Abstract
Asthma and allergic diseases have increased worldwide; however, etilogic factors for this increase are still poor. Prenatal consumptions of fatty acids are hypothesized, although few clinical trials in developing countries have been performed. This study was designed to identify predictors of immunoglobulin E (IgE) levels in cord blood of Mexican newborns. Total IgE was measured in umbilical cord blood from 613 infants whose mothers participated in a double-blind randomized controlled trial of 400 mg of docosahexaenoic acid or placebo from 18 to 22 weeks gestation through delivery. During pregnancy, information on sociodemographic characteristics, environmental exposures, and perceived maternal stress were obtained; a maternal blood sample was also collected to determine atopy via specific IgE levels. Logistic regression models were used to identify the main prenatal predictors of detectable total IgE levels in cord blood. IgE was detectable in cord blood from 344 (53.7%) infants; the main predictors in multivariate analyses were maternal atopy (odds ratio [OR] = 1.69; 95% CI, 1.19–2.42; p < 0.05) and pesticide use in the home (OR = 1.49; 95% CI, 1.04–2.14; p < 0.05). When stratified by maternal atopy, season of birth was a significant predictor in the atopic group only (OR = 2.48; 95% CI, 1.00–6.16; p < 0.05), and pesticide use was a significant predictor for infants born to nonatopic mothers (OR = 1.64; 95% CI, 1.07–2.51; p < 0.05). No differences were seen in the proportion of infants with detectable IgE by treatment group. Prenatal supplementation with omega-3 polyunsaturated fatty acid did not alter the detectable cord blood IgE levels. Maternal atopy and pesticide use during pregnancy are strong predictors of cord blood IgE levels in newborns. Clinical trial NCT00646360, www.clinicaltrials.gov.
Keywords: Clinical trial, cord blood, maternal atopy, newborn, omega-3 polyunsaturated fatty acid, pregnancy supplementation, total immunoglobulin E
Asthma and allergic diseases have been increasing in modern industrialized societies.1 The World Allergy Organization reports that over the past 30 years, the prevalence of allergic diseases has increased significantly; 400 million people worldwide have allergic rhinitis and 300 million have asthma,2 with estimated economic costs exceeding those of tuberculosis and acquired human immunodeficiency virus/immunodeficiency syndrome together.3 The prevalence of allergic rhinitis in Latin American is 7% in adults4 and in Mexico, asthma was among the top 20 causes of illness in 2006 and in Morelos State it holds the 15th place, with an incidence rate in the general population of 295.77 per 100,000 inhabitants.5
Allergic diseases result from an interaction between a genetic predisposition and environmental factors to produce high levels of IgE, which can activate the immune system.1 Maternal (history of atopy), environmental (passive smoke exposure and season of birth), and lifestyle (alcohol and caffeine consumption, tobacco use) factors have all been associated with increased cord blood IgE levels.6–8 Although measurement of total IgE in cord blood and its predictive value as a risk factor for atopy and/or asthma has been evaluated, results are discordant and remain difficult to compare because of different cutoff values used and differing definitions of atopy.9,10 In addition, not all asthma is associated with atopy, especially in developing countries.11 The predictors of cord blood IgE may differ by maternal atopic status.
Maternal consumption of omega-3 polyunsaturated fatty acid (n-3 PUFA) has been promoted as modifying neonatal immune responses.12 However, the biological mechanisms involved are not clearly understood. The present study made use of a randomized clinical trials in which mothers took n-3 PUFA or placebo during pregnancy to identify the main prenatal predictors of total IgE levels in cord blood of newborns and to determine whether these were influenced by n-3 PUFA supplementation.
MATERIALS AND METHODS
Study Design and Study Population
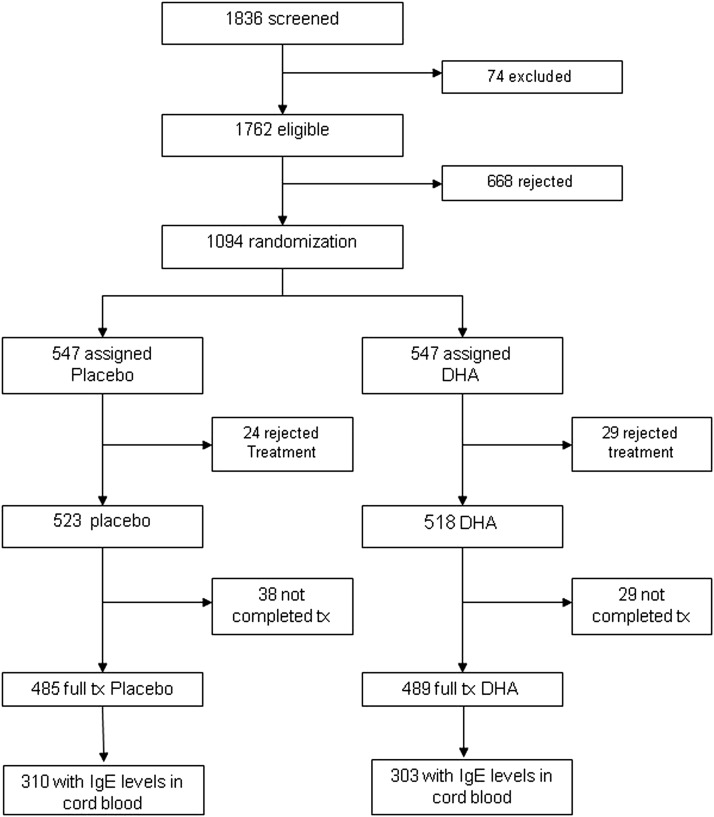
The study included a subset of mother–child who participated in the large randomized clinical trial (NCT00646360 “Effect of Prenatal Supplementation with Omega 3 Fatty Acids in the Infant's Neurobehavioral Development”). The methods of the clinical trial have been previously reported.13,14 For the present report 613 pairs of mother–child with complete information and cord blood IgE determination available were included in the analysis (Fig. 1). The study protocol was approved by Emory University's Human Investigations Board, the Ethic Committee of Instituto Nacional de Salud Publica, and the Instituto Mexicano del Seguro Social General Hospital's Human Subjects Boards. All procedures were explained to the participants, who signed an informed consent form.
Figure 1.
Consort diagram showing how subjects progressed through the study and how the final study sample was obtained. DHA = docosahexanoic acid.
Data Collection
During the prenatal period a general questionnaire was used to collect data on sociodemographic characteristics, health history, maternal stress, environmental exposures, and obstetric history and, as mentioned previously, the details have been described previously.13,14 Also, we collected blood samples to determine maternal-specific IgE levels in plasma and conducted anthropometric measures during pregnancy. During home visits an environmental exposure and stress questionnaire was administered. The stress questionnaire is validated and is designed to measure the degree to which situations in one's life are appraised as stressful, through the evaluation of 14 items.14 At delivery an umbilical cord blood sample was taken by highly trained study personnel to determine total IgE levels in newborns, using a standardized procedure to minimize potential contamination by maternal blood; also, we obtained determinations of total IgE levels in a subsample of maternal blood at delivery to evaluate the no concordance with the results of infants (data not shown).
Biomarkers
Maternal-specific IgE levels to 10 common allergens (milk, cat, egg, dust mite, wheat, Alternaria, grasses (Bermuda grass and Timothy grass), and pollen (mountain cedar and ragweed) were determined using the CAP technique (Pharmacia Diagnostic, Uppsala, Sweden). CAP IgE-specific levels were defined as follows: 0, <0.35 IU/mL, negative; 1, 0.35–0.69, very low; 2, 0.70–3.49, moderate; 3, 3.50–17.49, moderate to high; 4, 17.50–49.99, high; 5, 50–100, very high. Maternal atopy was defined as a CAP class of ≥1 for one or more of the allergens tested.15 Measurements of total IgE levels in cord blood samples were determined with the CAP system (Pharmacia Diagnostic), with a detection limit of 0.1 IU/mL, and for this study, we handle the total IgE levels in cord blood as a dichotomous variable, considering cutoff detection levels used. Some authors have noted that the range of values of total IgE level between 0.67 and 1.31 IU/mL have been shown to be predictors of atopy during childhood, especially in infants from atopic families16,17; however, we realized determinations in newborns, where the concentrations may be very low and can not be detected when considering cutoff point for childhood.
Statistical Analysis
First, a descriptive analysis of the data was conducted. We compared baseline characteristics for study population according to intervention and placebo groups. For our intention-to-treat analysis we included all children randomly assigned to our study groups. We then ran bivariate logistic regression models to explore the association between detectable cord blood total IgE (binary variable) and the main covariates including maternal age, body mass index, type of delivery, overweight, stress level during pregnancy, maternal and parental smoking, passive smoke exposure during the pregnancy, pesticide use at home, season of birth, and maternal atopy. We used multivariate logistic regression models to identify the main prenatal determinants of detectable levels of total IgE in cord blood considering the variables that were biologically plausible and statistically significant in the bivariate analysis. We also tested for interactions between selected variables and for pesticide use and mother atopy and pesticide use and treatment group in the multivariate analysis. The final multivariate model was obtained considering significance level of 0.05 and the biological plausibility and was stratified by maternal atopy. All analyses were conducted using Stata software Version 9.2 (Stata Corp., College, Station, TX).
RESULTS
Data from 613 mother–child pairs are included in this study. The sociodemographic characteristics of the study population and the comparability with those not included are presented in Table 1. There were no significant differences between participants and nonparticipants and between treatment groups (Tables 1 and 2). The mean maternal age at time of randomization was 26 years; 196 (32%) were atopic and 225 (36.7%) were primigravidas. Three hundred twenty-nine (53.7%) of the infants were boys, 309 (50.4%) were born by Cesarean section, with the highest percentage of births occurring in winter (29.5%) followed by summer (24.3%), autumn (23.52%), and spring (22.7%; Table 1).
Table 1.
Baseline characteristics of the total study population (n = 1041)
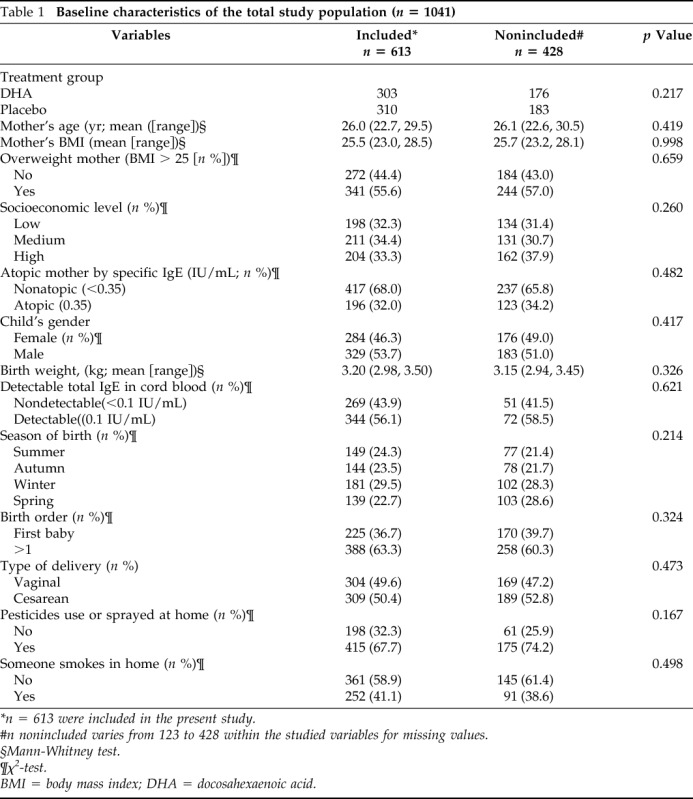
*n = 613 were included in the present study.
#n nonincluded varies from 123 to 428 within the studied variables for missing values.
§Mann-Whitney test.
¶χ2-test.
BMI = body mass index; DHA = docosahexaenoic acid.
Table 2.
Baseline characteristics of the study population by treatment group (n = 613)
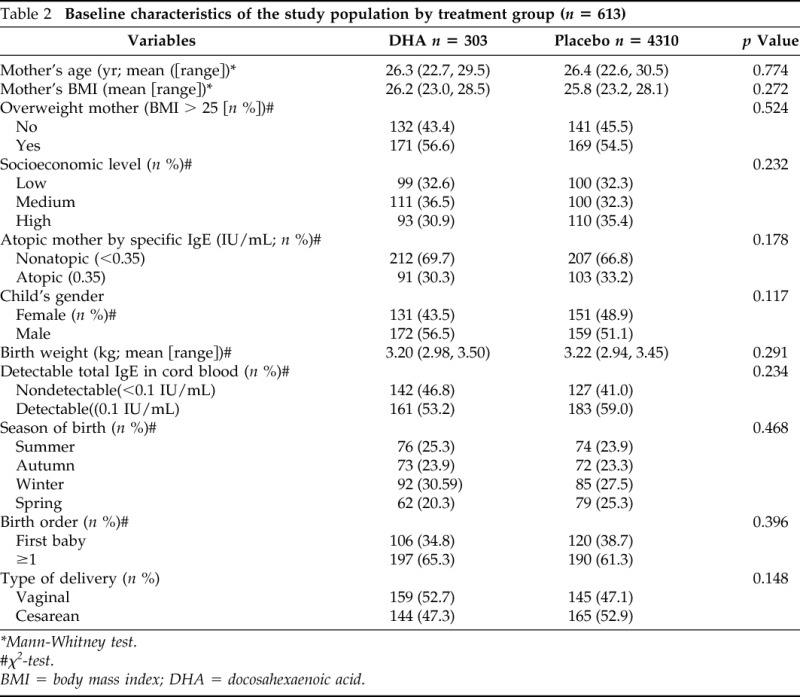
*Mann-Whitney test.
#χ2-test.
BMI = body mass index; DHA = docosahexaenoic acid.
Total IgE was detectable in cord blood of 344 (56.1%) infants of the samples (Table 1). Table 3 shows the results of bivariate and multivariate analyses on predictors of detectable cord blood total IgE levels. In the bivariate analyses, significant associations were observed between detectable cord blood IgE levels and pesticide use or sprayed pesticide at home during pregnancy (odds ratio [OR] = 1.44; 95% CI, 1.03, 2.03), maternal atopy (OR = 1.75; 95% CI, 1.23, 2.49), and season of birth (OR = 1.64; 95% CI, 1.03, 262 for spring compared with summer). Neither treatment group nor socioeconomic levels were predictors of detectable cord blood IgE (Table 2). In multivariate analyses (Table 3) only maternal atopy (OR = 1.69; 95% CI, 1.19–2.42) and pesticide use (OR = 1.49; 95% CI, 1.04–2.14) remained significant predictors of cord blood total IgE levels.
Table 3.
Main prenatal predictors of total IgE detectable levels* (n = 613) in cord blood
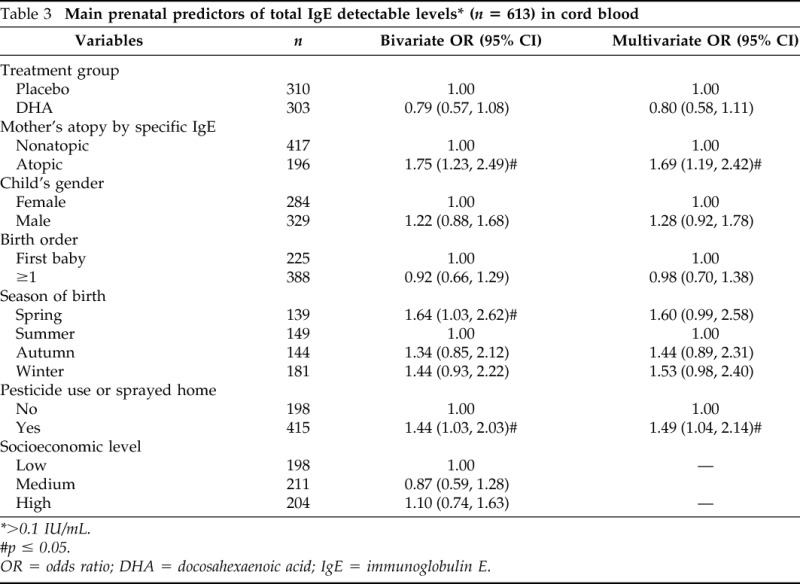
>0.1 IU/mL.
#p ≤ 0.05.
OR = odds ratio; DHA = docosahexaenoic acid; IgE = immunoglobulin E.
Results of analyses stratified by maternal atopy are shown in Table 4. In the multivariate analysis, we observed significant associations between detectable cord blood IgE and birth in spring (OR = 2.48; 95% CI 1.00, 6.16) in children from atopic mothers and pesticide use or spayed at home (OR = 1.64; 95% CI 1.07, 2.51) in children from nonatopic mothers (Table 4).
Table 4.
Main prenatal predictors of total IgE detectable levels*(n = 613) in cord blood stratified by atopic mother
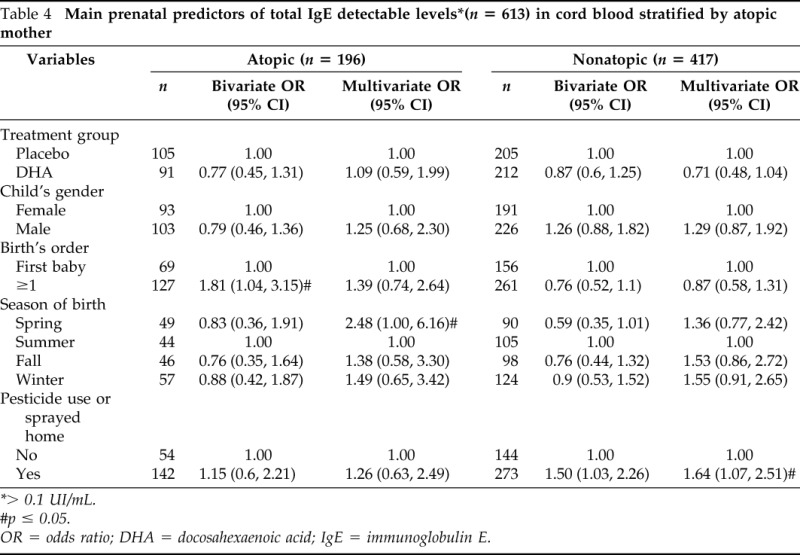
> 0.1 UI/mL.
#p ≤ 0.05.
OR = odds ratio; DHA = docosahexaenoic acid; IgE = immunoglobulin E.
DISCUSSION
Detectable levels of total IgE were found in 56% of Mexican infants. The most important prenatal determinants were maternal atopy and pesticide use in the home during pregnancy. The effect of pesticides was restricted to infants born to nonatopic mothers. Prenatal supplementation with n-3PUFA did not alter the likelihood of detectable cord blood total IgE and did not modify the prenatal predictors of detectable IgE.
Many studies have reported an association between family history of atopy6,7,15,18–20 and maternal IgE levels19–24 with total IgE levels in cord blood, suggesting that familial factors are important in stimulating the developing fetal immune system to produce IgE.6,22,25,26 The season of birth seems to influence the risk of developing allergic sensitization and atopic diseases; however, the results of studies linking IgE levels in cord blood and month of birth have produced inconsistent results.27 We found a positive influence on IgE detectable levels in infants born in spring (March to June in Mexico), which is consistent with findings by Kimpen and collaborators (Ecuador) who observed a seasonal trend in the concentration of IgE levels in cord blood, with a peak near the end of April.27
Previous studies have reported associations between exposure to environmental contaminants and components of the asthma/atopic phenotype. Reichrtova et al.28 reported an association between exposure to organochlorine compounds, including pesticides, and elevated levels of cord blood IgE in industrialized regions of Slovakia. In addition, Hoppin et al. reported that organophosphates can induce airway hyperreactivity, potentially at doses below those causing acetylcholinesterase inhibition and that certain metabolites of pesticides modulated endotoxin-induced macrophage activation through inhibition of nuclear factor kB activation.29 The primary metabolites of some pesticides have also been reported to enhance the endotoxin-induced interferon γ response in human blood cell cultures, suggesting that the pesticides, in combination with endotoxin, may lead to an increased type 1 immune response.30 Singh and Jiang31 have observed an increase in levels of proinflammatory cytokines, including tumor necrosis factor α, interferon γ, and inducible nitric oxide synthase in rats treated with organophosphate insecticide. In the present study we found a positive association between use of pesticides during pregnancy and detectable levels of total IgE that was restricted to infants born to nonatopic mothers. We did not have information on the type of pesticide used; however, our results are consistent with the reported association of household chemicals with wheeze in early life that was restricted to nonatopic children.
There is a precedent for the effects of chemical exposure during pregnancy being restricted to nonatopic subjects. The Avon Longitudinal Study of Parents and Children tested a composite index of household chemical use during pregnancy, including cleaning products, personal care products, and pesticides. Children born to mothers who used more chemicals during pregnancy had more wheezing during early childhood and lower lung function at the age of 7 years.32 This association was only seen in nonatopic children. Further research will be required to understand how these results ties in with our data showing an increase in detectable cord blood IgE in exposed infants but only if they were born to nonatopic mothers. One possibility is that the effects of atopic personal status or maternal atopy on detectable IgE levels in cord blood are strong and may mask the effects of household chemicals and pesticides.
A growing body of evidence suggests that maternal consumption of PUFAs has beneficial effects on immune function and neonatal inflammatory reactions.12,30,33–35 The suggestion has also been made that low levels of n-3PUFA in cord blood might be associated with the development of atopy in children, noting that a possible mechanism could be through the regulation of CD23 that influences IgE synthesis.36 However, in the present study, we did not observe a significant difference in the proportion of infants with detectable cord blood IgE between those born to mothers in the docosahexaenoic acid treatment and placebo groups and we did not observe any modification in the prenatal predictors of detectable IgE by treatment group. In a supplementation study conducted in Australia, infants whose mothers received n-3PUFA had lower IL-13 levels in plasma (p < 0.05) compared with the control group but no significant differences were found in levels of IgE.37,38
Some methodological considerations must be taken into account when interpreting our results. First, we excluded some participants with incomplete data from the analyses, which could lead to a possible selection bias. However, we did not observe differences on main characteristics between included versus nonincluded subjects and the reasons for exclusion were not related to the hypothesis of the study or had any connection with treatment assignment. Second, we could not include in the analysis levels of exposure to allergens during pregnancy, an important determinant of IgE. However, adjusting the analyses for pets, mice, or cockroaches did not modify our results.
In summary, our results support the growing evidence that maternal atopy and environmental exposures may influence IgE levels in cord blood. Our data add to the body of evidence suggesting that exposure to household chemicals, including pesticides during pregnancy, can have adverse health consequence for the infants and suggest that pregnant women should be advised to avoid exposure to these chemicals. This should be a general recommendation and not be restricted to nonatopic women.
Footnotes
Funded by National Council of Sciences and Technology CONACYT (Grant 14429) and the project described was supported by Award R01HD058818 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Rabinovich AG. Inmunointervención en la atopia. In Inmunopatología Molecular: Nuevas Fronteras de la Medicina. Rabinovich AG. (Ed). Editorial Médica Panamericana, S.A.: Buenos Aires, 537–542, 2004. [Google Scholar]
- 2. Pawankar R, Baena-Cagnani CE, Bousquet J, et al. State of world allergy report 2008: Allergy and chronic respiratory diseases. WAO J 2008:S3–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allergic Diseases Resource Center. Allergic asthma: Symptoms and treatment. Available online at www.worldallergy.org/professional/allergic_diseases_center/allergic_asthma/; accessed December 15, 2011.
- 4. Meltzer EO, Blaiss MS, Naclerio RM, et al. Burden of allergic rhinitis: Allergies in America, Latin America, and Asia-Pacific adult surveys. Allergy Asthma Proc 33:S113–S141, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Dirección General de Epidmeiología. Anuarios de morbilidad 1984–2010. Available online at www.dgepi.salud.gob.mx/anuario/html/anuarios.html; accessed December 11, 2011.
- 6. Bergmann RL, Schulz J, Günther S, et al. Determinants of cord-blood IgE concentrations in 6401 German neonates. Allergy 50:65–71, 1995. [DOI] [PubMed] [Google Scholar]
- 7. Bjerke T, Hedegaard M, Henriksen TB, et al. Several genetic and environmental factors influence cord blood IgE concentration. Pediatr Allergy Immunol 5:88–94, 1994. [DOI] [PubMed] [Google Scholar]
- 8. González-Quintela A, Vidal C, Gude FA. Alcohol-induced alterations in serum immunoglobulin e (IgE) levels in human subjects. Fron Biosci 7:3234–3244, 2002. [DOI] [PubMed] [Google Scholar]
- 9. Allam JP, Zivanovic O, Berg C, et al. In search for predictive factors for atopy in human cord blood. Allergy 60:743–750, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Chang WT, Sun HL, Lue KH, Chou MC. Predictability of early onset atopic dermatitis by cord blood IgE and parental history. Acta Paediatr Taiwan 46:272–277, 2005. [PubMed] [Google Scholar]
- 11. Pereira MU, Sly PD, Pitrez PM, et al. Nonatopic asthma is associated with helminth infections and bronchiolitis in poor children. Eur Respir J 29:1154–1160, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Blümer N, Renz H. Consumption of omega3-fatty acids during perinatal life: role in immuno-modulation and allergy prevention. J Perinat Med 35:S12–S18, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Imhoff-Kunsch B, Stein AD, Villalpando S, et al. Docosahexaenoic acid supplementation from mid-pregnancy to parturition influenced breast milk fatty acid concentrations at 1 month postpartum in Mexican women. J Nutr 141:321–326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramakrishnan U, Stein AD, Parra-Cabrera S, et al. Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: Randomized, double-blind, placebo-controlled trial in Mexico. Food Nutr Bull 31:S108–S116, 2010. [DOI] [PubMed] [Google Scholar]
- 15. Halonen M, Stern D, Taussig LM, et al. The predictive relationship between serum IgE levels at birth and subsequent incidences of lower respiratory illnesses and eczema in infants. Am Rev Respir Dis 146:866–870, 1992. [DOI] [PubMed] [Google Scholar]
- 16. Croner S, Kjellman NI, Eriksson B, Roth A. IgE screening in 1701 newborn infants and the development of atopic diseases during infancy. Arch Dis Child 57:364–368, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Michel FB, Bousquet J, Greillier P, et al. Comparison of cord blood immunoglobulin E concentrations and maternal allergy for the prediction of atopic disease in infancy. J Allergy Clin Immunol 65:422–430, 1980. [DOI] [PubMed] [Google Scholar]
- 18. Kaan A, Dimich-Ward H, Manfreda J, et al. Cord blood IgE: Its determinants and prediction of development of asthma and other allergic disorders at 12 months. Ann Allergy Asthma Immunol 84:37–42, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Shirakawa T, Morimoto K, Sasaki S, et al. Effect of maternal lifestyle on cord blood IgE factor. Eur J Epidemiol 13:395–402, 1997. [DOI] [PubMed] [Google Scholar]
- 20. Yang KD, Ou CY, Hsu TY, et al. Interaction of maternal atopy, CTLA-4 gene polymorphism and gender on antenatal immunoglobulin E production. Clin Exp Allergy 37:680–687, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Lin YC, Wen HJ, Lee YL, Guo YL. Are maternal psychosocial factors associated with cord immunoglobulin E in addition to family atopic history and mother immunoglobulin E? Clin Exp Allergy 34:548–554, 2004. [DOI] [PubMed] [Google Scholar]
- 22. Liu CA, Wang CL, Chuang H, et al. Prediction of elevated cord blood IgE levels by maternal IgE levels, and the neonate's gender and gestational age. Chang Gung Med J 26:561–569, 2003. [PubMed] [Google Scholar]
- 23. Liu CA, Wang CL, Chuang H, et al. Prenatal prediction of infant atopy by maternal but not paternal total IgE levels. J Allergy Clin Immunol 112:899–904, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Oryszczyn MP, Annesi-Maesano I, Campagna D, et al. Head circumference at birth and maternal factors related to cord blood total IgE. Clin Exp Allergy 29:334–341, 1999. [DOI] [PubMed] [Google Scholar]
- 25. Hansen LG, Høst A, Halken S, et al. Cord blood IgE. IgE screening in 2814 newborn children. Allergy 47:404–410, 1992. [DOI] [PubMed] [Google Scholar]
- 26. Karmaus W, Arshad H, Mattes J. Does the sibling effect have its origin in utero? Investigating birth order, cord blood immunoglobulin E concentration, and allergic sensitization at age 4 years. Am J Epidemiol 154:909–915, 2001. [DOI] [PubMed] [Google Scholar]
- 27. Kimpen J, Callaert H, Embrechts P, Bosmans E. Cord blood IgE and month of birth. Arch Dis Child 62:478–482, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reichrtová E, Ciznár P, Prachar V, et al. Cord serum immunoglobulin E related to the environmental contamination of human placentas with organochlorine compounds. Environ Health Perspect 107:895–899, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoppin JA, Umbach DM, London SJ, et al. Pesticides and atopic and nonatopic asthma among farm women in the Agricultural Health Study. Am J Respir Crit Care Med 177:11–18, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mihrshahi S, Peat JK, Webb K, et al. Effect of omega-3 fatty acid concentrations in plasma on symptoms of asthma at 18 months of age. Pediatr Allergy Immunol 15:517–522, 2004. [DOI] [PubMed] [Google Scholar]
- 31. Singh AK, Jiang Y. Lipopolysaccharide (LPS) induced activation of the immune system in control rats and rats chronically exposed to a low level of the organothiophosphate insecticide, acephate. Toxicol Ind Health 19:93–108, 2003. [DOI] [PubMed] [Google Scholar]
- 32. Henderson J, Sherriff A, Farrow A, Ayres JG. Household chemicals, persistent wheezing and lung function: Effect modification by atopy? Eur Respir J 31:547–554, 2008. [DOI] [PubMed] [Google Scholar]
- 33. de Matos OG, Amaral SS, Pereira da Silva PE, et al. Dietary supplementation with omega-3-PUFA-rich fish oil reduces signs of food allergy in ovalbumin-sensitized mice. Clin Dev Immunol 2012:236564, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kompauer I, Demmelmair H, Koletzko B, et al. Association of fatty acids in serum phospholipids with hay fever, specific and total immunoglobulin E. Br J Nutr 93:529–535, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Notenboom ML, Mommers M, Jansen EH, et al. Maternal fatty acid status in pregnancy and childhood atopic manifestations: KOALA Birth Cohort Study. Clin Exp Allergy 41:407–416, 2011. [DOI] [PubMed] [Google Scholar]
- 36. Byberg K, Øymar K, Aksnes L. Fatty acids in cord blood plasma, the relation to soluble CD23 and subsequent atopy. Prostaglandins Leukot Essent Fatty Acids 78:61–65, 2008. [DOI] [PubMed] [Google Scholar]
- 37. Dunstan JA, Mori TA, Barden A, et al. Maternal fish oil supplementation in pregnancy reduces interleukin-13 levels in cord blood of infants at high risk of atopy. Clin Exp Allergy 66:442–448, 2003. [DOI] [PubMed] [Google Scholar]
- 38. Mihrshahi S, Peat JK, Marks GB, et al. Eighteen-month outcomes of house dust mite avoidance and dietary fatty acid modification in the Childhood Asthma Prevention Study (CAPS). J Allergy Clin Immunol 111:162–168, 2003. [DOI] [PubMed] [Google Scholar]