TRIzol reagent and was precisely quantified. After reverse transcription, cDNA was used for real-time quantitative RT-PCR with Taqman PCR core reagent kit. Primers and protocols were referred to Gu et al. (37). Results were analyzed on the Macintosh Computer by using the sequence detector 1.6.3 program. The normalized values of the PML-RARα fusion transcript dose (abbreviated as PML-RARα DoseN) of patient samples and control RNA were calculated as follows: (PML-RARα fusion transcript copy number)/(GAPDH transcript copy number) × 1,000 (GAPDH was used for intersample normalization).
Western Blot Analysis. Protein extracts (50 μl) were loaded on a 10% SDS-polyacrylamide gel, electrophoresed, and transferred to a nitrocellulose membrane. Ponceau red staining (0.2%) was used to assure equal protein loading and transfer. After neutralization in 10% defatted milk powder, the membrane was incubated with anti-PML antibody and horseradish peroxidase-labeled second antibody sequentially as reported (38). The immunocomplex was visualized by the enhanced chemiluminescence kit (RPN2108; Amersham Pharmacia Life Science).
Statistical Analysis. Differences of quantitative variables, such as age, peripheral blood cell counts, BM blast cell counts, and copy number, as well as reduction fold of PML-RARα fusion transcripts, were compared with Wilcoxon rank-sum test, whereas the χ2 test (including Fisher's exact test) was used for categorical variables, including sex distribution, rate of toxic effects, relapse frequency, and so on. All statistical analyses were performed with the sas software package (SAS Institute, Cary, NC) with the only exception that Kaplan-Meier DFS survival curves was performed with the spss software package (SPSS, Chicago, IL).
Results
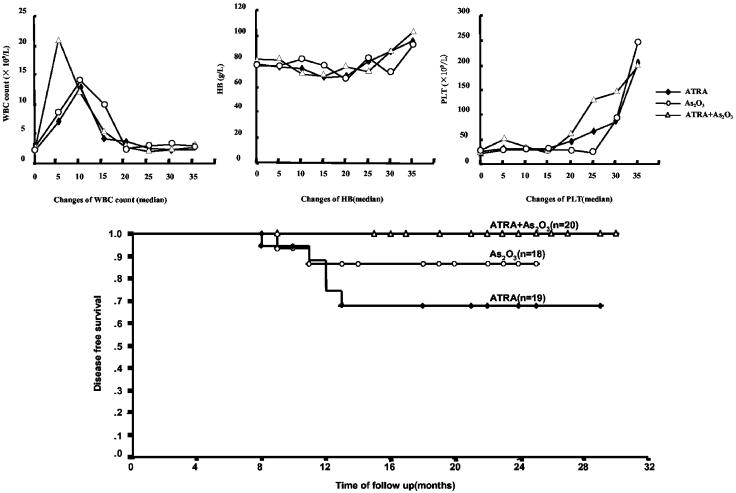
Remission Induction. As shown in Table 1 (Treatment outcome), 19 of 20 patients in group 1, 18 of 20 in group 2, and 20 of 21 in group 3 entered CR. The 4 cases who failed to enter CR all died of intracerebral hemorrhage on day 2, 1, 15, and 8, respectively, indicating that severe bleeding/coagulopathy was still a major contributor to treatment failure and early death. Although CR rates of the three groups were identical (group 1 vs. group 2, P = 0.548; group 1 vs. group 3, P = 0.972; group 2 vs. group 3, P = 0.520), the time to obtain CR showed statistically significant difference among three groups, with median time of 40.5 days (range, 25–65 days) in group 1, 31 days (range, 28–38 days) in group 2, and 25.5 days (range, 18–35 days) in group 3, respectively (group 1 vs. group 2, P = 0.0233; group 1 vs. group 3, P = 0.0003; group 2 vs. group 3, P = 0.0020). These differences could not be attributed to the chemotherapy added during remission induction because no statistical differences were found in the frequencies of patients receiving chemotherapy among the three groups (group 1 vs. group 2, P = 0.582; group 1 vs. group 3, P = 0.431; group 2 vs. group 3, P = 0.825). The above results therefore suggested superiority of combined therapy over mono-therapy on remission induction time. Examination of whole peripheral blood cell counts revealed earlier recovery of normal platelets counts (>100 × 109/liter) in group 3 (median, 22 days) over group 1 (median, 32 days, P = 0.03) as well as group 2 (median, 33 days, P = 0.031) whereas both the recovery time of Hb and that of white blood cell counts were found similar among three groups (Fig. 1 Upper). However, clinically, the time for coagulation and fibrinolysis parameters to return to normal in group 3 (median, 10.4 days; range, 6–19) was only slightly shorter than group 1 (median, 11.7 days; range, 7–22) and group 2 (median, 12.6 days; range, 8–24. Group 1 vs. group 2, P = 0.387; group 1 vs. group 3, P = 0.203; group 2 vs. group 3, P = 0.124). Hyperleukocytosis is a typical side effect occurring after treatment with ATRA and As2O3 (see Fig. 1 Upper Left). However, it is also considered as a sign of response to differentiation therapy in APL. Of note, the white blood cell count peak seemed to appear earlier in group 3 (median, 8.1 days) than in the other two groups (medians of group 1 and group 2: 9.4 days and 10.2 days, respectively), although these differences didn't reach to statistical significance (group 1 vs. group 2, P = 0.788; group 1 vs. group 3, P = 0.441; group 2 vs. group 3, P = 0.596).
Fig. 1.
(Upper) Examination of whole peripheral blood cell counts. Each point on the curves represents the median of the counts of all patients from three groups. (Lower) Kaplan-Meier DFS survival curves. The relapse rate of group 1 is significantly higher than that of group 3 (P = 0.0202, Fisher's exact test). When patients of the two mono-therapy groups are put together, the relapse rate is also statistically higher than that of group 3 (P = 0.038).
Real-Time Quantitative RT-PCR to Monitor the Residual Disease. Table 2 shows the changes of the PML-RARα DoseN at diagnosis, after remission induction, and after the chemotherapy consolidation. As previously reported by us and other groups, there was a big variation of the PML-RARα DoseN among different patients, ranging from 1111.2 to 681692.1. Therefore, we believed that not only the change of calibrated copy number (DoseN) but more importantly the fold change of PML-RARα DoseN in each patient should be taken into consideration while analyzing the data. As expected, no marked differences of PML-RARα DoseN were found among the three groups at diagnosis. When exposed to ATRA and/or As2O3 for 3 weeks (or shortly after CR), the median PML-RARα DoseN decreased quickly. Interestingly, the decrement was much stronger in the combination therapy group than that of the two monotherapy groups (group 1 vs. group 3, P = 0.0092; group 2 vs. group 3, P = 0.041), and group 2 also differed statistically from group 1 (P = 0.013). After chemotherapy consolidation, the PML-RARα DoseN decreased further. Although the median level seemed lower in the combination therapy group than those of the other two groups, a statistically significant difference was observed only between group 1 and group 3 (P = 0.0489). Changes with similar tendency were also observed in fold reduction of PML-RARα DoseN after CR, but the variations suggest deeper statistical implications than those observed at the level of copy number (group 1 vs. group 2, P = 0.0001; group 1 vs. group 3, P = 0.0001; group 2 vs. group 3, P = 0.0079). It is worth noting that the differences in terms of the degree of fold reduction persisted even after consolidation (group 1 vs. group 3, P = 0.018; group 2 vs. group 3, P = 0.036). Of note, the PML-RARα DoseN increased for a short period in some patients during induction therapy, but the peaks of copy number coincided with the peak of hyperleukocytosis and then decreased quickly in parallel to white blood cell counts after further treatment.
Table 2. Results of quantitative real time RT-PCR.
| Treatment options (group)
|
|||
|---|---|---|---|
| Sample collection | ATRA (1) | As2O3 (2) | ATRA/As2O3 (3) |
| Pretreatment | n = 19 | n = 18 | n = 20 |
| Median of copy number (range) | 4,595.6 (1,305.6-531,249) | 6,655.7 (1,777.2-681,692.1) | 5,155.3 (1,111.2-618,050) |
| After CR | n = 19 | n = 18 | n = 20 |
| Median of copy number (range) | 793.5 (37.5-26,680) | 286.3 (19.1-13,543)* | 177.3 (0.7-389.9)†‡ |
| Median of reduction fold (range) | 6.7 (1.1-1152.7) | 32.1 (2.4-993.5)§ | 118.9 (2.2-3,559.0)¶∥ |
| After consolidation | n = 14 | n = 11 | n = 14 |
| Median of copy number (range) | 71.6 (0.39-481.2) | 41.3 (0.39-362.5) | 15.2 (0.03-252.4)** |
| Median of reduction fold (range) | 369.5 (22.2-19683.4) | 521.3 (18.1-46899) | 800 (25.9-523008)††‡‡ |
, P = 0.013 vs. group 1; †, P = 0.0092 vs. group 1; ‡, P = 0.041 vs. group 2; §, P = 0.0001 vs. group 1; ¶, P = 0.0001 vs. group 1; ∥, P = 0.0079 vs. group 2; **, P = 0.0489 vs. group 1; ††, P = 0.018 vs. group 1; ‡‡, P = 0.036 vs. group 2.
Finally, among the 7 patients who developed relapse during 1st CR (see Fig. 1 Lower), their PML-RARα DoseN all increased to a level above 1,000 before the clinical manifestations occurred. Therefore, we confirmed the previous report that PML-RARα DoseN can be considered as a useful tool to predict pending relapse.
DFS. Until the time of the manuscript preparation, the median of follow-up was 18 months (range: 8–30 months). Fig. 1 Lower showed the Kaplan-Meier DFS survival curves. The median DFS were 13 months, 16 months, and 20 months for groups 1, 2, and 3, respectively. Of note, 5 cases in group 1 relapsed within 13 months after CR (5/19, 26.3%). Among these patients, 3 achieved second remission with As2O3-based reinduction therapy whereas the remaining 2 died of intracerebral hemorrhage. In group 2, 2 of 18 (2/18, 11.1%) cases relapsed within 1 year, of whom one obtained a second remission with As2O3 and the other achieved remission with combined As2O3 and ATRA reinduction regimen. None of the subjects in group 3 relapsed till the preparation of the manuscript. Although the follow-up should be continued, there was already a statistically significant difference between group 1 and group 3 (P = 0.0202, Fisher's exact test). A statistical difference in relapse rates was also observed when patients of the two mono-therapy groups were put together and compared with the combination therapy group (P = 0.038). No statistical difference was observed between group 2 and group 3 (P = 0.126). These data are consistent with those of the residual disease burden evaluated with PML-RARα DoseN.
Toxic Effects. Previous studies showed that, in the APL setting, the main side effect of ATRA is the hyperleukocytosis-related retinoic acid syndrome (RAS) whereas that of As2O3 application is liver dysfunction, although RAS-like syndrome can occasionally occur. In the present study, the combination therapy did not increase the frequency of hyperleukocytosis as compared with mono-therapy groups (group 1 vs. group 3, P = 0.557; group 2 vs. group 3, P = 0.825), nor did a difference exist between group 1 and group 2 (P = 0.385) (Table 1, Side effects). All cases with hyperleukocytosis returned to normal when treated with proper chemotherapy, and no one developed the adult respiratory distress syndrome (ARDS). Under close observation, 5 patients in group 1, 11 in group 2, and 13 in group 3 showed liver dysfunction. Among those patients, 19 returned to normal within 1 week, and the rest 10 recovered in 1–2 weeks. No one needed termination of As2O3 therapy because of severe liver lesion. Other side effects, including mouth dryness, headache, skin reactions, and gastrointestinal tract reactions, were mild and were overcome by administration of symptomatic medication.
In Vitro and In Vivo Effects of ATRA/AS2O3 Combination at the Cellular Level. To investigate possible mechanisms of in vivo synergistic effects between ATRA and As2O3, we carried out a series of in vitro studies. As reported previously, granulocytic differentiation revealed by CD11b expression and NBT reduction was increased when NB4 cells were exposed to 0.1 μM of ATRA for 3 days (92.7 ± 19.6% and 56.9 ± 12.9%, as compared with 15.0 ± 4.7% and 10.7 ± 3.9% in the control). In contrast, As2O3 at 0.25 μM displayed only weak effect on these differentiation parameters (29.4 ± 9.1% and 23.4 ± 8.7%). Similar results were observed with primary APL cells (Fig. 4, which is published as supporting information on the PNAS web site). When cells were treated with both drugs, As2O3 (0.25 μM) seemed to neither enhance nor decrease ATRA-induced CD11b expression and NBT reduction (90.3 ± 21.4% and 58.5 ± 15.7%). However, in situ terminal deoxynucleotidyltransferase labeling (also named TUNEL) results revealed more apoptotic cell percentage when NB4 cells or primary APL cells were exposed to the two drugs at the same concentrations for 6 to 10 days, as compared with those treated with As2O3 or ATRA alone (Table 3). BM smears from patients treated with different protocols revealed similar tendencies, in that significantly more apoptotic cells were found after 2 or 3 weeks of treatment in group 3 than in the other two groups (Table 4). Because ATRA is a well known differentiation agent whereas As2O3 may induce either differentiation or apoptosis depending on drug concentration and cellular context, the evident synergistic effect in the induction of apoptosis of APL leukemic cells shed light on the explanation of the synergistic clinical efficacy although the molecular mechanisms involved were not fully elucidated. Similarly, when cells with morphological characteristics of distinct differentiation stages along granulocytic lineage were analyzed, no significant differences were found among the three groups (Table 4). Hence, the dual treatment may promote blast or postmaturation apoptosis.
Table 3. Percentages of TUNEL positive cells in cultured cells.
| Treatment options (group)
|
|||
|---|---|---|---|
| Time after treatment, days | ATRA (1) | As2O3 (2) | ATRA/As2O3 (3) |
| Cultured NB4 cells | |||
| 2 | 1.5 ± 1.0 | 1.5 ± 1.0 | 2.0 ± 0.9 |
| 4 | 2.0 ± 1.0 | 2.5 ± 1.3 | 3.5 ± 2.3 |
| 6 | 2.0 ± 1.5 | 8.5 ± 2.6* | 14.5 ± 2.6† |
| 8 | 4.5 ± 0.9 | 12.0 ± 2.2 | 20.5 ± 4.8‡ |
| 10 | 5.0 ± 1.7 | 14.5 ± 3.1§ | 28.0 ± 5.2¶∥ |
| Cultured primary APL cells | |||
| 2 | 1.0 ± 0.5 | 1.0 ± 0.5 | 1.5 ± 0.9 |
| 4 | 1.3 ± 1.0 | 2.2 ± 1.2 | 3.8 ± 2.0 |
| 6 | 2.2 ± 0.8 | 5.8 ± 1.5 | 11.3 ± 3.5** |
| 8 | 3.8 ± 0.8 | 8.9 ± 2.5 | 17.2 ± 3.4†† |
| 10 | 4.0 ± 1.3 | 12.7 ± 2.4‡‡ | 24.3 ± 5.7§§¶¶ |
, P = 0.025 vs. group 1; †, P = 0.001 vs. group 1; ‡, P = 0.001 vs. group 1; §, P = 0.024 vs. group 1; ¶, P < 0.0001 vs. group 1; ∥, P = 0.02 vs. group 2; **, P = 0.01 vs. group 1; ††, P = 0.002 vs. group 1; ‡‡, P = 0.026 vs. group 1; §§, P < 0.0001 vs. group 1; ¶¶, P = 0.035 vs. group 2.
Table 4. Changes of bone marrow hematology and TUNEL-positive cells in APL patients.
| Treatment options (group)
|
Time after treatment, days
|
||||
|---|---|---|---|---|---|
| Cells | 0 | 13-16 | 19-23 | 30-35 | |
| ATRA (1) | |||||
| TUNEL-positive cells, % | 0 | 1.0 ± 0.5 | 0.5 ± 0.5 | 1.5 ± 1.0 | |
| Cell type, % | |||||
| Myeloblast | 2.6 ± 1.6 | 0.5 ± 0.5 | 0.5 | 2.3 ± 1.8 | |
| Promyelocyte | 81.2 ± 11.4 | 9.8 ± 3.5 | 6.2 ± 6.4 | 6 ± 4.6 | |
| Myelocyte | 4.5 ± 2.2 | 27.7 ± 6.9 | 27.3 ± 15.6 | 11 ± 8.0 | |
| Metamyelocyte | 1.2 ± 0.8 | 20.8 ± 5.3 | 16.3 ± 11.0 | 10.5 ± 7.5 | |
| Band | 1.2 ± 0.8 | 3.5 ± 2.1 | 7.3 ± 2.4 | 10.2 ± 4.0 | |
| Segmented | 4.5 ± 2.8 | 9 ± 5.8 | 12.3 ± 10.8 | ||
| Erythroid lineage | 3.5 ± 2.4 | 27.5 ± 11.5 | 21 ± 15.7 | 30.7 ± 5.9 | |
| Lymphoid lineage | 4.6 ± 1.5 | 7 ± 5.6 | 8.8 ± 9.4 | 9.3 ± 7.1 | |
| As2O3 (2) | |||||
| TUNEL-positive cells, % | 0 | 6.8 ± 2.2* | 12.6 ± 3.7† | 0.5 ± 0.5 | |
| Cell type, % | |||||
| Myeloblast | 3.0 ± 1.5 | 0.5 | 1 ± 0.7 | 0.7 ± 0.3 | |
| Promyelocyte | 78.2 ± 9.7 | 8.7 ± 6.1 | 4.3 ± 2.2 | 3.6 ± 1.8 | |
| Myelocyte | 5.4 ± 2.7 | 18.5 ± 5.2 | 19.3 ± 2.2 | 11.8 ± 4.7 | |
| Metamyelocyte | 2.5 ± 1.4 | 11.5 ± 6.1 | 13.4 ± 4.4 | 8.2 ± 3.6 | |
| Band | 0.6 ± 0.5 | 8.5 ± 3.1 | 14.6 ± 4.8 | 12.2 ± 3.1 | |
| Segmented | 3.2 ± 1.3 | 13.4 ± 4.9 | 11.4 ± 9.5 | ||
| Erythroid lineage | 4.5 ± 3.2 | 28.7 ± 4.3 | 25.3 ± 6.1 | 29.6 ± 10.6 | |
| Lymphoid lineage | 3.8 ± 2.2 | 14.2 ± 6.2 | 10.4 ± 6.1 | 15.6 ± 6.1 | |
| ATRA/As2O3 (3) | |||||
| TUNEL-positive cells, % | 0 | 16.3 ± 4.3‡§ | 25.4 ± 4.1¶∥ | 0.8 ± 0.5 | |
| Cell type, % | |||||
| Myeloblast | 2.8 ± 1.4 | 2.6 ± 2.0 | 0.5 | 1 ± 0.4 | |
| Promyelocyte | 82.8 ± 9.1 | 6.3 ± 6.1 | 2.8 ± 1.8 | 3 ± 0.7 | |
| Myelocyte | 3.8 ± 1.5 | 33.9 ± 11.7 | 6.8 ± 1.8 | 10.3 ± 4.2 | |
| Metamyelocyte | 2.0 ± 0.8 | 19.5 ± 10.8 | 21 ± 20.8 | 9.4 ± 4.8 | |
| Band | 1.0 ± 0.8 | 4.3 ± 3.4 | 13.5 ± 6.3 | 12.9 ± 2.9 | |
| Segmented | 7.9 ± 8.9 | 6.7 ± 2.1 | 14.6 ± 2.8 | ||
| Erythroid lineage | 2.2 ± 1.2 | 12.4 ± 9.4 | 36.8 ± 34.4 | 27.8 ± 3.5 | |
| Lymphoid lineage | 5.5 ± 3.6 | 8.4 ± 3.1 | 11.5 ± 9.3 | 16.2 ± 2.4 | |
, P = 0.034 vs. group 1; †, P = 0.001 vs. group 1; ‡, P < 0.0001 vs. group 1; §, P = 0.036 vs. group 2; ¶, P < 0.0001 vs. group 1; ∥, P = 0.021 vs. group 2.
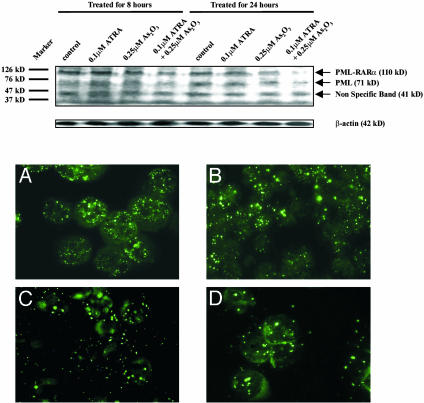
Examination of the PML-RARα Fusion Protein. Western blot analysis with anti-PML antibody was performed on fresh APL cells as well as NB4 cells after incubation with ATRA or As2O3 alone or in combination for different time courses. As shown in Fig. 2 Upper, after 8 h of incubation, As2O3 could weakly induce degradation of PML-RARα fusion protein, and the oncoprotein decreased more considerably when cells were treated with both drugs. At 24 h, the oncoprotein in cells exposed to two drugs was almost undetectable whereas the amount of PML-RARα was markedly reduced under incubation with As2O3 alone. These results indicated a synergistic effect between the two drugs in triggering degradation of the fusion protein. Restoration of the nuclear body (NB) structure was demonstrated to be the direct consequence of the degradation of the oncoprotein (25, 26, 38). As shown in Fig. 2 Lower, a large number of micropunctuates revealed by PML staining interspersed in the nucleus and cytoplasm of untreated primary APL cells. After 8 h of treatment with ATRA and/or As2O3, the micropunctuates were decreased in number whereas large PML speckles characteristic of normal NB appeared. Of note, the modulation of the PML staining patterns was more significant in cells treated by ATRA/As2O3 combination than that in cells treated by a single drug. NB4 cells revealed similar results (data not shown).
Fig. 2.
(Upper) Western blot analysis of PML-RARα fusion protein in cultured NB4 cells. Note that the intensity of the band corresponding to PML-RARα (arrow, 110 kDa) decreased on cotreatment with ATRA/As2O3 within 8 h. After 24 h of incubation, this band was almost undetectable in the eighth lane, and obvious degradation was observed in cells treated with 0.25 μM As2O3. (Lower) Immunofluorescence analysis of the subcellular localization of PML-RARa/PML in cultured fresh APL cells(original magnification ×1,000). (A) Untreated; (B) 8 h after 0.1 μM ATRA treatment; (C) 8 h after 0.25 μM As2O3 treatment; (D) 8 h after cotreatment with ATRA/As2O3 at the same concentrations.
Discussion
The major purpose of this study was to further improve the quality of the CR in the APL disease model by a knowledge-based, rational use of currently available therapeutic means. The results supported our assumption based on both theoretical and experimental evidence (19–22, 39, 40) because the ATRA/As2O3 combination obtained a quicker clinical response, a better reduction of disease burden, and 100% DFS rate over a follow-up of 8–30 months (median 18 months), showing a striking similarity of the results in patients and in mice with regard to disease eradication. Although the frequency of chemotherapy usage in the combination group during remission induction was slightly higher than that in the mono-therapy groups, a lack of statistical significance was not in favor of the point of view that additional chemotherapy might account for the superior results. It is obvious that our result, despite being highly promising, needs further confirmation in larger series and over longer time of follow-up. Nevertheless, in this case, two selective differentiation/apoptosis inducers showed cooperative effect in a combined induction and consolidation therapy protocol in a reasonable sample size of newly diagnosed human leukemia, which may represent an extension of the well established concept of cancer polychemotherapy since the 1960s (41).
Three points may be worth mentioning with regard to the clinical effect of ATRA/As2O3 combination therapy. First, in our hands, the beneficial effects were observed in a group of newly diagnosed APL, in contrast to a recent report on a trial of ATRA/As2O3 among relapsed APL patients where no obvious superiority was shown in the clinical outcome as compared with As2O3 monotherapy (42). Because most relapsed patients lost sensitivity to ATRA due to previous exposure, it would be hard to expect a full play of the synergism between ATRA and As2O3 in those patients. Second, ATRA/As2O3 induced earlier recovery of platelet counts, despite the fact that no obvious differences in the time course of recovery of coagulopathy were found among three groups. It is well known that the low platelet level in APL results from repression of the normal hematopoiesis and increased consumption/destruction level because of the coagulopathy. It is thus likely that combination therapy relieves the repression of hematopoiesis more significantly than mono-therapy (5, 43). Third, the side effects of combination therapy could be well controlled. Previous work showed that liver lesion was the most important toxic effect of As2O3 in newly diagnosed APL (14). However, in this work, neither liver toxicity nor general side effects were aggravated by the addition of ATRA. Moreover, the total dosage of the arsenic used in the combination group was lower than that in the arsenic mono-therapy group (Table 1, Treatment outcome). It is thus possible that the long-term side effects of arsenic could be even decreased in the combination group.
Unlike the previously used minimal residual disease (MRD) monitoring system (44), we used real-time quantitative RT-PCR, which was optimized in our laboratory as well as others to detect PML-RARα fusion transcripts to compare the effectiveness of distinct treatment protocols (37, 45, 46), and found that the beneficial effect in reducing tumor burden by combination therapy not only existed after remission induction, but also persisted after chemotherapy consolidation (Table 2). Even stronger evidence to support the superiority of ATRA/As2O3 combination therapy over mono-therapy is that the enhanced disease burden reduction as a result of combined treatment was translated into a better DFS curve. Therefore, we believe that chemotherapy is indispensable and that the ATRA/As2O3/chemotherapy triad could be the best choice at this moment.
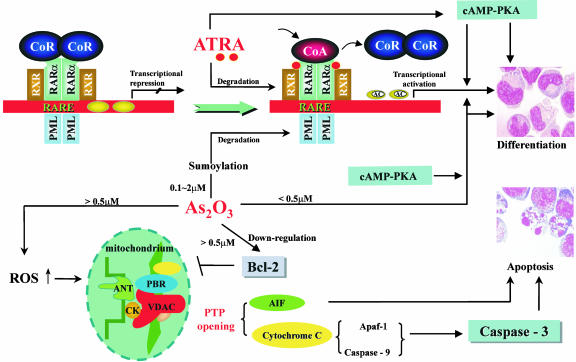
In this study, we also explored the possible cellular and molecular mechanisms of synergism between ATRA and As2O3. Although previous reports on the combined in vitro effects of the two drugs were controversial (17, 20, 47), we found no obvious inhibitory action of As2O3 on ATRA-mediated differentiation in our culture system. It has been demonstrated that, through decreasing mitochondrial transmembrane potentials (Δψm), opening mitochondrial permeability transition pore (PTP), and releasing cytochrome c and other apoptosis-inducing proteins, As2O3 induced apoptosis of APL cells as well as other cancer cells. It may be interesting to note that the voltage-dependant anion channel (VDAC), a component of the protein complex regulating PTP, was the target of As2O3 (48). The recently illustrated PML-dependent effect provides another mechanism of As2O3-induced apoptosis (49). In this study, ATRA displayed a slight apoptotic effect on APL cells in vivo and in vitro. However, it strengthened As2O3-mediated apoptosis even when As2O3 was used at low concentration (0.25 μM). The synergism in apoptosis not only provided a possible reason for superior clinical effects of the two drugs, but also suggested a paradigm shift from the previously established differentiation model to a combined differentiation/apoptosis induction model of APL therapy. At the molecular level, the chimeric PML-RARα protein has been shown to play a key role in the pathogenesis of APL through interaction with nuclear receptor corepressors, with abnormally high affinity leading to deacetylation of histones and transcription repression of target genes. In addition, PML-RARα, like the wild-type RARα, can form a complex with retinoid X receptor (RXR). Overexpression of PML-RARα may thus sequester RXR and abrogate RAR/RXR signaling. Both differentiation and apoptosis processes of granulocytes were thus interfered, and only pharmacological concentrations of ATRA (0.1–1 μM) could reverse these pathological processes by dissociating corepressor and recruiting coactivator (CoA), which possesses histone acetylase activity. Induction of PML-RARα sumoylation and subsequent proteolysis through proteosome system by As2O3 may relieve the transcriptional repression, which could be further enhanced by the cAMP/PKA pathway (Fig. 3) (39, 50–57). The results in this study indicated that the more rapid degradation of the fusion protein should underlie more profound and prompt reprogramming of APL cells. Large-scale transcriptome analysis of APL cells under ATRA and As2O3 may soon yield a better understanding of the possible synergistic pathways not necessarily affected by one drug individually.
Fig. 3.
Potential mechanisms of effects of ATRA/As2O3 on APL cells. ATRA induces differentiation in two major pathways. First, ATRA disassociates corepressor from the PML-RARα/RXR complex and recruits CoA, leading to transcription of target genes. Secondly, ATRA regulates the cAMP-PKA signaling pathway, which may also lead to the transcription activation and finally the differentiation of APL cells. Besides, ATRA could modulate and degrade the PML-RARα oncoprotein as well. As2O3 exerts dose-dependent dual effects. Low dose (<0.5 μM) of As2O3 mediates differentiation of APL cells, which may cross-talk with PKA-cAMP-signaling pathway(s) and RAR/RXR signaling pathway(s) and may ultimately facilitate histone acetylation. On the other hand, a high dose of As2O3 initiates apoptosis by means of decreasing mitochondrial transmembrane potentials (Δψm), opening mitochondrial permeability transition pore (PTP) and releasing cytochrome c and other pre-apoptotic factors. It is of note that As2O3 can trigger the modulation and/or degradation of PML-RARα oncoprotein under a wide range of dosage. →, stimulation; ┤, inhibition; AIF, apoptosis-inducing factor; ANT, adenosine nucleotide translocator; Apaf-1, apoptosis-activating factor-1; CK, cytokinin; CoA, coactivator; CoR, corepressor; VDAC, voltage-dependent anion channel; PBR, peripheral benzidiazepine receptor; PTP, permeability transition pore; PKA, protein kinase A; ROS, reactive oxygen species; AC, acetylation.
The successful implementation of the ATRA/As2O3 synergistic targeting therapy model represents a molecular triumph over an otherwise lethal human disease (Fig. 3). It has been over three decades since Leo Sachs first postulated the possibility of inducing leukemic blast cells to more mature or terminal blood cells through a normal differentiation process (3). Now, we know that differentiation induction is, after all, a mechanism by which key factors in differentiation arrest, growth advantage, and even apoptosis de-regulation could be targeted. The APL model also revived arsenic compounds, which, like a double-edged sword, have been used both as poison and as drug to treat a wide range of diseases since ancient times, particularly in traditional Chinese medicine (TCM). The potential of arsenic compounds in cancer therapy could be well beyond APL (58). To this end, it may be worth pointing out that the combined effects of ATRA and As2O3 in the induction and maintenance therapy for APL also mark a good example of the integration of oriental and occidental medicines, with As2O3 as an ancient TCM and ATRA as a synthesized compound of recent decades. Finally, it is important to strengthen the key role chemotherapy plays in the overall success of the therapy. It is our hope that the triad use of ATRA, As2O3, and chemotherapy will make APL a curable human acute leukemia in the end.
Supplementary Material
Acknowledgments
We acknowledge all members of the Shanghai Institute of Hematology and the State Key Laboratory of Medical Genomics for their support. This work was supported in part by the Chinese National Basic Research Program (973), National Natural Science Foundation of China, Chinese National High Tech Program (863), Shanghai Municipal Commission for Science and Technology, Shanghai Leading Academic Discipline, and the Pôle Sino-Français en Sciences du Vivant et en Génomique.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 29, 2003.
Abbreviations: APL, acute promyelocytic leukemia; ara-C, cytosine arabinoside; DFS, disease-free survival; ATRA, all-trans retinoic acid; CR, complete remission; PML-RARα, promyelocytic leukemia-retinoic acid receptor α; BM, bone marrow; SIH, Shanghai Institute of Hematology; NBT, nitroblue tetrazolium; RXR, retinoid X receptor; WBC, white blood cell; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
See accompanying Biography on page 5325.
References
- 1.Brown, D., Kogan, S., Lagasse, E., Weissman, I., Alcalay, M., Pelicci, P. G., Atwater, S. & Bishop, J. M. (1997) Proc. Natl. Acad. Sci. USA. 94, 2551–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avvisati, G., Petti, M. C., Lo-Coco, F., Vegna, M. L., Amadori, S., Baccarani, M., Cantore, N., Di Bona, E., Ferrara, F. & Fioritoni, G., et al. (2002) Blood 100, 3141–3146. [DOI] [PubMed] [Google Scholar]
- 3.Paran, M., Sachs, L., Barak, Y. & Resnitzky, P. (1970) Proc. Natl. Acad. Sci. USA 67, 1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breitman, T. R., Collins, S. J. & Keene, B. R. (1981) Blood 57, 1000–1004. [PubMed] [Google Scholar]
- 5.Huang, M. E., Ye, Y. C., Chen, S. R., Chai, J. R., Lu, J. X., Zhoa, L., Gu, L. J. & Wang, Z. Y. (1988) Blood 72, 567–572. [PubMed] [Google Scholar]
- 6.Castaigne, S., Chomienne, C., Daniel, M. T., Ballerini, P., Berger, R., Fenaux, P. & Degos, L. (1990) Blood 76, 1704–1709. [PubMed] [Google Scholar]
- 7.Warrell, R. P., Jr., Frankel, S. R., Miller, W. H., Jr., Scheinberg, D. A., Itri, L. M., Hittelman, W. N., Vyas, R., Andreeff, M., Tafuri, A., Jakubowski, A., et al. (1991) N. Engl. J. Med. 324, 1385–1393. [DOI] [PubMed] [Google Scholar]
- 8.Fenaux, P., Chevret, S., Guerci, A., Fegueux, N., Dombret, H., Thomas, X., Sanz, M., Link, H., Maloisel, F., Gardin, C., et al. (2000) Leukemia 14, 1371–1377. [DOI] [PubMed] [Google Scholar]
- 9.Wang, Z. Y., Sun, G. L., Shen, Z .X., Chen, S. J. & Chen, Z. (1999) Chin. Med. J. 112, 963–967. [PubMed] [Google Scholar]
- 10.Tallman, M. S., Andersen, J. W., Schiffer, C. A., Appelbaum, F. R., Feusner, J. H., Woods, W. G., Ogden, A., Weinstein, H., Shepherd, L., Willman, C., et al. (2002) Blood 100, 4298–4302. [DOI] [PubMed] [Google Scholar]
- 11.Hu, J., Shen, Z. X., Sun, G. L., Chen, S. J., Wang, Z. Y. & Chen, Z. (1999) Int. J. Hematol. 70, 248–260. [PubMed] [Google Scholar]
- 12.Shen, Z. X., Chen, G. Q., Ni, J. H., Li, X. S., Xiong, S. M., Qiu, Q. Y., Zhu, J., Tang, W., Sun, G. L., Yang, K. Q., et al. (1997) Blood 89, 3354–3360. [PubMed] [Google Scholar]
- 13.Soignet, S. L., Maslak, P., Wang, Z. G., Jhanwar, S., Calleja, E., Dardashti, L. J., Corso, D., DeBlasio, A., Gabrilove, J., Scheinberg, D. A., et al. (1998) N. Engl. J. Med. 339, 1341–1348. [DOI] [PubMed] [Google Scholar]
- 14.Niu, C., Yan, H., Yu, T., Sun, H. P., Liu, J. X., Li, X. S., Wu, W., Zhang, F. Q., Chen, Y., Zhou, L., et al. (1999) Blood 94, 3315–3324. [PubMed] [Google Scholar]
- 15.Shen, Y., Shen, Z. X., Yan, H., Chen, J., Zeng, X. Y., Li, J. M., Li, X. S., Wu, W., Xiong, S. M., Zhao, W. L., et al. (2001) Leukemia 15, 735–741. [DOI] [PubMed] [Google Scholar]
- 16.Lazo, G., Kantarjian, H., Estey, E., Thomas, D., O'Brien, S. & Cortes, J. (2003) Cancer 97, 2218–2224. [DOI] [PubMed] [Google Scholar]
- 17.Shao, W., Fanelli, M., Ferrara, F. F., Riccioni, R., Rosenauer, A., Davison, K., Lamph, W. W., Waxman, S., Pelicci, P. G., Lo Coco, F., et al. (1998) J. Natl. Cancer Inst. 90, 124–133. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy, G. A., Marlton, P., Cobcroft, R. & Gill, D. (2000) Br. J. Haematol. 111, 1103–1105. [DOI] [PubMed] [Google Scholar]
- 19.Lallemand-Breitenbach, V., Guillemin, M. C., Janin, A., Daniel, M. T., Degos, L., Kogan, S. C., Bishop, J. M. & de Thé, H. (1999) J. Exp. Med. 189, 1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jing, Y., Wang, L., Xia, L., Chen, G. Q., Chen, Z., Miller, W. H. & Waxman, S. (2001) Blood 97, 264–269. [DOI] [PubMed] [Google Scholar]
- 21.Visani, G., Piccaluga, P. P., Martinelli, G., Rossi, M., Malagola, M. & Baccarani, M. (2003) Haematologica 88, ELT15. [PubMed] [Google Scholar]
- 22.Au, W. Y., Chim, C. S., Lie, A. K., Liang, R. & Kwong, Y. L. (2002) Br. J. Haematol. 117, 130–132. [DOI] [PubMed] [Google Scholar]
- 23.Zhao, Y. Z., Li, H. Q., Li, D. P., Li, R., Qi, J. Y., Wan, C. C., Zhou, C. L., Wang, Z. Q. & Qian, L. S. (2003) Chin. J. Hematol. 24, 32–34. [PubMed] [Google Scholar]
- 24.Yoshida, H., Kitamura, K., Tanaka, K., Omura, S., Miyazaki, T., Hachiya, T., Ohno, R. Y Naoe, T. (1996) Cancer Res. 56, 2945–2948. [PubMed] [Google Scholar]
- 25.Raelson, J. V., Nervi, C., Rosenauer, A., Benedetti, L., Monczak, Y., Pearson, M., Pelicci, P. G. & Miller, W. H., Jr. (1996) Blood 88, 2826–2832. [PubMed] [Google Scholar]
- 26.Nervi, C., Ferrara, F. F., Fanelli, M., Rippo, M. R., Tomassini, B., Ferrucci, P. F., Ruthardt, M., Gelmetti, V., Gambacorti-Passerini, C., Diverio, D., et al. (1998) Blood 92, 2244–2251. [PubMed] [Google Scholar]
- 27.Zhu, J., Koken, M. H., Quignon, F., Chelbi-Alix, M. K., Degos, L., Wang, Z. Y., Chen, Z. & de The, H. (1997) Proc. Natl. Acad. Sci. USA 94, 3978–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu, J., Gianni, M., Kopf, E., Honore, N., Chelbi-Alix, M., Koken, M., Quignon, F., Rochette-Egly, C. & de Thé, H. (1999) Proc. Natl. Acad. Sci. USA 96, 14807–14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu, J., Lallemand-Breitenbach, V. & de Thé, H. (2001) Oncogene 20, 7257–7265. [DOI] [PubMed] [Google Scholar]
- 30.Jaffe, E. S., Harris, N. L., Stein, H. & Vardiman, J. W., eds. (2001) Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues (IARC Press, Lyon), pp. 75–107.
- 31.Tallman, M. S., Andersen, J. W., Schiffer, C. A., Appelbaum, F. R., Feusner, J. H., Ogden, A., Shepherd, L., Willman, C., Bloomfield, C. D., Rowe, J. M., et al. (1997) N. Engl. J. Med. 337, 1021–1028. [DOI] [PubMed] [Google Scholar]
- 32.Chen, G. Q., Shen, Z. X., Wu, F., Han, J. Y., Miao, J. M., Zhong, H. J., Li, X. S., Zhao, J. Q., Zhu, J., Fang, Z. W., et al. (1996) Leukemia 10, 825–828. [PubMed] [Google Scholar]
- 33.Miller, A. B., Hoogstraten, B., Staquet, M. & Winkler, A. (1981) Cancer 47, 207–214. [DOI] [PubMed] [Google Scholar]
- 34.ISCN (1978) Cytogenet. Cell Genet. 21, 309–409. [DOI] [PubMed] [Google Scholar]
- 35.ISCN (1995) Guidelines for Cancer Cytogenetics (Karger, Basel).
- 36.Zhu, J., Shi, X. G., Chu, H. Y., Tong, J. H., Wang, Z. Y., Naoe, T., Waxman, S., Chen, S. J. & Chen, Z. (1995) Leukemia 9, 302–309. [PubMed] [Google Scholar]
- 37.Gu, B. W., Hu, J., Xu, L., Yan, H., Jin, W. R., Zhu, Y. M., Zhao, W. L., Niu, C., Cao, Q., Su, X. Y., et al. (2001) Hematol. J. 2, 330–340. [DOI] [PubMed] [Google Scholar]
- 38.Chen, G. Q., Shi, X. G., Tang, W., Xiong, S. M., Zhu, J., Cai, X., Han, Z. G., Ni, J H., Shi, G. Y., Jia, P. M., et al. (1997) Blood 89, 3345–3353. [PubMed] [Google Scholar]
- 39.Zhu, J., Chen, Z., Lallemand-Breitenbach, V. & De Thé, H. (2002) Nat. Rev. Cancer 2, 705–714. [DOI] [PubMed] [Google Scholar]
- 40.Fang, J., Chen S. J., Tong, J. H., Wang, Z. G., Chen, G. Q. & Chen, Z. (2002) Cancer Biol. Ther. 1, 614–620. [DOI] [PubMed] [Google Scholar]
- 41.Thompson, I., Hall, T. C. & Moloney, W. C. (1965) N. Engl. J. Med. 273, 1302–1307. [DOI] [PubMed] [Google Scholar]
- 42.Raffoux, E., Rousselot, P., Poupon, J., Daniel, M. T., Cassinat, B., Delarue, R., Taksin, A. L., Rea, D., Buzyn, A., Tibi, A., et al. (2003) J. Clin. Oncol. 21, 2326–2334. [DOI] [PubMed] [Google Scholar]
- 43.Zhu, J., Guo, W. M., Yao, Y. Y., Zhao, W. L., Pan, L., Cai, X., Ju, B., Sun, G. L., Wang, H. L., Chen, S. J., et al. (1999) Leukemia 13, 1062–1070. [DOI] [PubMed] [Google Scholar]
- 44.Huang, W., Sun, G. L., Li, X. S., Cao, Q., Lu, Y., Jang, G. S., Zhang, F. Q., Chai, J. R., Wang, Z. Y., Waxman, S., et al. (1993) Blood 82, 1264–1269. [PubMed] [Google Scholar]
- 45.Gallagher, R. E., Yeap, B. Y., Bi, W., Livak, K. J., Beaubier, N., Rao, S., Bloomfield, C. D., Appelbaum, F. R., Tallman, M. S., Slack, J. L., et al. (2003) Blood 101, 2521–2528. [DOI] [PubMed] [Google Scholar]
- 46.Schnittger, S., Weisser, M., Schoch, C., Hiddemann, W., Haferlach, T. & Kern, W. (2003) Blood 102, 2746–2755. [DOI] [PubMed] [Google Scholar]
- 47.Gianni, M., Koken, M. H., Chelbi-Alix, M. K., Benoit, G., Lanotte, M., Chen, Z. & de Thé, H. (1998) Blood 91, 4300–4310. [PubMed] [Google Scholar]
- 48.Zheng, Y. H., Shi, Y., Tian, C. H., Jiang, C. S., Jin, H. J., Chen, J. J., Almasan, A., Tang, H. & Chen, Q. (2004) Oncogene 23, 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puccetti, E., Beissert, T., Guller, S., Li, J. E., Hoelzer, D., Ottmann, O. G. & Ruthardt, M. (2003) Oncogene 22, 6900–6908. [DOI] [PubMed] [Google Scholar]
- 50.Lin, R. J., Nagy, L., Inoue S., Shao, W., Miller, W. H., Jr. & Evans, R. M. (1998) Nature 391, 811–814. [DOI] [PubMed] [Google Scholar]
- 51.Grignani, F., De Matteis, S., Nervi, C., Tomassoni, L., Gelmetti, V., Cioce, M., Fanelli, M., Ruthardt, M., Ferrara, F. F., Zamir, I., et al. (1998) Nature 391, 815–818. [DOI] [PubMed] [Google Scholar]
- 52.Wang, Z. G., Delva, L., Gaboli, M., Rivi, R., Giorgio, M., Cordon-Cardo, C., Grosveld, F. & Pandolfi, P. P. (1998) Science 279, 1547–1551. [DOI] [PubMed] [Google Scholar]
- 53.Cheng, G. X., Zhu, X. H., Men, X. Q., Wang, L., Huang, Q. H., Jin, X. L., Xiong, S. M., Zhu, J., Guo, W. M., Chen, J. Q., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 6318–6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu, Q., Zhang, J. W., Zhu, H. Q., Shen, Y. L., Flexor, M., Jia, P. M., Yu, Y., Cai, X., Waxman, S., Lanotte, M., et al. (2002) Blood 99, 1014–1022. [PubMed] [Google Scholar]
- 55.Zhao, Q., Tao, J., Zhu, Q., Jia, P. M., Dou, A. X., Li, X., Cheng, F., Waxman, S., Chen, G. Q., Chen, S. J., et al. (2004) Leukemia, 18, 285–292. [DOI] [PubMed] [Google Scholar]
- 56.Chen, Z. & Wang, Z. Y. (2003) in Treatment of Acute Leukemias: New Directions for Clinical Research, Current Clinical Oncology, ed. Pui, C.-H. (Humana, Totowa, NJ), pp. 291–308.
- 57.Lallemand-Breitenbach, V., Zhu, J., Puvion, F., Koken, M., Honore, N., Doubeikovsky, A., Duprez, E., Pandolfi, P. P., Puvion, E., Freemont, P., et al. (2001) J. Exp. Med. 193, 1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen, Z., Chen, G. Q., Shen, Z. X., Sun, G. L., Tong, J. H., Wang, Z. Y. & Chen, S. J. (2002) Semin. Hematol. 39, 22–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.