Highlights
-
•
Endometrioid adenocarcinoma may develop during the long-term follow-up of APA.
-
•
Atypical polypoid adenomyoma is a precursor of endometrioid adenocarcinoma.
-
•
Careful follow-up is needed for the conservative management of APA.
Keywords: Atypical polypoid adenomyoma, Endometrioid adenocarcinoma, Fertility preservation
Background
Atypical polypoid adenomyoma (APA) is a rare uterine tumor; less than 200 cases have been reported in the literature, and it most often occurs in young nulliparous women (Young et al., 1986; Longacre et al., 1996; Heatley, 2006; Matsumoto et al., 2013). APA is a benign tumor, and fertility-sparing management is considered for those who desire pregnancy. Recurrent or persistent lesions after conservative management are common, and coexisting endometrioid adenocarcinoma at the time of initial treatment of APA may be observed in some cases. However, to the best of our knowledge, the development of endometrioid adenocarcinoma during the long-term follow-up period after conservative management has not been reported yet. We report a case of endometrioid adenocarcinoma that developed in a young woman 8 years after the diagnosis of APA.
Case report
A 36-year-old nulligravida Japanese woman, with a body mass index of 20 kg/m2 and regular menstrual periods, had a history of APA. According to her family history, her grandmother was diagnosed with uterine cancer in her eighties. Our patient was clinically diagnosed with endometrial polyps and underwent a dilation and curettage procedure at the age of 28 years. The histopathological diagnosis of endometrial specimens was APA with a markedly complex architecture of the glands (Fig. 1). After the dilation and curettage procedure, there were no abnormal findings on ultrasonography and endometrial biopsies. For 8 years, she was carefully followed up with semi-annual ultrasonography and cytological examinations.
Fig. 1.
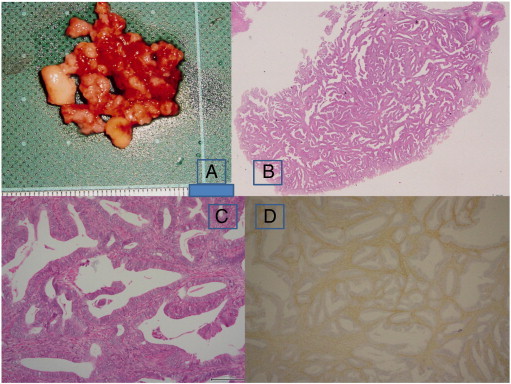
Macroscopic and microscopic findings of the dilation and curettage specimens. Gray and yellowish polypoid masses (panel A). H&E stain of a polypoid mass (panel B, loupe). Endometrioid type glands within a myomatous stroma (panel C, H&E, × 100). Immunohistochemical staining of αSMA is positive in the stroma (× 40).
H&E, hematoxylin and eosin; αSMA, alpha-smooth muscle actin.
She visited her gynecologic clinic with a chief complaint of abnormal uterine bleeding 3 months after her regular follow-up visit, which had shown no abnormal findings. Transvaginal ultrasonography showed a tumor in the lower uterine segment and cervix. The endometrial biopsy showed grade 2 endometrioid adenocarcinoma with notable nuclear atypia, and findings of magnetic resonance imaging suggested myometrial invasion of a tumor in the cervix. Informed consent for hysterectomy was obtained. Abdominal type II hysterectomy with bilateral salpingo-oophorectomy, omentectomy, and pelvic and para-aortic lymphadenectomy were performed.
Macroscopic examination of hysterectomy specimens showed an exophytic polypoid tumor in the fundus and infiltrative growth in the cervix. Histopathological findings showed that the vast majority of the tumor, including the infiltrative mass in the cervix, was grade 2 endometrioid adenocarcinoma (architectural grade 1, nuclear grade 2; Fig. 2) with positive lymphovascular invasion, negative peritoneal cytology, no lymph node metastases, and International Federation of Gynecology and Obsteterics FIGO stage II.
Fig. 2.
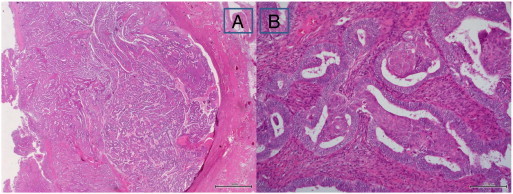
Microscopic findings of the tumor in the lower uterine segment of the hysterectomy specimen. Myometrial invasion of endometrioid adenocarcinoma in the lower uterine segment (panel A, H&E, loupe). Nuclear grade 2 with enlarged and rounded nuclei and small nucleoli (panel B, H&E, × 100).
H&E, hematoxylin and eosin.
A small part of the polypoid mass in the fundus was APA that was adjacent and transitioned to endometrial adenocarcinoma (Fig. 3). Immunohistochemical staining for alpha-smooth muscle actin was positive in the myomatous stroma of APA and negative around the glands of endometrioid adenocarcinoma. Squamous morule formation was present in the area of APA.
Fig. 3.
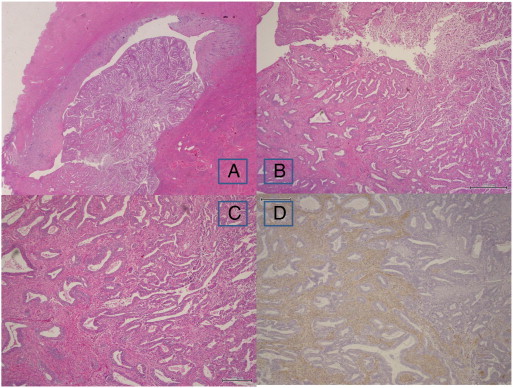
Microscopic findings of the tumor in the fundus of the hysterectomy specimen. A polypoid mass in the fundus (panel A, H&E, loupe). Area of transition from APA to endometrioid adenocarcinoma (panel B, H&E, × 40). APA on the left side of the panel and endometrioid adenocarcinoma on the right side (panel C, H&E, × 200). Immunohistochemical staining of αSMA is positive in the stroma of APA and negative in the stroma of endometrioid adenocarcinoma (panel D, × 4).
H&E, hematoxylin and eosin; APA, atypical polypoid adenomyoma; αSMA, alpha-smooth muscle actin.
The patient's postoperative outcome was uneventful, and she was discharged on postoperative day 10.
Discussion
APA is generally benign and mostly occurs in young women; therefore, fertility-sparing management should be considered. However, careful follow-up is required because there is a risk of recurrence of APA or transition to endometrioid adenocarcinoma. Longacre et al. (1996) reported that the recurrence of APA among patients treated with conservative management was 45% (13/29). Matsumoto et al. (2013) recently reported that the recurrence rate was 23.8% (5/21), and that the rate was lower (10%, 1/10) in patients treated with transcervical resection (TCR) and higher (36.4%, 4/11) among those treated with dilation and curettage or other therapies. Longacre et al. (1996) reported that 2 of 55 patients with APA showed myometrial invasion, and Matsumoto et al. (2013) reported that 17.2% (5/29) showed coexistence of endometrioid adenocarcinoma with APA. However, there have been no case reports of endometrioid adenocarcinoma that developed after a long-term follow up of APA.
Longacre et al. (1996) proposed that atypical polypoid adenomyofibroma of low malignant potential (APA-LMP) has a higher rate of recurrence and progression to carcinoma than does APA. The dilation and curettage specimen obtained during the initial treatment in this case showed APA with a markedly complex architecture of the glands, which can be equivalent to APA-LMP. The hysterectomy specimen showed endometrioid adenocarcinoma in continuity with APA, which may suggest progression from APA to carcinoma in addition to recurrence of APA after the long-term follow up. Thus, APA should be considered as one of the precursors of endometrioid adenocarcinoma, and APAs, especially those with complex architecture, should be managed with caution because of a higher risk of cancer. Moreover, APAs are often located in the lower uterine segment (Young et al., 1986; Longacre et al., 1996; Matsumoto et al., 2013), and endometrial adenocarcinoma with lower uterine segment involvement has poorer recurrence- and survival-related outcomes (Kizer et al., 2011; Madom et al., 2007). Early invasion of the cervix will make it difficult for young women to preserve the uterus.
For patients who wish to undergo conservative management for APA, TCR has been recommended for the diagnosis and treatment (Matsumoto et al., 2013; Di Spiezio Sardo et al., 2008; Guida et al., 2008; Inoue et al., 2012). Careful follow-up with ultrasonography, hysteroscopy, and endometrial biopsy should be performed, although appropriate intervals of examinations have not been clearly determined.
In conclusion, APA, especially APA with complex architecture, may have a risk of recurrence or malignant transformation into endometrioid adenocarcinoma, and careful management is required if fertility is to be preserved. Only a few reports have shown a long-term follow up of APA after fertility-sparing management. Retrospective accumulations of case reports are mandatory.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
Our special gratitude goes to those who have made valuable contributions, including Dr Yuriko Nakagawa at Takemura Ladies Clinic, Dr Ayako Kakuno, Ms Yuki Suzuki, Mr Kenji Matsubayashi, Mr Masahiro Shibutani, and Dr Kiyohiko Kishi at Meiwa Hospital.
References
- Di Spiezio Sardo A., Mazzon I., Gargano V., Di Carlo C., Guida M., Mignogna C. Hysteroscopic treatment of atypical polypoid adenomyoma diagnosed incidentally in a young infertile woman. Fertil. Steril. 2008;89:456. doi: 10.1016/j.fertnstert.2007.02.061. (e9–12) [DOI] [PubMed] [Google Scholar]
- Guida M., Greco E., Di Spiezio Sardo A., Di Carlo C., Lavitola G., Tarsitano F. Successful pregnancy after four-step hysteroscopic technique for the treatment of atypical polypoid adenomyoma. Fertil. Steril. 2008;89:1283–1284. doi: 10.1016/j.fertnstert.2008.01.093. [DOI] [PubMed] [Google Scholar]
- Heatley M.K. Atypical polypoid adenomyoma: a systematic review of the English literature. Histopathology. 2006;48:609–610. doi: 10.1111/j.1365-2559.2005.02315.x. [DOI] [PubMed] [Google Scholar]
- Inoue K., Tsubamoto H., Honda O., Kato T., Wakimoto Y., Ogino M. Fertility-preserving management of atypical polypoid adenomyoma (APAM) in two young women. Adv. Obstet. Gynecol. 2012;64:483–489. (in Japanese) [Google Scholar]
- Kizer N.T., Gao F., Guntupalli S., Thaker P.H., Powell M.A., Goodfellow P.J. Lower uterine segment involvement is associated with poor outcomes in early-stage endometrioid endometrial carcinoma. Ann. Surg. Oncol. 2011;18:1419–1424. doi: 10.1245/s10434-010-1454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longacre T.A., Chung M.H., Rouse R.V., Hendrickson M.R. Atypical polypoid adenomyofibromas (atypical polypoid adenomyomas) of the uterus. A clinicopathologic study of 55 cases. Am. J. Surg. Pathol. 1996;20:1–20. doi: 10.1097/00000478-199601000-00001. [DOI] [PubMed] [Google Scholar]
- Madom L.M., Brown A.K., Lui F., Moore R.G., Granai C.O., Disilvestro P.A. Lower uterine segment involvement as a predictor for lymph node spread in endometrial carcinoma. Gynecol. Oncol. 2007;107:75–78. doi: 10.1016/j.ygyno.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Hiura M., Baba T., Ishiko O., Shiozawa T., Yaegashi N. Clinical management of atypical polypoid adenomyoma of the uterus. A clinicopathological review of 29 cases. Gynecol. Oncol. 2013;129:54–57. doi: 10.1016/j.ygyno.2012.12.040. [DOI] [PubMed] [Google Scholar]
- Young R.H., Treger T., Scully R.E. Atypical polypoid adenomyoma of the uterus. A report of 27 cases. Am. J. Clin. Pathol. 1986;86:139–145. doi: 10.1093/ajcp/86.2.139. [DOI] [PubMed] [Google Scholar]


