Abstract
IL-5 plays important roles in eosinophil differentiation, expansion, and recruitment. The regulation of IL-5 seems critical for the treatment of eosinophil-mediated allergic reactions. However, the precise mechanisms for IL-5 regulation remain unknown. In this study, we investigated how IL-5 production is regulated. The transduction of GATA-3 into a murine T cell hybridoma resulted in acquiring the ability to produce IL-5 in response to an antigenic stimulus like Th2 cells. This production was dependent on the cAMP-PKA pathway, but not on p38 activation. Transduction of NIK largely impaired IL-5 production. RelA and RelB similarly impaired IL-5 production. RelA decreased not only IL-5 protein amount but mRNA. RelA also inhibited Il5-luciferase reporter activity. The transduction of GATA-3 decreased the expression of Tbx21 and Eomes, but the additional transduction of RelA abrogated the decreased expression of GATA-3-induced Tbx21 and Eomes. Furthermore, the transduction of T-bet or Eomes into the GATA-3-transduced T cell hybridoma impaired IL-5 production. These results suggested that strong enhancement of the NFκB pathway downregulates IL-5 production and upregulates T-box protein expression to shift an immune response from Th2 to inflammatory Th1.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-013-9585-z) contains supplementary material, which is available to authorized users.
Keywords: IL-5, GATA-3, NFκB, Rel, T-bet, Eomes
Introduction
Helper T cells (Th) are classified into at least 3 types: T helper 1 (Th1), Th2, and Th17. Th1 cells produce IFN-γ and evoke immunity against intracellular pathogens. Th2 cells produce IL-4, IL-5, and IL-13 and participate in immunity against parasites and allergic responses. Th17 cells produce IL-17A, IL-17F, and IL-22 and induce immunity against extracellular pathogens and certain autoimmune diseases. Th differentiation and functions are tightly controlled by specific transcription factors. Th1 differentiation is favored by T-bet (Szabo et al. 2000), Th2 is enhanced by GATA-3 (Zheng and Flavell 1997), and Th17 is controlled by RORγ (Ivanov et al. 2006).
IL-5 was initially identified as a mouse B cell growth and differentiation factor, and subsequent studies revealed that IL-5 plays important roles in the differentiation, function, and recruitment of eosinophils (Rothenberg and Hogan 2006; Kouro and Takatsu 2009; Takatsu et al. 2009). IL-5 is produced by Th2 cells, Tc2 cells, mast cells, eosinophils, NK cells, NKT cells, and natural helper cells/innate lymphoid cells (Mosmann and Coffman 1989; Tominaga et al. 1988; Desreumaux et al. 1992; Warren et al. 1995; Sakuishi et al. 2007; Moro et al. 2010; Ikutani et al. 2012; Kuraoka et al. 2004). The expression of IL-5 is induced by bt2cAMP, a chemical analogue of cAMP (Snijdewint et al. 1993; Lee et al. 1993). Activation of adenylate cyclase by cAMP leads to protein kinase A (PKA) activation, resulting in IL-5 production in a murine T cell line, EL4, and some other types of cells (Lee et al. 1993). Physiological stimuli such as TCR stimulation induce IL-5 expression in Th2 cells. Recently, IL-33, the IL-1 family cytokine was shown to robustly induce IL-5 (Schmitz et al. 2005). p38 upregulates IL-5 production in a murine Th2 clone (Chen et al. 2000) and in human T cells (Mori et al. 1999). cAMP elevates the p38 level and p38 phosphorylates GATA-3 (Chen et al. 2000). A large amount of IL-5 production seems to be restricted to highly differentiated cells, such as repeatedly induced Th2 cells (Islam et al. 2011). IL-5 production is regulated by GATA-3 (Zhang et al. 1997), a transcription factor that is induced by the IL-4-Stat6 signaling pathway (Ouyang et al. 1998). GATA-3 regulates not only the generation of T cells but also Th2 differentiation (Zheng and Flavell 1997). GATA-3 participates in the remodeling of chromatin at Th2 cytokine gene loci (Ansel et al. 2006; Wilson et al. 2009), which may induce IL-4 expression. IL-5 expression is directly transactivated at the Il5 promoter region by GATA-3, whereas IL-4 expression does not necessarily require GATA-3 (Zhang et al. 1997; Zhang et al. 1998; Ranganath et al. 1998; Lee et al. 2000). On the other hand, mechanisms for the downregulation of IL-5 have not been completely elucidated. Only T-box transcription factor has been reported; Eomes downregulates IL-5 expression in memory Th2 cells (Endo et al. 2011).
In this study, we examined the regulation mechanism of IL-5 production. We show that Gata3 transduction into a T cell hybridoma led to IL-5 production in a cAMP-PKA pathway-dependent manner. We also show that high levels of Rel proteins reduce the Gata3-induced IL-5 production and induce T-box protein in Th2-like T cells.
Materials and methods
Mice
OT-II mice transgenic for chicken ovalbumin 323–339 (cOVA323-339)-specific I-Ab-restricted T cell receptor (Barnden et al. 1998) were provided by Dr William R. Heath (The Walter and Eliza Hall Institute of Medical Research, Parkville VIC, Australia) through Dr. Nakayama (Chiba University, Chiba, Japan). Mice were housed under specific-pathogen free conditions in Laboratory Animal Research Center, Dokkyo Medical University (Mibu, Japan) and were handled according to the Guidelines for the Care and Use of Laboratory Animal Research Center, Dokkyo Medical University (protocol #0663).
Cells and cell culture
DO11.10 T cell hybridoma (Haskins et al. 1983) was maintained in RPMI 1640 (Wako, Osaka, Japan) containing 5 % FCS (Equitech-Bio, Kerrville, TX, USA) supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA). Cells were stimulated with immobilized anti-CD3 mAb (10 μg/ml; 145-2C11 (BioLegend, San Diego, CA, USA)) or the combination of 0.01–1 mM bt2cAMP (Sigma-Aldrich), 10 ng/ml PMA (Sigma-Aldrich), and 0.5 μM ionomycin (Merck-Millipore, Billerica, MA, USA). For some experiments, 10 μM PKA inhibitor H-89 (Sigma-Aldrich) or 10 μM p38 inhibitor SB203580 (Merck-Millipore) was added. OT-II splenocytes were stimulated with 1 μM cOVA323-339 supplemented with 10 ng/ml rhIL-2 (PeproTech, Rocky Hill, NJ, USA).
Gene transduction
Murine Gata3, Tbx21, Eomes, Rel, Rela and Relb, and the wild-type and loss-of-function forms of human NIK (MAP3K14) were obtained by RT-PCR. IκBαM, a suppressor mutant of human IκBα (NFKAIA) in pMX-IG (Inami et al. 2004) was a gift from Dr. T. Nakayama, Chiba University. Full-length cDNA was inserted into pMXs, pMXs-IRES-EGFP (pMXs-IG, kindly provided by Dr. T. Kitamura, University of Tokyo) (Nosaka et al. 1999), or pMXs-IRES-human nerve growth factor receptor (hNGFR, kindly provided by Dr. T. Nakayama, Chiba University) (Shinnakasu et al. 2008). Human NFATc1 cDNA (Northrop et al. 1994) was inserted into pEGFP-C1, and the EGFP-hNFATc1 region was inserted into pMXs. PLAT-E cells (kindly provided by Dr. T. Kitamura (Morita et al. 2000)) were transfected with retroviral vector (kindly provided by Dr. T. Kitamura (Nosaka et al. 1999)) using FuGENE HD (Roche (Basel, Switzerland) or Promega (Fitchburg, WI, USA)) and the supernatant at day 2 was mixed with the cell suspension in the presence of 8 μg/ml polybrene (Sigma-Aldrich). OT-II CD4+ cells stimulated for 1 day were transduced using PLAT-E supernatant on RetroNectin (Takara, Otsu, Japan)-coated wells by incubation for over night. Cells were washed and expanded in the presence of 10 ng/ml rhIL-2 for 2–4 days.
Flow cytometry
Fluorochrome-conjugated mAbs were purchased from BioLegend or eBioscience (San Diego, CA, USA) unless otherwise described. hNGFR was stained by biotinylated anti-hNGFR mAb (C40-1457, BD Biosciences, Franklin Lakes, NJ, USA) followed by streptavidin-PE or -APC (BioLegend). For intracellular cytokine staining, cells were fixed with 4 % paraformaldehyde (WAKO, Osaka, Japan), permeabilized with 0.1 % saponin, stained with anti-IL-5-PE, anti-IL-4-PE, anti-IL-13-PE, or anti-IFN-γ-PE or –APC. Cells were analyzed on a FACSCalibur (BD Biosciences) using the FlowJo software (TreeStar). In some experiments, cells were sorted on a FACSAria (BD Biosciences).
ELISA
IL-5 concentration in culture supernatant of DO11.10 T cell hybridoma was determined by sandwich ELISA using specific mAbs (TRFK5 (capture antibody) and TRFK4 (antibody for detection), BioLegend).
RT-Quantitative-PCR (RT-qPCR)
Total RNA was prepared with ISOGEN (Nippon Gene, Tokyo, Japan) and converted to cDNA using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA). qPCR was performed then using Thunderbird SYBR qPCR mix (Toyobo, Japan, Tokyo) on an ABI5700 (Invitrogen). Specific primers are listed in a table in the Supplementary Methods section. Data were normalized to Actb and Hprt.
Luciferase assay
DO11.10 T cell hybridoma were electroporated with 20 μg of 3.6 kb Il5 promoter-inserted pGL4 and 0.5 μg of phRL-TK (thymidine kinase promoter-driven Renilla luciferase expression vector for internal control) (Promega, Tokyo, Japan) at 250 V, 975 μF on a Gene Pulser II (BioRad, Hercules, CAn USA). Cells were left unstimulated or stimulated with 10 ng/ml PMA (Sigma-Aldrich), 0.5 μg/ml ionomycin (Sigma-Aldrich) and 1 mM bt2cAMP (Sigma-Aldrich) for 6 h, and harvested. Luciferase activities were measured by Dual Luciferase Reporter Assay System (Promega) according to the manufacturer’s protocol.
Results
GATA-3 induces IL-5 production in T cell hybridoma
Because GATA-3 is known as a master regulator of Th2 cytokines, we initially tried to investigate IL-4 production mechanisms by the transduction of Gata3. Retroviral transduction of Gata3 bicistronically expressing egfp into DO11.10 T cell hybridoma, however, did not lead to IL-4 production (data not shown). Interestingly, it did lead to IL-5 production in response to PMA/ionomycin/bt2cAMP stimulus (Fig. 1a), which supports the findings of previous reports (Zhang et al. 1998; Ranganath et al. 1998; Lee et al. 2000). Gata3 transduction into 2B4 T cell hybridoma or OT-II CD4+ T cells similarly led to IL-5 production to a lesser extent (Supplementary Figure S1a). On the other hand, retroviral transduction of Tbx21, a master regulator of Th1 cytokines, into DO11.10 T cell hybridoma resulted in IFN-γ production (Supplementary Figure S2). Gata3- and Tbx21-transduced DO11.10 cells seemed similar to Th2-like and Th1-like cells, respectively, according to the cytokines produced. In order to investigate what molecule regulates IL-5 production, DO11.10 was transduced with pMXs-Gata3, without any marker such as EGFP, and then cloned and selected for IL-5 production.
Fig. 1.

Gata3 transduction leads to IL-5 production in T cell hybridoma. a DO11.10 T cell hybridoma was retrovirally transduced with control pMXs-IG or pMXs-Gata3-IG (Gata3), and IL-5 production in response to PMA/ionomycin/bt2cAMP was measured by intracellular staining. b–d Gata3-transduced DO11.10 T cell hybridoma was treated with various stimuli (b), with fixed doses of PMA + ionomycin and various concentrations of bt2cAMP (c), or with PMA + ionomycin + bt2cAMP in the absence or presence of H89 or SB203580 (d), and IL-5 production was measured by intracellular staining. d Gata3-transduced DO11.10 T cell hybridoma was stimulated as in (d), except without the addition of inhibitors, and IL-5, IL-4, and IL-13 were measured. Numbers in figures indicate mean fluorescence intensity (MFI). Data are representative of 2 (c–e) or 3 (a, b) independent experiments
PMA and, ionomycin alone did not induce IL-5 production (Fig. 1b and data not shown, respectively), but bt2cAMP alone led to IL-5 production from the Gata3-transduced DO11.10 hybridoma. PMA upregulated and PMA plus ionomycin maximized the bt2cAMP-induced production of IL-5. The production of IL-5 induced by bt2cAMP was dose dependent, and it reached the maximum when 0.3 mM bt2cAMP was added (Fig. 1c). Previous reports demonstrated that IL-5 production is regulated by the cAMP-PKA pathway (Lee et al. 1993; Klein-Hessling et al. 2003) or induced by p38 activation (Mori et al. 1999; Chen et al. 2000). As expected, the addition of H89, a PKA inhibitor, impaired IL-5 production, but the addition of SB203580, a p38 inhibitor, did not affect the production (Fig. 1d). These data confirmed that bt2cAMP-induced production of IL-5 by DO11.10 was mediated by PKA. The production of Th2-type cytokines, IL-4, IL-5, and IL-13, are relatively linked. The production of IL-13 was similarly detected, but that of IL-4 was not detected (Fig. 1e).
NFκB pathway downregulates IL-5 production in T cell hybridoma
In order to identify the key modulator(s) for IL-5 production, signaling molecules were tested by retroviral transductions. According to the transcription factor-binding search (http://www.cbrc.jp/research/db/TFSEARCH.html), many AP-1 recognition sites are present in the Il5 promoter. Thus, the MAPK pathway was first examined. Transduction of wild-type or a gain-of-function Xenopus MEK (map2k1) did not alter IL-5 production (Supplementary Figure S3a). GATA3 and NFAT are both implicated in IL-5 gene transcription (Rao et al. 1997; Zhang et al. 1997; Zhang et al. 1999), and an NFAT-binding site exists in the Il5 promoter region (Mordvinov and Sanderson 2001). Thus, EGFP-fused human NFATc1 was retrovirally transduced to Gata3-transduced DO11.10 and the IL-5 production was examined. However, IL-5 production in response to bt2cAMP and the combination with PMA was comparable to the EGFP control transduction (Supplementary Figure S3b). The transcription factor-binding search also showed NFκB binding sites near the Il5 transcription initiation site. Human NIK was retrovirally transduced to Gata3-transduced DO11.10 bicistronically with EGFP. The transduction efficiency of NIK was relatively low, but EGFP+ cells showed drastically decreased IL-5 production (Fig. 2a). On the other hand, loss-of-function human NIK slightly decreased IL-5 production (Supplementary Figure S4). A gain-of-function human IκBα (constitutively attenuating the NFκB pathway) transduction slightly decreased IL-5 production. These results imply that attenuation of the NFκB pathway enhances, but a complete shutdown of the NFκB pathway impairs, IL-5 production.
Fig. 2.

NFκB activation impairs IL-5 production. a Gata3-transduced DO11.10 T cell hybridoma was retrovirally transduced with pMXs-IG (control) or pMXs-human NIK-IG (MAP3K14), and IL-5 production in response to bt2cAMP, bt2cAMP + PMA, bt2cAMP + ionomycin, or bt2cAMP PMA + ionomycin + bt2cAMP was measured by intracellular staining. EGFP+ cells were gated (1st column) and analyzed for IL-5 production (2nd to 5th columns). b Gata3-transduced DO11.10 T cell hybridoma was retrovirally transduced with pMXs-IG (control), pMXs-c-Rel-IG (Rel), pMXs-RelA-IG (Rela), or pMXs-RelB-IG (Relb), and IL-5 production in response to anti-CD3 mAb or PMA + ionomycin + bt2cAMP was measured by intracellular staining. EGFP+ cells were gated (1st column) and analyzed for IL-5 production (2nd and 3rd columns). c OT-II CD4+ T cells were retrovirally transduced with Gata3 (bicistronically with hNGFR) together with control (pMXs-IG) or Rela. hNGFR+ (GATA-3+) and IL-5 production in response to PMA + ionomycin + bt2cAMP was measured by intracellular staining. EGFPhi cells were gated and analyzed. Histograms show IL-5 production with isotype control staining (left). Bars show mean fluorescence intensity (MFI) + SD of triplicate wells with a statistical difference (Student’s t test) (right). Numbers indicate MFI (a, b) and percentages of IL-5 positive cells (c). Data are representative of at least 2 independent experiments
Next, the Rel family molecules were tested. Murine c-Rel (Rel), RelA (Rela), or RelB (Relb) was transduced to Gata3-transduced DO11.10. Although the transduction level of Rel and the suppressive effects were relatively low, all Rel-related molecules tested showed impaired IL-5 production against both anti-CD3 and PMA/ionomycin/bt2cAMP stimuli (Fig. 2b). When 2B4 used, the inhibition of IL-5 production by Rela or Relb was similarly observed, although IL-5 production levels were lower (Supplementary Figure S5). We also observed that double transduction of Gata3 and Rela substantially lowered the IL-5 production from OT-II CD4+ T cells (Fig. 2c).
RelA transcriptionally inhibits IL-5 expression
Next, we examined whether the inhibition caused by Rela transduction happened at a transcriptional level. Control (pMXs-IG) or RelA (Rela) was transduced to Gata3-transduced DO11.10, sorted for EGFP+ and expanded. The cells were stimulated with PMA + ionomycin + bt2cAMP and IL-5 protein amount in the culture supernatant was measured by ELISA. The IL-5 amount produced by Rela-transduced cells was drastically lower compared to control transduction. (Fig. 3a). Then, the cells were stimulated to examine Il5 transcript by qPCR. Similarly, the Il5 transcript level was severely impaired in Rela-transduced cells compared to pMXs-IG transduced cells (control) (Fig. 3b). Furthermore, Il5 transcription was studied using a 3.6 kb Il5 promoter linked to luciferase. The cells were transfected with the reporter plasmid and stimulated. As expected luciferase activity of Rela-transduced cells was lower compared to the control transduction (Fig. 3c). These results demonstrate that RelA inhibits IL-5 production at a transcriptional level.
Fig. 3.
Rel transcriptionally suppresses IL-5 expression. Gata3-transduced DO11.10 T cell hybridoma was retrovirally transduced with pMXs-IG (control) or pMXs-RelA-IG (Rela), and IL-5 production in response to PMA + ionomycin + bt2cAMP was measured by ELISA (a), Il5 transcript by RT-qPCR (b), and Il5 promoter activity by luciferase assay (c). Il5 transcript was normalized by Hprt. Il5 promoter activity was normalized by Renilla luciferase activity. Bars show Mean + SD of triplicate samples. *p < 0.05 (Student’s t test). Data are representative of 2 independent experiments
RelA upregulates T-box protein expression
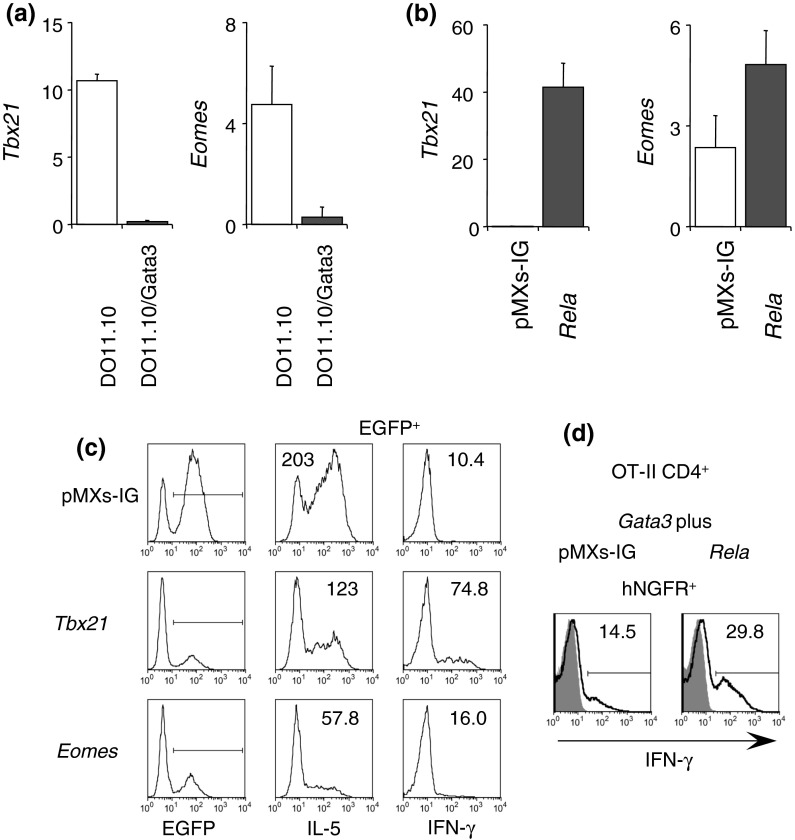
Because the NFκB pathway downregulated IL-5 but upregulated IFN-γ production, we hypothesized that GATA-3 downregulates Rel molecules. However, all Rel, Rela, and Relb levels were comparable or rather enhanced in Gata3-transduced DO11.10 and Th2 cells as compared to non-transduced DO11.10 and Th1 cells, respectively (Supplementary Figure S6). This finding suggested that Rel indirectly regulates IL-5 expression. It is well known that T-bet and GATA-3 reciprocally regulate the level of the other one (Usui et al. 2003, 2006). GATA-3 also suppressed Eomes expression (Yagi et al. 2010). Thus, T-box protein expression in Gata3-transduced DO11.10 was examined. As expected, Tbx21 and Eomes levels were severely decreased in Gata3-transduced DO11.10 (Fig. 4a). Then, we investigated whether Rel increases T-box protein expression. Transduction of Rela considerably enhanced Tbx21 and Eomes expression (Fig. 4b). To test whether these T-box proteins impair IL-5 production, Tbx21 or Eomes were transduced into Gata3-transduced DO11.10 cells. Transduction of Tbx21 moderately impaired the IL-5 production, whereas the production was severely impaired by Eomes (Fig. 4c). At the same time, Tbx21 and Eomes induced IFN-γ production. However, knockdown of Eomes or Tbx21 did not alter the decreased IL-5 production (Supplementary Figure S7). These results suggest that Rel does not simply induce T-box family protein to directly inhibit IL-5 production. We also examined IFN-γ production in GATA-3-expressing cells. Rela transduction resulted in enhanced IFN-γ production in GATA-3-expressing OT-II CD4+ T cells (Fig. 4d). These results suggested that strong NFκB signals favors Th1 responses in T cells.
Fig. 4.
Rel transduction restores T-box molecule expression, which in turn decrease IL-5 expression. a Gata3 transduction decreased T-box molecule expression. Tbx21 (left) and Eomes (right) mRNA levels in Gata3-transduced DO11.10 T cell hybridoma were measured by RT-qPCR. b Gata3-transduced DO11.10 T cell hybridoma was retrovirally transduced with pMXs-IG (control) or pMXs-RelA-IG (Rela), sorted for EGFP+, and Tbx21 and Eomes mRNA levels were measured by RT-qPCR. c Gata3-transduced DO11.10 T cell hybridoma was retrovirally transduced with pMXs-IG (control), pMXs-Tbx21-IG (Tbx21), or pMXs-Eomes-IG (Eomes), and IL-5 and IFN-γ production were measured by intracellular staining as described in Fig. 1. EGFP+ cells were gated (1st column) and analyzed for IL-5 and IFN-γ production (2nd and 3rd column, respectively). Numbers in histograms indicate MFI. d OT-II CD4+ T cells were retrovirally transduced with Gata3 (bicistronically with hNGFR) together with Empty (pMXs-IG) or Rela and IFN-γ production in response to PMA + ionomycin + bt2cAMP was measured by intracellular staining. hNGFR+ (GATA-3+) and EGFP+ cells were gated and analyzed. Numbers indicate percentages of IFN-γ+ cells. Data are representative of 2 independent experiments
Discussion
We demonstrated that Rel molecules downregulate IL-5 production by upregulating T-box protein expression. Previous reports have demonstrated that Rel supports the Th1 response. c-Rel-deficient mice have slight symptoms of myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis, and MOG-immunized T cells result in normal IL-4 production but impaired IFN-γ production (Hilliard et al. 2002). c-Rel-deficient T cells also showed impaired IFN-γ production against Toxoplasma antigen (Mason et al. 2004). RelB-deficient CD4+ T cells have defects in Th1 differentiation (Corn et al. 2005). RelA-deficiency has a mild effect on Th1 differentiation, but RelA-deficient mice are susceptible to Leishmania major infection (Mise-Omata et al. 2009). A previous report has suggested that RelA regulates T-bet function through heterodimerization of these proteins (Hwang et al. 2005a). On the other hand, NFκB1 (p50/p105) is reciprocally important for IL-5 production. p50-deficient T cells show impaired IL-5 production (Barnes and Karin 1997; Yang et al. 1998; Das et al. 2001). This impaired production is caused by the fact that p50 deficiency impairs GATA-3 expression (Das et al. 2001). NFκB family proteins dimerize to exert their function. Rel proteins have a transactivation domain, whereas p50 and p52 do not. Constituents of the dimers could be important irrespective of whether the outcome is activation or inhibition of gene expression.
From our results, it seems that Rel does not directly regulate IL-5 production, but indirectly modulates IL-5 expression by upregulating T-box proteins. T-bet is induced under Th1-inducing conditions (Szabo et al. 2000). Eomes is induced by Runx3 (Yagi et al. 2010). The functions of T-box proteins and GATA-3 are reciprocal (Usui et al. 2003; Usui et al. 2006). T-bet downregulates GATA-3 expression (Usui et al. 2006) as well as the binding of GATA-3 to DNA (Szabo et al. 2000; Hwang et al. 2005b). Similarly, Eomes regulates GATA-3 binding to the Il5 promoter by direct binding through the T-box region (Endo et al. 2011). It is possible that Rel protein-induced T-box proteins inhibit GATA-3 binding to DNA. Putative NFκB binding sites are present at approximately −7.2, −6.5, −6.4, −5.2, −2.8, −1.7, and 0.5 kb and −9.7, −8.4, −6.8, −6.6, and −0.1 kb in the Tbx21 and Eomes promoters, respectively. Thus, Rel protein may regulate Tbx21 and Eomes. However, knockdown either of Eomes or Tbx21 did not restore IL-5 production. Either of them might be enough to suppress IL-5 production. Also, the mechanisms by which Rel induces T-bet and Eomes need to be elucidated.
Our data do not exclude the possibility that IL-5 induction does not require the Rel family molecules. Transduction of the loss-of-function NIK lowered the IL-5 production (Supplementary Figure S4), suggesting that diminished NFκB activation hampers the IL-5 expression. p50 plays an essential role in IL-5 production (Barnes and Karin 1997; Yang et al. 1998; Das et al. 2001). Diminished activation of NFκB activation including p50 may decrease IL-5 production. Our data suggested that neither strong NFAT- nor strong MAPK- pathway activation is required for IL-5 production. However, our data also do not exclude the possibility that NFAT and MAPK proteins are not required for IL-5 production. NFAT-binding sites are present in the Il5 promoter region, and previous data demonstrate that NFAT plays important roles in IL-5 production (De Boer et al. 1999). Certain amounts of these proteins would be sufficient for IL-5 expression.
Our data demonstrate that Rel molecules downregulate IL-5 production. This seems to conflict with a previous report showing that activation of the NFκB pathway enhances IL-5 production (Inami et al. 2004). This enhancement was mediated by a locus controlled by histone hyperacetylation of the Il5 locus. In the report, NFκB molecule(s) involved in this enhancement were not identified. There is the possibility that this can be attributed to the presence of p50 and/or p52. Our data suggest that Rel effects on the production of IL-5 are indirect. It is still possible that the NFκB pathway controls accessibility to the gene locus during functional development of T cells and modulates expression after functional maturation.
The regulation of IL-5 is thought to be important for eosinophilic allergic responses. Thus far, the specific regulation of IL-5 is only mediated by mAb. Although the precise mechanisms of IL-5 production have to be clarified, our data shed light on new targets for IL-5 regulation.
Electronic supplementary material
Acknowledgments
We thank to Dr. T. Kitamura, Institute of Medical Science, the University of Tokyo, for providing the retroviral vector pMXs-IG and packaging cell, PLAT-E; Dr. T. Nakayama, Chiba University, for providing IκBαM, the repressor mutant of IκBα and the retroviral vector pMXs-IRES-hNGFR and for transferring OT-II mice; Dr. William R. Heath, The Walter and Eliza Hall Institute of Medical Research, for providing the OT-II mice; Ms. Y. Nonaka for cell sorting; Ms. Y. Nitta for secretary assistance. This study was in part supported by a Grant-in-aid by the Japan Society for the Promotion of Science (24580196) and by a grant from the Minato Seki Foundation.
References
- Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Nuclear Factor-κB—a pivotal transcription factor in chronic inflammatory diseases. New Eng J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Chen CH, Zhang DH, LaPorte JM, Ray A. Cyclic AMP activates p38 mitogen-activated protein kinase in Th2 cells: phosphorylation of GATA-3 and stimulation of Th2 cytokine gene expression. J Immunol. 2000;165:5597–5605. doi: 10.4049/jimmunol.165.10.5597. [DOI] [PubMed] [Google Scholar]
- Corn RA, Hunter C, Liou HC, Siebenlist U, Boothby MR. Opposing roles for RelB and Bcl-3 in regulation of T-box expressed in T cells, GATA-3, and Th effector differentiation. J Immunol. 2005;175:2102–2110. doi: 10.4049/jimmunol.175.4.2102. [DOI] [PubMed] [Google Scholar]
- Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NF-κB in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- De Boer ML, Mordvinov VA, Thomas MA, Sanderson CJ. Role of nuclear factor of activated T cells (NFAT) in the expression of interleukin-5 and other cytokines involved in the regulation of hemopoetic cells. Int J Biochem Cell Biol. 1999;31:1221–1236. doi: 10.1016/S1357-2725(99)00069-2. [DOI] [PubMed] [Google Scholar]
- Desreumaux P, Janin A, Colombel JF, Prin L, Plumas J, Emilie D, Torpier G, Capron A, Capron M. Interleukin 5 messenger RNA expression by eosinophils in the intestinal mucosa of patients with coeliac disease. J Exp Med. 1992;175:293–296. doi: 10.1084/jem.175.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Iwamura C, Kuwahara M, Suzuki A, Sugaya K, Tumes DJ, Tokoyoda K, Hosokawa H, Yamashita M, Nakayama T. Eomesodermin controls interleukin-5 production in memory T helper 2 cells through inhibition of activity of the transcription factor GATA3. Immunity. 2011;35:733–745. doi: 10.1016/j.immuni.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Haskins K, Kubo R, White J, Pigeon M, Kappler J, Marrack P (1983) The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med 157:1149–1169 [DOI] [PMC free article] [PubMed]
- Hilliard BA, Mason N, Xu L, Sun J, Lamhamedi-Cherradi SE, Liou HC, Hunter C, Chen YH. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J Clin Invest. 2002;110:843–850. doi: 10.1172/JCI0215254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, Hong JH, Glimcher LH. IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J Exp Med. 2005;202:1289–1300. doi: 10.1084/jem.20051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, Takatsu K. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol. 2012;188:703–713. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- Inami M, Yamashita M, Tenda Y, Hasegawa A, Kimura M, Hashimoto K, Seki N, Taniguchi M, Nakayama T. CD28 costimulation controls histone hyperacetylation of the interleukin 5 gene locus in developing Th2 cells. J Biol Chem. 2004;279:23123–23133. doi: 10.1074/jbc.M401248200. [DOI] [PubMed] [Google Scholar]
- Islam SA, Chang DS, Colvin RA, Byrne MH, McCully ML, Moser B, Lira SA, Charo IF, Luster AD. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ TH2 cells. Nat Immunol. 2011;12:167–177. doi: 10.1038/ni.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγ directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Klein-Hessling S, Jha MK, Santner-Nanan B, Berberich-Siebelt F, Baumruker T, Schimpl A, Serfling E. Protein kinase A regulates GATA-3-dependent activation of IL-5 gene expression in Th2 cells. J Immunol. 2003;170:2956–2961. doi: 10.4049/jimmunol.170.6.2956. [DOI] [PubMed] [Google Scholar]
- Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21:1303–1309. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- Kuraoka M, Hashiguchi M, Hachimura S, Kaminogawa S. CD4−c-kit−CD3ε−IL-2Rα+ Peyer’s patch cells are a novel cell subset which secrete IL-5 in response to IL-2: implications for their role in IgA production. Eur J Immunol. 2004;34:1920–1929. doi: 10.1002/eji.200324696. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Koyano-Nakagawa N, Naito Y, Nishida J, Arai N, Arai K-i, Takashi Yokota. cAMP activates the IL-5 promoter synergistically with phorbol ester through the signaling pathway involving protein kinase A in mouse thymoma line EL-4. J Immunol. 1993;151:6135–6142. [PubMed] [Google Scholar]
- Lee HJ, Takemoto N, Kurata H, Kamogawa Y, Miyatake S, O’Garra A, Arai N. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J Exp Med. 2000;192:105–115. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason NJ, Liou HC, Hunter CA. T cell-intrinsic expression of c-Rel regulates Th1 cell responses essential for resistance to Toxoplasma gondii. J Immunol. 2004;172:3704–3711. doi: 10.4049/jimmunol.172.6.3704. [DOI] [PubMed] [Google Scholar]
- Mise-Omata S, Kuroda E, Sugiura T, Yamashita U, Obata Y, Doi TS. The NF-κB RelA subunit confers resistance to Leishmania major by inducing nitric oxide synthase 2 and Fas expression but not Th1 differentiation. J Immunol. 2009;182:4910–4916. doi: 10.4049/jimmunol.0800967. [DOI] [PubMed] [Google Scholar]
- Mordvinov VA, Sanderson CJ. Regulation of IL-5 expression. Arch Immunol Ther Exp. 2001;49:345–351. [PubMed] [Google Scholar]
- Mori A, Kaminuma O, Miyazawa K, Ogawa K, Okudaira H, Akiyama K. p38 mitogen-activated protein kinase regulates human T cell IL-5 synthesis. J Immunol. 1999;163:4763–4771. [PubMed] [Google Scholar]
- Morita S, Kojima T, Kitamura T (2000) Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther 7:1063–1066 [DOI] [PubMed]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Northrop JP, Ho SN, Chen L, Thomas DJ, Timmerman LA, Nolan GP, Admon A, Crabtree GR. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- Nosaka T, Kawashima T, Misawa K, Ikuta K, Mui AL, Kitamura T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999;18:4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/S1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- Ranganath S, Ouyang W, Bhattarcharya D, Sha WC, Grupe A, Peltz G, Murphy KM. GATA-3-dependent enhancer activity in IL-4 gene regulation. J Immunol. 1998;161:3822–3826. [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Sakuishi K, Oki S, Araki M, Porcelli SA, Miyake S, Yamamura T. Invariant NKT cells biased for IL-5 production act as crucial regulators of inflammation. J Immunol. 2007;179:3452–3462. doi: 10.4049/jimmunol.179.6.3452. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Shinnakasu R, Yamashita M, Kuwahara M, Hosokawa H, Hasegawa A, Motohashi S, Nakayama T. Gfi1-mediated stabilization of GATA3 protein is required for Th2 cell differentiation. J Biol Chem. 2008;283:28216–28225. doi: 10.1074/jbc.M804174200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–5329. [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/S0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Takatsu K, Kouro T, Nagai Y. Interleukin 5 in the link between the innate and acquired immune response. Adv Immunol. 2009;101:191–236. doi: 10.1016/S0065-2776(08)01006-7. [DOI] [PubMed] [Google Scholar]
- Tominaga A, Matsumoto M, Harada N, Takahashi T, Kikuchi Y, Takatsu K. Molecular properties and regulation of mRNA expression for murine T cell-replacing factor/IL-5. J Immunol. 1988;140:1175–1181. [PubMed] [Google Scholar]
- Usui T, Nishikomori R, Kitani A, Strober W. GATA-3 suppresses Th1 development by downregulation of Stat4 and not through effects on IL-12Rβ2 chain or T-bet. Immunity. 2003;18:415–428. doi: 10.1016/S1074-7613(03)00057-8. [DOI] [PubMed] [Google Scholar]
- Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream JH, O’Shea JJ, Strober W. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203:755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren HS, Kinnear BF, Phillips JH, Lanier LL. Production of IL-5 by human NK cells and regulation of IL-5 secretion by IL-4, IL-10, and IL-12. J Immunol. 1995;154:5144–5152. [PubMed] [Google Scholar]
- Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- Yagi R, Junttila IS, Wei G, Urban JF, Jr, Zhao K, Paul WE, Zhu J. The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity. 2010;32:507–517. doi: 10.1016/j.immuni.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor κB in the induction of eosinophilia in allergic airway inflammation. J Exp Med. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- Zhang DH, Yang L, Ray A. Differential responsiveness of the IL-5 and IL-4 genes to transcription factor GATA-3. J Immunol. 1998;161:3817–3821. [PubMed] [Google Scholar]
- Zhang DH, Yang L, Cohn L, Parkyn L, Homer R, Ray P, Ray A. Inhibition of allergic inflammation in a murine model of asthma by expression of a dominant-negative mutant of GATA-3. Immunity. 1999;11:473–482. doi: 10.1016/S1074-7613(00)80122-3. [DOI] [PubMed] [Google Scholar]
- Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/S0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.