Abstract
Bone marrow (BM) has been considered as a reservoir of stem/progenitor cells which are able to differentiate into ectodermal, endodermal, and mesodermal origins in vitro as well as in vivo. Following adequate stimulation, such as granulocyte stimulating factor (G-CSF) or AMD3100, BM resident stem/progenitor cells (BMSPCs) can be mobilized to peripheral blood. Several host-related factors are known to participate in this mobilization process. In fact, a significant number of donors are resistant to G-CSF induced mobilization protocols. AMD3100 is currently used in combination with G-CSF. However, information regarding host-related factors which may influence the AMD3100 directed mobilization is extremely limited. In this study, we were to get some more knowledge on the host-related factors that affect the efficiency of AMD3100 induced mobilization by employing in vivo mobilization experiments. As a result, we found that C57BL/6J mice are more sensitive to AMD3100 but less sensitive to G-CSF which promotes the proliferation of BMSPCs. We excluded S1P as one of the host related factor which influences AMD3100 directed mobilization because pre-treatment of S1P receptor antagonist FTY720 did not inhibit BMSPC mobilization. Further in vitro experiments revealed that BALB/c mice, compared to C57BL/6J mice, have less BMSPCs which migrate in response to host related factors such as sphingosine-1-phosphate (S1P) and to CXCL12. We conclude that AMD3100-directed mobilization depends on the number of BMSPCs rather than on the host-related factors. These results suggest that the combination of AMD3100 and G-CSF is co-operative and is optimal for the mobilization of BMSPCs.
Keywords: Bone marrow stem/progenitor cells, Mobilization, AMD3100, G-CSF, Host-related factors
Bone marrow (BM) is a privileged tissue where stem/progenitor cells (SPCs) that can be used for cellular therapy reside. BM has been considered as a reservoir of hematopoietic stem/progenitor cells (HSPCs), which have the potential to differentiate into all myeloid and lymphoid cell lineages in vitro and can reconstitute the entire hematopoietic systems following transplantation in vivo [1]. More recently, BM resident non-hematopoietic SPCs also have been identified. These BMSPCs are able to differentiate into other cell types including endothelial progenitor cells (EPCs) [2], contributing to the neovascularization of ischemic tissues; and mesenchymal stem cells (MSCs) [3], which are able to differentiate into many cells of ectodermal, endodermal, and mesodermal origins in vitro as well as in vivo. In fact, adult BMSPCs are known to participate in recovery from chemical diabetes through neogenesis of insulin-producing cells. They home efficiently to the injured islets, differentiate to produce insulin, and restore almost euglycemic levels through contribution of insulin-producing cells [4].
BMSPCs can be forced out of the BM to circulate into the peripheral blood, following adequate stimulation, a phenomenon called "mobilization". Mobilization is preferred to BM aspiration for collection of BMSPCs because of the advantages such as a relatively safe procedure to collect transplantable SPCs from the donor [5]. Mobilized SPCs are harvested by apheresis from the peripheral blood so that they can be concentrated, enriched, and stored for transplantation.
On the other hand, the number of available SPCs is a critical issue for successful cell therapy, such as tissue regeneration and hematopoietic recovery [6]. In fact, the dose of SPCs infused correlates directly with the speed of hematopoietic recovery and the overall survival of patients after transplantation [7].
Granulocyte colony stimulating factor (G-CSF) was the most frequently used clinical drug that may efficiently direct the SPCs out of the BM after a few consecutive daily injections [8]. Nevertheless, a significant number of donors are resistant to G-CSF mobilization [9,10] because several host-related factors also participate in the process of BMSPC mobilization. This is the reason why new pro-mobilizing compounds are tested as being used alone or in combination with G-CSF.
One such compound is AMD3100, which blocks the interaction between CXCR4 and CXCL12 that is crucial for the retention of PCs in BM. AMD3100 induces the release of stem/progenitor cells from BM niches into the blood stream within 1 h in experimental animals after injection [11,12]. Recently, it has been reported that AMD3100 induced mobilization depends on sphnigosine-1-phosphate (S1P) [13]. However, information regarding host-related factors which may influence AMD3100 directed mobilization is extremely limited.
In this study, we evaluated which of host-related factors influence the efficiency of AMD3100 induced mobilization by employing an in vivo mobilization experiment with C57BL/6J and BALB/c mice.
Materials and Methods
Animals
C57BL/6J mice and BALB/c mice were purchased from Orient-Bio (Korea) and adopted at 1-2 weeks and were used for experiments at ages8 to 12 weeks. Mice were housed in a SPF state under adequate temperature (23±3℃) and relative humidity (55±5%) control with a 12h light/12h dark cycle, and provided with free access to food and water. Animal studies were approved by the Animal Care and Use Committee of the Institute of Laboratory Animal Resources, Seoul National University (SNU-121112-1). All efforts were made to minimize animal suffering as well as the number of animals used.
Mobilization
Mice were injected subcutaneously with 5 mg/kg of AMD3100. For G-CSF mobilization, mice were injected 250 g/kg of human G-CSF (Amgen, Thousand Oaks, CA, USA, http://www.amgen.com) daily for 6 days. One hour after AMD3100 injection or at 6 h after the last G-CSF administration, mice were bled from the retro-orbital plexus for a complete blood count, and Peripheral blood (PB) was obtained from the vena cava with a 25-G needle and 1 mL syringe containing 50 l of 100 mM EDTA. In some experiment, PBS or FTY720 (1.5 mg/kg) were injected intra-peritoneally 1 h before AMD3100 administration.
Evaluation of HSPC mobilization
The following formula was used for evaluation of circulating CFU-M cells: [number of white blood cells (WBCs)×number of CFU-M colonies]/number of WBCs plated=number of CFU-M per microliter of PB.
Complete blood counts
Fifty microliters of PB were taken from the retro-orbital plexus of mice and collected into ethylenediaminetetra acetic acid-coated tubes (Sarstedt Inc., Newton, NC, http://www.sarstedt.com/php/main.php). Samples were run within 2 h of collection on VetScan HM2 (ABXIS, CA94587, USA).
Colony forming unit assay
Red blood cells were lysed with BD Pharm Lyse buffer. Nucleated cells were subsequently washed twice and used for CFU-M colonies. Bone marrow nucleated cells (BMNCs) were resuspended in human methylcellulose base media provided by the manufacturer (R&D Systems, Minneapolis, MN, USA) supplemented with 10 ng/mL recombinant murine macrophage stimulating factor (mM-CSF) for CFU-M. Cultures were incubated for 7 days, at which time they were scored for the number of CFU-M colonies under an inverted microscope.
Bone marrow nucleated cells (BMNCs)
BMNCs were prepared by flushing femurs and tibias with phosphate buffered saline from pathogen-free, 8- to 12-week-old mice without enzymatic digestion. They were lysed with BD Pharm Lyse buffer (BD Biosciences, San Jose, CA, US) to remove red blood cells (RBCs), washed, and resuspended in appropriate media for further analysis.
Trans-well migration assay
The trans-well migration assay was performed as described elsewhere. Briefly, unless otherwise indicated, RBC-lysed BMNCs from C57BL/6J mice were resuspended in assay media (RPMI 1640 containing 0.5% bovine serum albumin) and equilibrated for 30 min at 37℃. Six hundred-fifty microliters of assay media in the presence or absence of CXCL12 (50 ng/mL, PeproTech, Rocky Hill, NJ, USA) or S1P (100 nM) were added to the lower chambers of a Trans-well 24-well plate (Costar Corning, Cambridge, MA, USA). Aliquots of freshly prepared BMNCs (1×106 cells/100 µL) were loaded onto the upper chambers with 5 µm-pore filters, which were incubated for 3 h at 37℃, 95% humidity, and 5% CO2. Cells from the lower chambers were scored using a CFU-M assay.
Statistical analysis
The data are presented as mean and SD, unless otherwise noted. All differences were analyzed using Student's t-test. A P value less than 0.05 was considered statistically significant.
Results
BALB/c mice are poor mobilizer to AMD3100 mobilization protocol
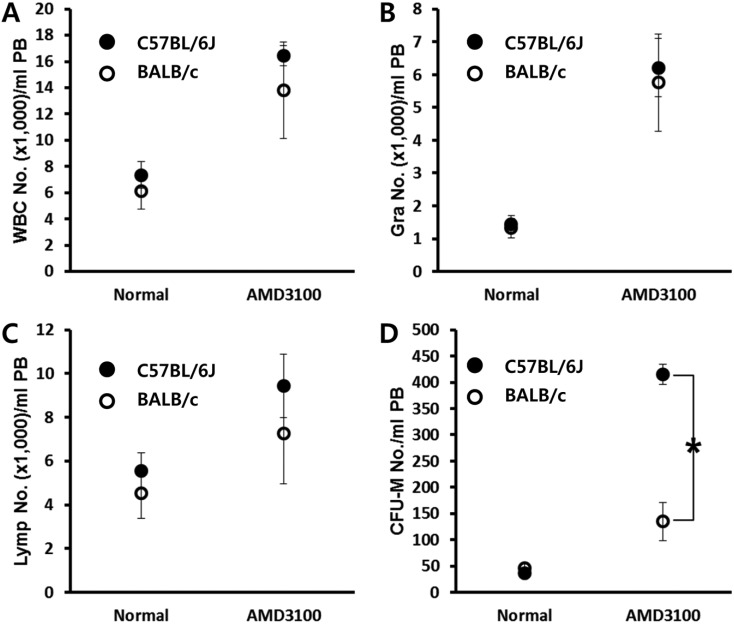
AMD3100 is known to block the interaction between CXCR4 and CXCL12 which is crucial for the retention of SPCs in BM. We first evaluated mobilization efficiency after AMD3100 administration in mice which are a genetically different background to test whether any of the host-related factors influence AMD3100-induced mobilization. Both C57BL/6J and BALB/c mice were mobilized by a short-term (1 h) AMD3100-induced protocol. One hour after AMD3100 administration, mobilization was evaluated by counting the number of circulating total white blood cells (Figure 1A), granulocyte (Figure 1B), lymphocytes (Figure 1C), and CFU-M clonogenic cells (Figure 1D) from mobilized animals. We could see a dramatic increase of circulating white blood cells after AMD3100 administration in both strains. More interestingly, we noticed that BALB/c mice showed impaired mobilization response to AMD3100 administration.
Figure 1.
BALB/c mice are poor mobilizer to AMD mobilization protocol. Age- and sex-matched C57BL/6J and BALB/c mice were mobilized with AMD3100 (5 mg/kg s.c.) for 1 h. The numbers of circulating white blood cells (A-C) and CFU-M clonogenic cells (D) per mililiter of PB are depicted. Data (mean±SD) show representative results from three independent experiments with five animals per group. *P<0.05 between C57BL/6J and BALB/c mice. CTL, counts in steady state condition; AMD3100, counts after AMD3100 induced mobilization; WBC, white blood cells; Gra, granulocytes; Lymp, lymphocytes; CFU-M, colony forming unit macrophages.
BALB/c mice are easy mobilizer to G-CSF mobilization protocol
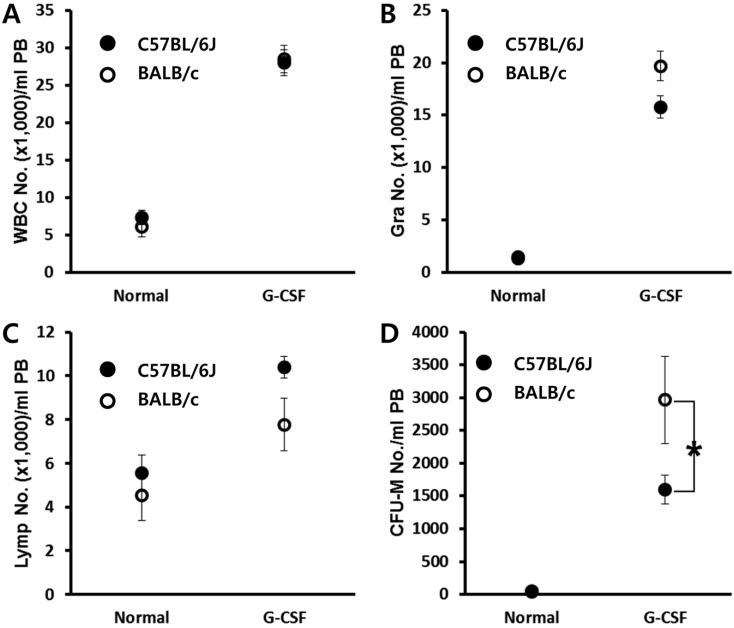
Contrary to short-term AMD3100-induced mobilization, G-CSF induced mobilization needs consecutive daily injection for 3-6 days, and this is known to be affected by several host-related factors. C57BL/6J and BALB/c mice were mobilized with a daily injection of G-CSF for 6 days, and 6 h after the last G-CSF treatment, we evaluated the number of circulating white blood cells and clonogenic CFU-M cells. As a result, we noticed that BALB/c mice are easy mobilizer compared to C57BL/6J in this protocol (Figure 2). This result is contrary to that of AMD3100 induced mobilization (Figure 1).
Figure 2.
BALB/c mice are easy mobilizer to G-CSF mobilization protocol. Age- and sex-matched C57BL/6J and BALB/c mice were mobilized with G-CSF for 6 days. The numbers of circulating white blood cells (A-C) and CFU-M clonogenic cells (D) per mililiter of peripheral blood (PB) are shown. Data (mean±SD) show representative results from three independent experiments with five animals per group. *P<0.05 between C57BL/6J and BALB/c mice. CTL, counts in steady state condition; AMD3100, counts after AMD3100 induced mobilization; WBC, white blood cells; Gra, granulocytes; Lymp, lymphocytes; CFU-M, colony forming unit macrophages.
Plasma S1P does not affect the mobilization of BMSPCs after AMD3100 administration
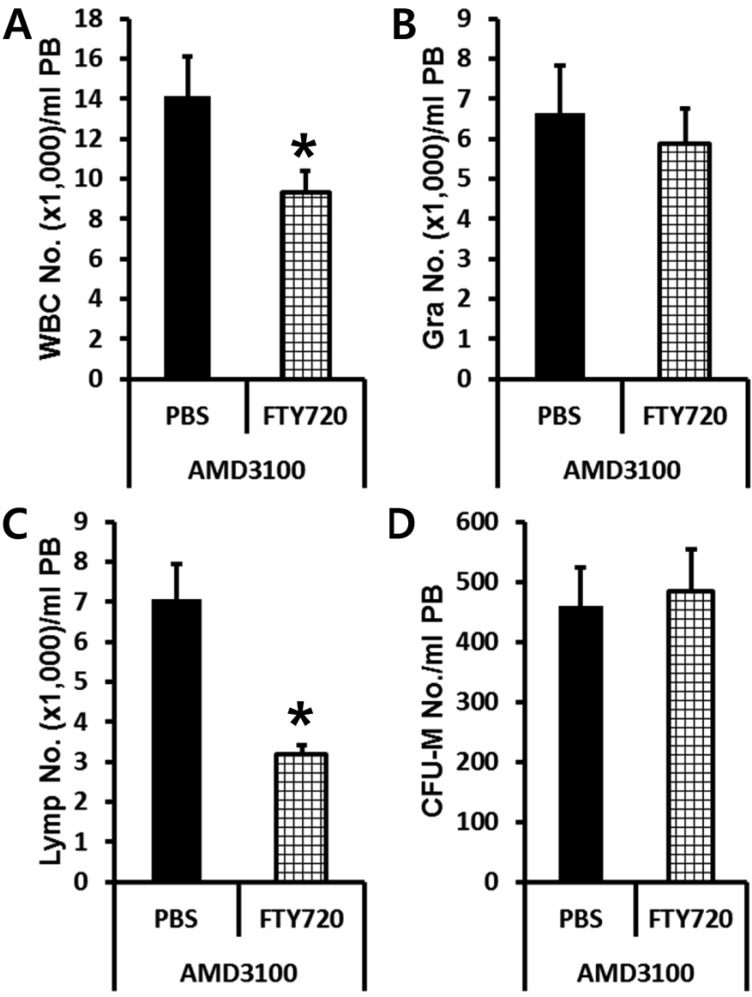
Previously, it had been reported that plasma S1P is a major chemoattractant that directs the egress of SPCs from BM to circulation. So we tested if plasma S1P and its receptor expressed in BMSPCs are the main host-related factors modulating AMD3100-induced mobilization by using S1P receptor antagonist FTY720. Normal or FTY720 pre-treated C57BL/6J mice were subjected to AMD3100 induced mobilization. PBS or FTY720 (1.5 mg/kg) were injected (I.P.) 1 h before AMD3100 administration. We evaluated the number of circulating total white blood cells (Figure 3A), granulocyte (Figure 3B), lymphocytes (Figure 3C), and CFU-M clonogenic cells (Figure 3D) in AMD3100 treated animals. As we expected, FTY720 pre-treatment decreased the number of circulating white blood cells (Figure 3A) caused by lymphocyte homing (Figure 3C). However, FTY720 pre-treatment did not change the number of circulating BMSPCs after AMD3100 administration suggesting that S1P-S1P receptor interaction is not a crucial host-related factor which can affect AMD3100 induced mobilization.
Figure 3.
Plasma S1P does not affect the mobilization of BMSPCs in the AMD3100 protocol. Normal (PBS treated) or FTY720 (1.5 mg/kg i.p.) pre-treated C57BL/6J mice were mobilized with AMD3100 for 1 h and the numbers of circulating white blood cells (A-C) and CFU-M clonogenic cells (D) per mililiter of peripheral blood (PB) are shown. Data (mean±SD) show representative results from three independent experiments with five animals per group. *P<0.05 between normal and FTY720 pre-treated C57BL/6J mice. Closed bars, PBS pretreated; Bars with strip, FTY720 pre-treated; PBS, counts in PBS pre-treated mice after AMD3100 mobilization; FTY720, counts in FTY720 pre-treated mice after AMD3100 mobilization; WBC, white blood cells; Gra, granulocytes; Lymp, lymphocytes; CFU-M, colony forming unit macrophages.
BALB/c mice have fewer BMSPCs compared to C57BL/6J
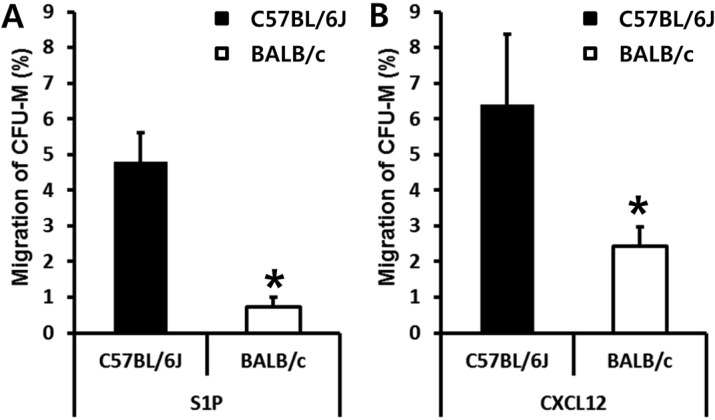
AMD3100 administration induced relatively rapid egress of BMSPCs into circulation (within 1 h). This led us to test if C57BL/6J and BALB/c mice have different numbers of BMSPCs. We isolated BM nucleated cells (BMNCs) from C57BL/6J and BALB/c mice in a steady state condition and scored the number of CFU-M clonogenic cells which are known to migrate in response to the gradient of S1P or CXCL12. As shown in figure 4, the numbers of BMSPCs that migrated to S1P (Figure 4A) and to CXCL12 (Figure 4B) were significantly lower in BALB/c mice. These data clearly suggest than AMD3100 induced mobilization is dependent on the number of BMSPCs before AMD3100 administration.
Figure 4.
BALB/c mice have fewer BMSPCs than C57BL/6J. The migration of BALB/c derived BMSPCs in response to S1P (100 nM) gradient (A) or in response to CXCL12 (50 ng/mL) gradient (B) are less than that of C57BL/6J. Values are the percent (%) of migrated BMSPCs compared to each input of BMSPCs (100%). The data (mean±SD) shown represent the combined results from three independent experiments carried out in triplicate per group. *P<0.05 between C57BL/6J and BALB/c mice.
Discussion
BM has been considered as a reservoir of SPCs which have the potential to differentiate not only into the hematopoietic lineage but also into many cells of ectodermal, endodermal, and mesodermal origins in vitro as well as in vivo. After transplantation, they home efficiently to the damaged tissue/organs to rescue loss of normal cells [4].
Although the use of mobilized peripheral blood is a preferred approach for the collection of BMSPCs rather than BM aspiration, the mobilization of adult BMSPCs in sufficient numbers for practical, clinical applications remains a challenge because a significant number of donors are resistant to mobilization. In fact, the dose of SPCs infused correlates directly with the overall survival of patients after transplantation [7]. So the innovative strategy that promotes the mobilization of BMSPCs is crucial for the collection of sufficient SPCS not only for hematopoietic transplantation but also for cell therapies in the field of regenerative medicine.
Although G-CSF is frequently used as a clinical drug that directs the SPCs out of the BM [8] a significant number of donors are known to be resistant to this protocol [9,10]. This is the reason why new promobilizing compounds are being used alone or in combination with G-CSF.
Increasing evidence suggests that G-CSF induced mobilization efficiency is influenced by elements of innate immunity [14]. However, knowledge regarding host-related factors which affect AMD3100 induced mobilization is extremely limited.
For this reason, we first evaluated mobilization efficiency after AMD3100 administration in mice which have a genetically different background to test whether any of the host-related factors influence AMD3100 induced mobilization. As a result, we found that BALB/c mice are poor mobilizers for the AMD3100 protocol (Figure 1). Since AMD3100 is usually used for short-term (1 h) protocol, we employed a G-CSF induced mobilization protocol which requires consecutive daily injection for 6 days. Contrary to AMD3100 induced mobilization, the G-CSF protocol was much more efficient in BALB/c mice (Figure 2). This difference allowed us to become more interested in the mechanism by which both mobilizing agents direct egress of BMSPCs into circulation.
We noticed previous report that stated S1P receptor type 1, which is expressed on the surface of BMSPCs, is also responsible for the egress into peripheral blood in response to plasma S1P gradient [13]. So we tested if S1P1 receptor antagonist pre-treatment attenuates BMSPC mobilization after administration by employing FTY720. As shown in figure 3, FTY720 pre-treatment did not attenuate AMD3100 induced mobilization suggesting that both S1P1 receptor and plasma S1P are not host-related factors affecting AMD3100 induced mobilization efficiency.
Compared to G-CSF induced mobilization, which needs consecutive daily injections for 3 to 6 days, AMD3100 induces release of SPCs from BM into circulation within 1 h after injection in an experimental setting [11,12]. This suggests that host-related factors may not affect the efficiency of AMD3100 induced mobilization. So we finally compared the number of BMSPCs between C57BL/6J and BALB/c mice by performing in vitro migration assay. To our surprise, BALB/c mice have much less clonogenic BMSPCs than C57BL/6J (Figure 4). This can be the explanation for the impaired mobilization in BALB/c mice after AMD3100 administration.
Based on these results, we would like to conclude that AMD3100-directed mobilization depends on the number of BMSPCs rather than on host-related factors. These results also suggest that the combination of AMD3100 and G-CSF is co-operative and is optimal for the mobilization of BMSPCs.
Acknowledgments
This work was supported by the Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0009550) and by a grant from the Innovative Research Institute for Cell Therapy (A062260) by the Ministry of Health and Welfare, Republic of Korea.
References
- 1.Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102(4):1249–1253. doi: 10.1182/blood-2003-01-0318. [DOI] [PubMed] [Google Scholar]
- 2.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105(1):71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal 'stem' cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121(2):368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 4.Iskovich S, Goldenberg-Cohen N, Stein J, Yaniv I, Fabian I, Askenasy N. Elutriated stem cells derived from the adult bone marrow differentiate into insulin-producing cells in vivo and reverse chemical diabetes. Stem Cells Dev. 2012;21(1):86–96. doi: 10.1089/scd.2011.0057. [DOI] [PubMed] [Google Scholar]
- 5.Körbling M, Anderlini P. Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood. 2001;98(10):2900–2908. doi: 10.1182/blood.v98.10.2900. [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Che JH, Lee JC, Chung SS, Jung HS, Park KS. Granulocyte-derived cationic Peptide enhances homing and engraftment of bone marrow stem cells after transplantation. Lab Anim Res. 2011;27(2):133–140. doi: 10.5625/lar.2011.27.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siena S, Schiavo R, Pedrazzoli P, Carlo-Stella C. Therapeutic relevance of CD34 cell dose in blood cell transplantation for cancer therapy. J Clin Oncol. 2000;18(6):1360–1377. doi: 10.1200/JCO.2000.18.6.1360. [DOI] [PubMed] [Google Scholar]
- 8.Papayannopoulou T, Nakamoto B, Andrews RG, Lyman SD, Lee MY. In vivo effects of Flt3/Flk2 ligand on mobilization of hematopoietic progenitors in primates and potent synergistic enhancement with granulocyte colony-stimulating factor. Blood. 1997;90(2):620–629. [PubMed] [Google Scholar]
- 9.Hosing C, Saliba RM, Ahlawat S, Körbling M, Kebriaei P, Alousi A, De Lima M, Okoroji JG, McMannis J, Qazilbash M, Anderlini P, Giralt S, Champlin RE, Khouri I, Popat U. Poor hematopoietic stem cell mobilizers: a single institution study of incidence and risk factors in patients with recurrent or relapsed lymphoma. Am J Hematol. 2009;84(6):335–337. doi: 10.1002/ajh.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gertz MA, Wolf RC, Micallef IN, Gastineau DA. Clinical impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. Bone Marrow Transplant. 2010;45(9):1396–1403. doi: 10.1038/bmt.2009.370. [DOI] [PubMed] [Google Scholar]
- 11.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cashen AF, Nervi B, DiPersio J. AMD3100: CXCR4 antagonist and rapid stem cell-mobilizing agent. Future Oncol. 2007;3(1):19–27. doi: 10.2217/14796694.3.1.19. [DOI] [PubMed] [Google Scholar]
- 13.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24(5):976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reca R, Cramer D, Yan J, Laughlin MJ, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. A novel role of complement in mobilization: immunodeficient mice are poor granulocyte-colony stimulating factor mobilizers because they lack complement-activating immunoglobulins. Stem Cells. 2007;25(12):3093–3100. doi: 10.1634/stemcells.2007-0525. [DOI] [PubMed] [Google Scholar]