Abstract
Cytokines of the IL-6 family have been related to infarct size and prognosis in patients with myocardial infarction. The aims of the present study were to elucidate possible associations between myocardial necrosis and left ventricular impairment and members of the IL-6 transsignalling system including soluble (s) IL-6R and (s) glycoprotein 130 (sgp130) in patients with ST-elevation myocardial infarction (STEMI) treated with primary PCI.
In blood samples from 1028 STEMI patients, collected in-hosptial, we found significant correlations between peak TnT and IL-6 and CRP (p < 0.001, all) and between IL-6 and CRP and LV ejection fraction and NT-proBNP (p < 0.001, all). On the contrary, no significant associations were found between peak TnT and sgp130 or sIL-6R. Furthermore sgp130 was significantly elevated in diabetic patients and also associated with the glucometabolic state.
In conclusion, circulating levels of IL-6 and CRP, but not the soluble forms of the receptor (sIL-6R) or the receptor signalling subunit (sgp130) were associated with the extent of myocardial necrosis. The biological importance of the IL-6/gp130-mediated signalling pathways in patients with acute myocardial infarction and dysglycemia should be further elucidated.
Keywords: ST-elevation myocardial infarction, IL-6, sgp130, sIL-6R, Myocardial necrosis
Abbreviations: sgp130, soluble glycoprotein 130; sIL-6R, soluble interleukin -6 receptor; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; LVEF, left ventricular ejection fraction
Highlights
-
•
Circulating levels of IL-6 and CRP, but not sgp130 and sIL-6R were associated with the extent of myocardial necrosis.
-
•
Circulating levels of sgp130 were weakly associated with impaired LV function and glucometabolic state.
-
•
Circulating levels of sIL-6R were not associated with either impaired LV function or glucometabolic state.
1. Introduction
Atherosclerosis is now regarded as an inflammatory disease. Inflammation in the vessel wall seems to be important for the development of the atherosclerotic plaque as well as for plaque destabilisation [1,2]. Inflammation also seems to be involved in the remodelling process after acute STEMI [3]. The complexity of the atherosclerotic process and the regulatory mechanisms involving inflammation are only partly understood [3,4]. However, cytokines of the IL-6 family have been shown to play a role in all stages of the development and progression of atherosclerosis, from early inflammatory lesions to destabilisation of the plaque [5]. Pro-inflammatory cytokines are thought to be involved in reperfusion injury, repair processes and scar tissue formation after myocardial infarction [3]. Circulating levels of IL-6 and C-reactive protein (CRP) have been shown to correlate with infarct size [6], whereas the knowledge of other novel members of the IL-6 family like soluble interleukin-6 receptor (sIL-6R) and soluble glycoprotein 130 (sgp130) is limited in patients with myocardial infarction.
The IL-6 signalling is activated through two different ways and it has been a matter of discussion whether this results in both a pro- and anti-inflammatory effect of IL-6 [7].
In the classical IL-6 signalling pathway IL-6 binds to IL-6R, which is a membrane-bound receptor on the cell surface [8]. The receptor–ligand complex then associates with the common signal transducing receptor gp130, initiating activation of intracellular signalling pathways. The membrane-bound IL-6R is present in cells like hepatocytes, monocytes, inactive B and T-lymphocytes [8] and cardiac myocytes [9] whereas gp130 is widely expressed in most cell types in the human body. In the second way of IL-6 signalling, which has been named the transsignalling system, IL-6 binds to a soluble form of IL-6R (sIL-6R), and this complex binds subsequently to gp130 on cells. The gp130/IL6/sIL-6R complex activates intracellular pathways, and exerts its function in cells not expressing the IL-6R [10]. This IL-6 transsignalling pathway may be more important for the pro-inflammatory effect of IL-6 than the classical receptor signalling [7]. The soluble form of gp130 (sgp130) is the natural inhibitor of this transsignalling system [11]. It inhibits the ability of the circulating IL-6/sIL-6R complex to bind to membrane bound gp130.
These less studied members of the IL-6 signalling system, i.e. sgp130 and sIL-6R, might give additional mechanistic insights into the atherosclerotic process associated with STEMI.
The aims of the present study were to elucidate possible associations between members of the IL-6 transsignalling system including sIL-6R and sgp130 and (1) the degree of myocardial necrosis, (2) left ventricular (LV) impairment, and (3) dysglycemia as well as conventional risk factors in patients with ST-elevation myocardial infarction (STEMI).
2. Methods
2.1. Study population
This study was a cross-sectional cohort study of STEMI patients admitted to Oslo University Hospital Ullevål, Norway and treated with primary PCI. From June 2007 to August 2011 a total of 1028 patients with diagnosed STEMI were included consecutively during weekdays after written informed consent was obtained. The study was approved by the Regional Ethics Committee.
STEMI was defined as electrocardiographic ST segment elevation of >2?mm in two or more contiguous chest leads or >1?mm in two or more limb leads or new onset of left bundle-branch block, together with chest pain or other typical symptoms and elevated troponin levels above the 99th percentile.
Patients below 18 years old or patients unable or unwilling to give written informed consent were not included. Patients were routinely included after the primary PCI-procedure, the following morning after the admission.
Clinical information was collected from hospital records and questionnaires obtained at the time of inclusion.
2.2. Laboratory methods
Blood samples were collected at median time of 24?h after symptoms and 18?h after the PCI procedure, between 08:00 and 10:00?a.m. the following morning, except those with infarction during the weekend, which were included the following monday morning. In order to standardize blood sampling, and also to avoid any influence of diurnal variations and food intake, all samples were taken after an overnight fast. Serum was prepared by centrifugation for 10?min at 2000g and samples were stored at -80?°C until analysed. Circulating levels of sgp130, sIL-6R and IL-6 were determined by commercial ELISA (R&D Systems, Abingdon, Oxon, UK) and CRP with kits from DRG Instruments (Marburg/Lahn, Germany). In our laboratory, the inter-assay coefficients of variation (CV) were 5.2%, 3.6%, 10.5% and <5%.
Routine blood samples were analysed by use of commercial methods. Electrochemiluminescence technology for quantitative measurement was used for repeated measures of TnT (3rd generation cTroponinT, Elecsys 2010, Roche, Mannheim, Germany). The lower detection limit of the assay is 10?ng/L with a recommended diagnostic threshold of 30?ng/L. The inter-assay coefficient of variation was 7%. TnT levels were determined in blood samples collected immediately after the acute PCI and in repetitive samples with 4?h between the two first samples and then following every 12?h until a rise and fall curve was observed. The level used in this study was the highest measured during hospital stay for each patient.
NT-proBNP was measured in serum using Elecsys proBNP sandwich immunoassay on Elecsys 2010 (Roche Diagnostics, Indianapolis, USA). The inter-assay coefficient of variation was 7%.
2.3. Left ventricular function
Left ventricular ejection fraction (LVEF) was measured by echocardiography a.m. Simpson or by visual judgement before hospital discharge or at clinical follow-up within 3 months after the myocardial infarction. In case of repeated examinations, an average value was used. LVEF obtained after discharge was only included if the patient was hemodynamically stable and without rehospitalisation between the index myocardial infarction and the examination.
2.4. Statistics
Continuous variables are presented as median values (25, 75 percentiles) and categorical variables as proportions.
As most measured biomarkers were skewly distributed, non-parametric methods were used throughout. Correlation analyses were performed by Spearman's rho and the Mann–Whitney test was used for group comparisons.
The 75th percentile was chosen as cut-off points for max TnT and NT-proBNP in the present material. The LVEF cut-off level was set to 40% according to clinical practice. Glucometabolic classification was defined according to the American Diabetes Association criteria [12]. We dichotomised the following variables into high and low values using cut-off levels of 11.1?mmol/L for admission glucose, 7.0?mmol/L for fasting glucose, and 6.5% for HbA1c. A p-value of <0.05 was considered statistically significant. All analyses were performed by SPSS Software, version 18.0 (SPSS Inc., USA).
3. Results
Baseline characteristics of the total study population (n = 1028) are shown in Table 1. The cohort was relatively young (median 61 years) with medium size infarction (measured by peak TnT), 80% were male and 47% were current smokers. Only 12% had previous myocardial infarction, 13% had known diabetes. Half of the patients had single vessel disease.
Table 1.
Baseline characteristics.
| Patients (n = 1028) | |
|---|---|
| Age (years)a | 61 (24/94) |
| Male | 823 (80) |
| Weight (kg) | 83 (74, 93) |
| Waist circumference (cm) | 97 (90, 104) |
| Systolic blood pressure (mmHg) | 140 (120, 157) |
| Smokers | 485 (47.2) |
| Previous disorders | |
| Angina pectoris | 88 (8.6) |
| Myocardial infarction | 125 (12.2) |
| Hypertension | 351 (34.1) |
| Heart failure | 20 (1.9) |
| Cerebrovascular disease | 47 (4.6) |
| Diabetes mellitus | 132 (12.8) |
| Insulin treated diabetes | 33 (3.2) |
| Atrial fibrillation | 42 (4.1) |
| Laboratory analyses at admission | |
| Hemoglobin (g/100 mL) | 13.4 (12.6, 14.3) |
| Total cholesterol (mmol/L) | 4.8 (4.1, 5.6) |
| Triglycerides (mmol/L) | 1.3 (0.9, 1.8) |
| Admission glucose (mmol/L) | 7.4 (6.3, 9.0) |
| Fasting glucose (mmol/L) | 5.8 (5.2, 6.7) |
| HbA1c (%) | 5.9 (5.6, 6.3) |
| TnT max (ng/L) | 3800 (1710, 7140) |
| NT-proBNP (ng/L) | 33 (11, 122) |
| Prehospital thrombolysis | 119 (11.6) |
| Time interval from symptoms to blood sampling (h)a | 24 (2/264) |
| Anterior infarction | 438 (42.6) |
| Number of vessels with significant stenoses | |
| 1 | 546 (53.3) |
| 2 | 295 (29.1) |
| 3 | 172 (16.9) |
| LVEF ( %)b | 50 (44, 55) |
Data are presented as median (25, 75 percentiles) or numbers ( %) if not otherwise stated, for details see Section 2.
Range.
Left ventricular ejection fraction.
The included patients were on average hemodynamically stable with only slightly reduced LVEF and normal NT-proBNP levels.
3.1. Circulating levels of IL-6, gp130, IL-6R and CRP
There were weak, but statistically significant correlations between age and IL-6 and between systolic blood pressure and IL-6, sgp130 and sIL-6R (Table 2). IL-6 and CRP, as well as sgp130 and sIL-6R, were significantly intercorrelated (p < 0.001, all), however, the correlation between sgp130 and sIL-6R was weak (Table 2). There was no correlation between IL-6 and sIL-6R while there was a weak, inverse correlation between CRP and sIL-6R (p = 0.001). Smokers had significant lower levels of sgp130 levels compared to non-smokers, whereas no gender differences were found (Table 3).
Table 2.
Correlation between inflammatory biomarkers and patients characteristics.
| IL-6 | sgp130 | sIL-6R | CRP | |
|---|---|---|---|---|
| Age | 0.095, 0.002 | 0.05, 0.106 | 0.002, 0.953 | 0.032, 0.311 |
| Systolic BPa | 0.139, <0.001 | 0.081, 0.01 | 0.069, 0.028 | -0.23, 0.458 |
| TnT max | 0.282, <0.001 | 0.06, 0.054 | -0.051, 0.106 | 0.302, <0.001 |
| NT-proBNP | 0.217, <0.001 | 0.058, 0.066 | -0.020, 0.535 | 0.328, <0.001 |
| LVEFb | -0.189, <0.001 | -0.085, 0.018 | 0.018, 0.610 | -0.274, <0.001 |
| HbA1c | 0.059, 0.065 | 0.092, 0.004 | 0.036, 0.264 | 0.064, 0.045 |
| Admission glucose | 0.058, 0.063 | 0.132, <0.001 | 0.032, 0.307 | 0.067, 0.032 |
| Fasting glucose | 0.124, <0.001 | 0.082, 0.009 | 0.028, 0.370 | 0.133, <0.001 |
| Timec | 0.077, 0.014 | -0.049, 0.119 | -0.109. <0.001 | 0.452, <0.001 |
| IL-6 | 1 | 0.042, 0.176 | 0.007, 0.832 | 0.418, <0.001 |
| sgp130 | – | 1 | 0.182, <0.001 | -0.040, 0.205 |
| sIL-6R | – | – | 1 | -0.103, 0.001 |
| CRP | – | – | – | 1 |
Data are presented as coefficients of correlation and p-values.
Systolic blood pressure.
Left ventricular ejection fraction.
Time interval from symptom onset to blood sampling in hours.
Table 3.
Circulating levels of the IL-6 family members in STEMI patients (n = 1028), related to patient characteristics.
| IL-6 pg/mL | sgp130 ng/mL | sIL-6R ng/mL | CRP mg/L | |
|---|---|---|---|---|
| Female | 19.4 (13.3, 31.4) | 240 (218, 264) | 39,1 (30.9, 46.9) | 16.3 (8.2, 38.3) |
| Male | 18.7 (14.1, 29.8) | 240 (219, 259) | 39.4 (31.2, 47.8) | 13.0 (6.9, 31.1) |
| p-Value | 0.7 | 0.09 | 0.9 | 0.09 |
| No smokers | 18.5 (14.1, 29.3) | 244 (222, 265) | 39.3 (31.5, 47.7) | 12.0 (6.2, 34.2) |
| Smokers | 19.1 (13.8, 30.2) | 238 (217, 258) | 39.2 (30.8, 47.5) | 14.2 (7.1, 31.5) |
| p-Value | 0.5 | 0.005 | 0.7 | 0.2 |
| Low TnT maxa | 18.0 (13.2, 26.2) | 240 (217, 259) | 39.7 (30.9, 47.8) | 11.7 (6.2, 26.5) |
| High TnT max | 25.0 (17.3, 36.5) | 240 (222, 265) | 38.6 (31.3, 47.3) | 22.1 (10.5, 49.5) |
| p-Value | <0.001 | 0.2 | 0.8 | <0.001 |
| Low NT-proBNPb | 18.1 (13.0, 26.8) | 238 (218, 258) | 39.4 (31.1, 48.1) | 11.6 (6.0, 23.3) |
| High NT-proBNP | 24.3 (17.4, 34.5) | 246 (221, 268) | 38.7 (30.4, 46.3) | 31.1 (11.6, 82.0) |
| p-Value | <0.001 | 0.007 | 0.2 | <0.001 |
| LVEF <40% | 29.0 (17.9, 37.5) | 240 (223, 265) | 37.9 (29.5, 49.1) | 36.1 (4.11, 9.5) |
| LVEF =40% | 18.6 (13.3, 29.0) | 239 (218, 259) | 39.3 (30.9, 47.3) | 13.3 (6.8, 29.4) |
| p-Value | <0.001 | 0.2 | 0.8 | <0.001 |
| No diabetics | 18.7 (14.1, 29.7) | 239 (218, 259) | 39.2 (31.2, 47.5) | 13.1 (7.0, 31.0) |
| Diabetics | 19.1 (12.7, 33.7) | 247 (226, 270) | 39.7 (30.6, 47.8) | 16.6 (7.5, 58.5) |
| p-Value | 0.5 | 0.002 | 1.0 | 0.04 |
| Low HbA1cc | 18.6 (14.0, 29.0) | 238 (217, 258) | 39.1 (30.9, 47.4) | 13.1 (6.9, 30.7) |
| High HbA1c | 20.0 (13.9, 33.3) | 246 (229, 273) | 40.6 (31.7, 47.9) | 17.0 (7.8, 49.6) |
| p-Value | 0.056 | <0.001 | 0.4 | 0.03 |
| Low admission glucosed | 18.7 (13.9, 29.2) | 239 (218, 260) | 39.2 (31.2, 47.6) | 12.8 (6.9, 29.3) |
| High admission glucose | 20.6 (15.6, 34.8) | 246 (224, 273) | 39.1 (30.3, 49.1) | 23.3 (10.2, 68.9) |
| p-Value | 0.016 | 0.02 | 0.7 | <0.001 |
| Low fasting glucosee | 18.7 (13.9, 29.0) | 239 (218, 259) | 39.2 (30.9, 47.6) | 12.7 (6.9, 29.2) |
| High fasting glucose | 19.7 (14.3, 33.8) | 243 (220, 270) | 39.3 (31.7, 47.2) | 18.3 (8.4, 52.8) |
| p-Value | 0.07 | 0.05 | 0.7 | <0.001 |
Medians (25,75 percentiles) are given.
TnT max low <7140 ng/L and high =7140 ng/L.
Low NT-proBNP <122 ng/L and high =122 ng/L.
Low HbA1c <6.5% and high =6.5%.
Low admission glucose <11.1 mmol/L and high =11.1 mmol/L.
Low fasting glucose <7.0 mmol/L and high =7.0 mmol/L.
3.2. Association with max troponin T
There were significant correlations between peak TnT and IL-6, and between peak TnT and CRP (Table 2). When dichotomising TnT values at the 75th percentile (7140?ng/L) (quartiles shown in Fig. 1), IL-6 and CRP were found to be significantly elevated in the upper quartiles compared to the lower three quartiles (p < 0.001, both) (Table 3). On the other hand no associations were found between peak TnT levels and sgp130 or sIL-6R levels (Tables 2 and 3, Fig. 1).
Fig. 1.
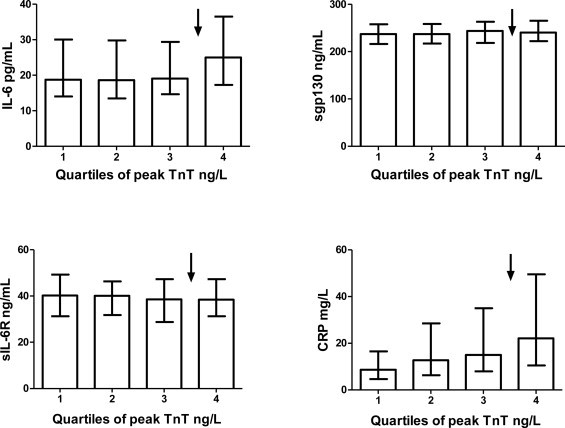
Inflammatory biomarkers related to TnT levels. The different variables (IL-6, sgp130, sIL-6R and CRP) as related to quartiles of peak TnT Levels are given as median values with interquartile range.
3.3. Association with LVEF and NT-proBNP
We found significant inverse correlations between LVEF and levels of IL-6, sgp130 and CRP. There were also significant correlations between NT-proBNP levels and IL-6 and CRP (Table 2).
When dichotomized into groups of high (=40%) and low LVEF, we found significantly elevated levels of IL-6 and CRP in the lower LVEF group (Table 3). When dichotomizing NT-proBNP at the 75th percentile (=122?ng/L), IL-6, sgp130 and CRP were significantly elevated in the upper quartile compared to the lower three quartiles (p < 0.01, all) (Table 3). However sIL-6R was neither associated with LVEF nor with NT-proBNP levels (Table 3).
3.4. Association with glucometabolic state
Weak, but statistically significant correlations were found for sgp130 and CRP levels and both HbA1c and admission glucose (Table 2). IL-6, sgp130 and CRP were also significantly correlated to fasting glucose levels (Table 2). Dichotomized into groups of known diabetes or not, high (=6.5%) or low HbA1c levels, high (=11.1?mmol/L) or low admission-glucose, and high (=7.0?mmol/L) or low fasting glucose levels, sgp130 and CRP levels were significantly elevated in the groups of patients with known diabetes, and high HbA1c and glucose levels (Table 3). IL-6 was significantly elevated in the group of high admission glucose levels only. sIL-6R did not show any association to the glucometabolic state (Table 3).
4. Discussion
The main results of the present study were that patients with the most extensive myocardial necrosis defined as the upper quartile of peak TnT, had elevated circulating levels of IL-6 and CRP, whereas levels of circulating receptor and receptor unit, sIL-6R and sgp130, were not related to the degree of myocardial necrosis. Furthermore, IL-6, sgp130 and CRP were elevated in patients with high NT-proBNP levels and IL-6 and CRP were associated with reduced LVEF. Finally, circulating levels of sgp130 were significantly elevated in STEMI patients with diabetes and were associated with glucometabolic variables measured during the acute STEMI.
Neither CRP, sgp130 nor sIL-6R were associated with age or gender in this STEMI population. IL-6 was weakly associated with age, and interestingly, smokers had significantly reduced sgp130 levels, which to our knowledge has not previously been shown. This is not easily explainable, but it might fit with the assumption that sgp130 has antiinflammatory properties [7] and that smokers are prone to inflammation. When we compared the measured inflammatory biomarkers to each other, IL-6 was significantly correlated to CRP as expected. However sgp130 and sIL-6R were weakly intercorrelated, whereas sIL-6R was weakly inversely correlated to CRP. As sgp130 may act as an inhibitor of the sIL-6R/IL-6 complex [7,13], binding of sgp13 may inhibit the activation of the transsignalling cascade, and thereby possibly reducing the levels of CRP.
The relation between IL-6, CRP and myocardial necrosis or infarct size is well known. Our results showing an association between IL-6 and CRP with high levels of peak TnT are thus in accordance with other studies, confirming the connection between inflammation and infarct size [6,14,15]. A novel observation in STEMI patients was the lack of association between peak TnT and sgp130 or sIL-6R. There is limited knowledge of the role of these circulating members of the IL-6 receptor complex in patients with STEMI. Significantly higher levels of sIL-6R in a group of patients with acute myocardial infarction compared to stable angina patients and controls has been reported [16]. In contrast, in the study of Kaminski et al., no differences were observed in the levels of neither sIL-6R nor sgp130 in patients with myocardial infarction (MI) compared to patients with stable angina or healthy controls [17]. They showed, however, a possible relation between concentrations of IL-6 and sIL-6R in the coronary sinus and cardiovascular complications in patients with MI [17]. The levels of sIL-6R and sgp130 in this study were of similar magnitude as was found in the present study. There is limited knowledge on sgp130 levels in MI patients, although an inverse association between sgp130 levels and CRP has been demonstrated [18], indicating high levels of sgp130 to be protective in this setting. One might speculate that the complex orchestration of the IL-6 system does not happen in the acute phase, but might – especially in the case of sgp130 – happen after the initial burst of pro-inflammatory stimuli as an anti-inflammatory compensatory mechanism in the subacute phase after AMI. It should also be emphasized that the variables measured in the circulation may not reflect or correlate to the tissue consentration.
The importance of inflammation in congestive heart failure has been consistently reported over the last decades. Our results, showing significantly elevated levels of IL-6 and CRP in patients with high NT-proBNP values and low LVEF, are consistent with other reports [6,19]. We found no direct correlation between sgp130 and NT-proBNP, but when NT-proBNP was dichotomised at the 75th percentile, we found significantly elevated levels of sgp130 in the upper quartile vs. the lower three quartiles. This may reflect an up regulation of sgp130 in patients with failing post-ischemic myocardium, as also suggested by others [20]. This association, which seems to be independent of the extent of myocardial necrosis, is to our knowledge not previously described in STEMI patients. However, sgp130 levels were not related to low LVEF. It has previously also been shown that elevated sgp130 levels are associated with cardiovascular mortality and death from worsening of heart failure [21] as well as with the severity of congestive heart failure [20,22]. No significant association between sIL-6R and heart failure could be demonstrated in our population. This is in line with a previous report [20].
There are to our knowledge no previous reports of the association between sgp130 and glucometabolic disturbances in a STEMI population. We found significantly higher levels of sgp130 in STEMI patients with known diabetes or high HbA1c values. Significant, although weak correlations were also found between sgp130 and both admission glucose, and fasting glucose. It is possible that MI patients with impaired glucose regulation have increased sgp130 levels related to insulin resistance and endothelial dysfunction, although this hypothesis must be investigated in further studies. The association regarding fasting glucose should be interpreted with caution as glucose levels in the acute phase might be influenced by myocardial necrosis or inflammation. A possible influence of glucose per se should nevertheless be further explored. A significant relationship between sgp130 and the metabolic syndrome and insulin resistance discussed to be related to endothelial dysfunction, has previously been reported [23,24]. Again, a compensatory up-regulation of sgp130 might be suggested.
5. Limitations
One limitation in our study is the lack of repeated blood sampling in order to study the time-course of the measured variables. Also the variability in the time frame from symptoms onset to blood sampling may play a role. We do not know, from the present study, the time profile of sgp130 and sIL-6R after a STEMI and we may have missed both a peak value as well as possible transient changes, as discussed in previous reports [25,26].
There was, however, a relatively large number of patients included with relatively large differences in both time from symptoms and time from PCI, to blood sampling and any correlations between time and measured parameters were weak except for CRP, suggesting that the measured variables were representative for the levels in a broad population of patients with PCI treated STEMI.
The median time from start of symptoms to blood sampling was 24?h. IL-6 was weakly correlated to time, which is in accordance with other reports describing peak IL-6 in patients with STEMI occurring after the first day [27,28]. For CRP there was a stronger association between time from start of symptoms to blood sampling and the measured levels, despite the fact that peak CRP value may occur as late as 72?h after PCI [27,28]. Secondly, our STEMI cohort was a low-risk population with subnormal LVEF and few complications, which may have influenced the results.
6. Conclusion
In our STEMI-population circulating levels of IL-6 and CRP were elevated in patients with high peak TnT, confirming previous reports. In contrast we found no association between circulating levels of gp130 or IL-6R and myocardial necrosis. Finally sgp130 levels were weakly, but statistically significantly associated with measures of heart failure and glucometabolic disturbance and sIL-6R did not show any associations with these parameters.
The biological importance of the IL-6/IL-6R/gp130-mediated transsignalling pathway in patients with acute myocardial infarction and dysglycemia should be further elucidated.
Acknowledgements
This work was supported by the Stein Erik Hagen Foundation for Clinical Heart Research, Oslo, Norway. We thank the study nurses and the staff at the Coronary Intensive Care Unit and Center for Clinical Heart Research for excellent assistance and medical technologist Sissel Åkra for laboratory analyses. The study was a part of the Biobanking in myocardial infarction (BAMI) project at Oslo University Hospital Ullevål, which is lead by a Steering committee including Arild Mangschau, Reidar Bjørnerheim, Dan Atar, as well as the following authors: Seljeflot, Arnesen (Chair), Eritsland, Halvorsen and Andersen.
Contributor Information
Vibeke N. Ritschel, Email: Vibeke.Ritschel@ous-hf.no.
Ingebjørg Seljeflot, Email: Ingebjorg.Seljeflot@ous-hf.no.
Harald Arnesen, Email: Harald.Arnesen@ous-hf.no.
Sigrun Halvorsen, Email: Sigrun.Halvorsen@ous-hf.no.
Thomas Weiss, Email: thomas.weiss@meduniwien.ac.at.
Jan Eritsland, Email: Jan.Eritsland@ous-hf.no.
Geir Ø Andersen, Email: Geir.Oystein.Andersen@ous-hf.no.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovascular Research. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 4.Aukrust P, Yndestad A, Smith C, Ueland T, Gullestad L, Damas JK. Chemokines in cardiovascular risk prediction. Thrombosis and Haemostasis. 2007;97:748–754. [PubMed] [Google Scholar]
- 5.Young JL, Libby P, Schonbeck U. Cytokines in the pathogenesis of atherosclerosis. Thrombosis and Haemostasis. 2002;88:554–567. [PubMed] [Google Scholar]
- 6.Karpinski L, Plaksej R, Kosmala W, Witkowska M. Serum levels of interleukin-6, interleukin-10 and C-reactive protein in relation to left ventricular function in patients with myocardial infarction treated with primary angioplasty. Kardiologia polska. 2008;66:1279–1285. [PubMed] [Google Scholar]
- 7.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thrombosis and Haemostasis. 2009;102:215–222. doi: 10.1160/TH09-05-0297. [DOI] [PubMed] [Google Scholar]
- 9.Szabo-Fresnais N, Lefebvre F, Germain A, Fischmeister R. Pomerance M. A new regulation of IL-6 production in adult cardiomyocytes by beta-adrenergic and IL-1 beta receptors and induction of cellular hypertrophy by IL-6 trans-signalling. Cell Signal. 2010;22:1143–1152. doi: 10.1016/j.cellsig.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. Journal of Leukocyte Biology. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 11.Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. European Journal of Biochemistry. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 12.Diabetes American. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl. 1):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rose-John S. Interleukin-6 biology is coordinated by membrane bound and soluble receptors. Acta Biochimica Polonica. 2003;50:603–611. [PubMed] [Google Scholar]
- 14.Orn S, Manhenke C, Ueland T, Damas JK, Mollnes TE, Edvardsen T. C-reactive protein, infarct size, microvascular obstruction, and left-ventricular remodelling following acute myocardial infarction. European Heart Journal. 2009;30:1180–1186. doi: 10.1093/eurheartj/ehp070. [DOI] [PubMed] [Google Scholar]
- 15.Okin PM, Bang CN, Wachtell K, Hille DA, Kjeldsen SE, Dahlof B. Relationship of sudden cardiac death to new-onset atrial fibrillation in hypertensive patients with left ventricular hypertrophy. Circulation: Arrhythmia and Electrophysiology Archives. 2013;6:243–251. doi: 10.1161/CIRCEP.112.977777. [DOI] [PubMed] [Google Scholar]
- 16.Bossowska A, Kiersnowska-Rogowska B, Bossowski A, Galar B, Sowinski P. Cytokines in patients with ischaemic heart disease or myocardial infarction. Kardiologia Polska. 2003;59:105–114. [PubMed] [Google Scholar]
- 17.Kaminski KA, Kozuch M, Bonda T, Wojtkowska I, Kozieradzka A, Dobrzycki S. Coronary sinus concentrations of interleukin 6 and its soluble receptors are affected by reperfusion and may portend complications in patients with myocardial infarction. Atherosclerosis. 2009;206:581–587. doi: 10.1016/j.atherosclerosis.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 18.Ichiki T, Jougasaki M, Setoguchi M, Shimokawahara H, Nakashima H, Matsuoka T. Plasma levels of soluble glycoprotein 130 in acute myocardial infarction. Journal of Cardiology. 2007;50:101–119. [PubMed] [Google Scholar]
- 19.van Diepen S, Roe MT, Lopes RD, Stebbins A, James S, Newby LK. Baseline NT-proBNP and biomarkers of inflammation and necrosis in patients with ST-segment elevation myocardial infarction: insights from the APEX-AMI trial. Journal of Thrombosis and Thrombolysis. 2012;34:106–113. doi: 10.1007/s11239-012-0691-0. [DOI] [PubMed] [Google Scholar]
- 20.Aukrust P, Ueland T, Lien E, Bendtzen K, Muller F, Andreassen AK. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. American Journal of Cardiology. 1999;83:376–382. doi: 10.1016/s0002-9149(98)00872-8. [DOI] [PubMed] [Google Scholar]
- 21.Askevold ET, Nymo S, Ueland T, Gravning J, Wergeland R, Kjekshus J. Soluble glycoprotein 130 predicts fatal outcomes in chronic heart failure: analysis from the controlled rosuvastatin multinational trial in heart failure (CORONA) Circulation: Heart Failure. 2013;6:91–98. doi: 10.1161/CIRCHEARTFAILURE.112.972653. [DOI] [PubMed] [Google Scholar]
- 22.Hirota H, Izumi M, Hamaguchi T, Sugiyama S, Murakami E, Kunisada K. Circulating interleukin-6 family cytokines and their receptors in patients with congestive heart failure. Heart Vessels. 2004;19:237–241. doi: 10.1007/s00380-004-0770-z. [DOI] [PubMed] [Google Scholar]
- 23.Zuliani G, Galvani M, Maggio M, Volpato S, Bandinelli S, Corsi AM. Plasma soluble gp130 levels are increased in older subjects with metabolic syndrome. The role of insulin resistance. Atherosclerosis. 2010;213:319–324. doi: 10.1016/j.atherosclerosis.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss TW, Arnesen H, Seljeflot I. Components of the Interleukin-6 transsignalling system are associated with the metabolic syndrome, endothelial dysfunction and arterial stiffness. Metabolism. 2013;62:1008–1013. doi: 10.1016/j.metabol.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Bonda T, Kaminski KA, Kozuch M, Kozieradzka A, Wojtkowska I, Dobrzycki S. Circadian variations of interleukin 6 in coronary circulations of patients with myocardial infarction. Cytokine. 2010;50:204–209. doi: 10.1016/j.cyto.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Hartford M, Wiklund O, Mattsson Hulten L, Perers E, Person A, Herlitz J. CRP, interleukin-6, secretory phospholipase A2 group IIA, and intercellular adhesion molecule-1 during the early phase of acute coronary syndromes and long-term follow-up. International Journal of Cardiology. 2006;108:55–62. doi: 10.1016/j.ijcard.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Theroux P, Armstrong PW, Mahaffey KW, Hochman JS, Malloy KJ, Rollins S. Prognostic significance of blood markers of inflammation in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty and effects of pexelizumab, a C5 inhibitor: a substudy of the COMMA trial. European Heart Journal. 2005;26:1964–1970. doi: 10.1093/eurheartj/ehi292. [DOI] [PubMed] [Google Scholar]
- 28.Solheim S, Grogaard HK, Hoffmann P, Arnesen H, Seljeflot I. Inflammatory responses after percutaneous coronary intervention in patients with acute myocardial infarction or stable angina pectoris. Scandinavian Journal of Clinical and Laboratory Investigation. 2008;68:555–562. doi: 10.1080/00365510701884584. [DOI] [PubMed] [Google Scholar]


