Highlights
-
•
A 59-year-old postmenopausal woman had ovarian clear cell adenocarcinoma producing AFP.
-
•
The tumor lacked a yolk sac component and formed ducts similar to the fetal gut.
Keywords: Ovary, Postmenopausal, AFP-producing, Clear cell adenocarcinoma, Fetal gut appearance
Introduction
Alpha-fetoprotein (AFP) was first described in the human fetus in 1956 (Bergstrand and Czar, 1956). AFP is synthesized by the developing liver by at least 6 weeks of gestation. After birth, AFP rapidly disappears and is not detected in the serum of healthy persons. This protein is a useful tumor marker for detecting and monitoring malignant tumors, such as germ cell tumors, especially yolk sac tumor (YST), and hepatoid carcinoma, and is also significant for confirming the diagnosis. Epithelial ovarian carcinoma rarely secretes AFP, especially in postmenopausal women. We report here a 59-year-old postmenopausal woman with AFP producing ovarian clear cell adenocarcinoma (CCA) with germ cell tumor differentiation, simulating YST of fetal gut type. The serum AFP level was elevated to 1606 ng/ml preoperatively, with a subsequent decrease to the normal range after treatment. The tumor was composed of conventional clear cells and those similar to the fetal gut. Immunohistochemically, the cellular cytoplasm was diffusely positive for AFP and GPC3, and focally positive for SALL4, which are known as oncofetal proteins expressed in germ cell tumors.
Case
A 59-year-old postmenopausal woman, gravida 2, parity 2, was admitted to our hospital, with the chief complaints with urinary incontinence, frequent urination, lower back pain, and left lower-limb edema. A transvaginal ultrasonographic examination showed a multilocular cystic mass, with a heterogeneous solid part and fluid (Fig. 1A). Magnetic resonance imaging (MRI) showed a multicystic pelvic mass, with a stained-glass pattern, measuring 14 cm in diameter. T1-weighted gadolinium-enhanced MRI showed an enhanced solid mass at the head of the tumor (Fig. 1B, C). Computed tomography did not detect any signs of distant metastasis or lymphadenopathy. Serum tumor markers including carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), CA125, and squamous cell carcinoma antigen (SCC) were within the normal range. Only serum AFP level was elevated to 1606 ng/ml.
Fig. 1.
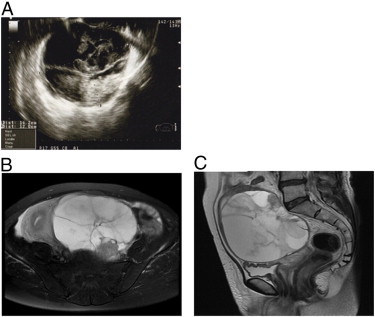
Ultrasonography showing a multilocular, heterogeneous tumor (A). MRI showing a multicystic pelvic mass with a stained-glass pattern: T1W1 image (B), T2W1 image (C).
The patient underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy, omentectomy, appendectomy, pelvic lymphadenectomy, and para-aortic lymphadenectomy. The surgery was optimal, that is, all gross tumors were resected almost completely. Ascitic fluid was cytologically negative. Histologically, the predominant pattern in the tumor was characterized by the presence of cuboidal or polygonal cells having a clear cytoplasm in solid nests and intermediate nuclear grade (Fig. 2A). No typical hobnail cells of conventional CCA or Schiller Duval bodies commonly observed in YST were detected. We also observed a fetal gut-like appearance of a clear cell pattern, showing a tubular structure lined by clear columnar cells (Fig. 2B).
Fig. 2.
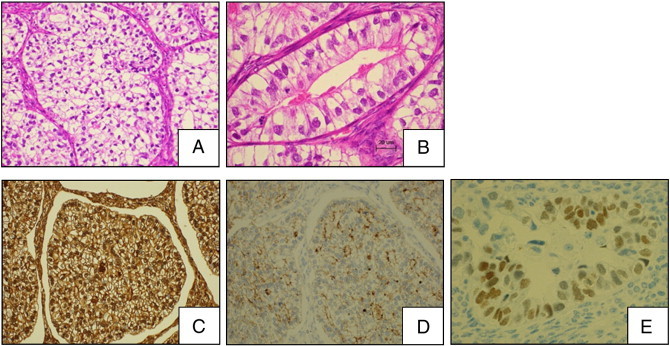
Microscopic examination. (A) Solid area composed of a sheet of polygonal or cuboidal clear cells with intermediate nuclear grade. (H&E × 200) (B) Tubular structure lined by clear columnar cells having subnuclear or supranuclear vacuoles, characterized by fetal gut appearance. (C) Diffuse positive reaction for AFP in the cytoplasm. (D) Diffuse positive reaction for GPC3 in the cytoplasm. (E) Focal positive reaction for SALL4 in the nucleus.
The immunohistochemical staining results are shown in Table 1. The tumor cells were diffusely positive for AFP (Fig. 2C) and GPC3 (Fig. 2D) and were focally positive for SALL4 (Fig. 2E). No apparent expression of epithelial membrane antigen, cytokeratin 7, inhibin-α, calretinin, CD30, human chorionic gonadotropin, phospholipase A2-activating protein, C-kit, or hepatocyte nuclear factor-1beta (HNF-1β) was observed. The combination of the morphological findings and immunohistochemical expression profiles confirmed the diagnosis of ovarian CCA partially simulating YST of fetal gut type. The patient was staged as pT2bN0M0.
Table 1.
Immunohistochemical staining results: −, negative; 1 +, < 25%; 2 +, 25–49%; 3 +, 50% or greater.
| AE1/3 | 3 + | CD30 | − |
| EMA | − | HCG | − |
| CK7 | − | PLAP | − |
| LeuM1 | 1 + | C-kit | − |
| CEA | 1 + | Ki-67 labeling index | 40% |
| Inhibin-α | − | SALL4 | 1 + |
| Calretinin | − | GPC3 | 3 + |
| AFP | 3 + | HNF-1β | − |
Following an operation, the patient underwent adjuvant chemotherapy consisting of six cycles of paclitaxel and carboplatin. Serum AFP level eventually decreased to the normal value after the surgery. AFP level in the normal range has been maintained up to the present day (Supplemental Table. 1). She is currently clinically free of the tumor, 23 months postoperatively.
Following an operation, the patient underwent adjuvant chemotherapy consisting of six cycles of paclitaxel and carboplatin. Serum AFP level eventually decreased to the normal value after the surgery. AFP level in the normal range has been maintained up to the present day (Supplemental Table. 1). She is currently clinically free of the tumor, 23 months postoperatively.
Discussion
Most of the AFP-producing ovarian tumors occurring in young women are germ cell tumors, specifically YSTs. However in elderly, postmenopausal women, AFP-producing ovarian malignancies are rarely encountered and their histology varies. Some AFP-producing ovarian tumors in postmenopausal women have been documented, such as endometrioid adenocarcinoma with a yolk sac component, mucinous adenocarcinoma with a yolk sac component, CCA with a serous adenocarcinoma component, and endometrioid adenocarcinoma with a clear cell component (Abe et al., 2008; Kamoi et al., 2002; Esheba et al., 2008; Cetin et al., 2007; Maida et al., 1998). However, the ovarian tumor in our case, which was largely composed of clear cells, was different from these other tumors histopathologically in terms of lacking a YST component and forming glands similar to a gland-like fetal gut. The clear cells were the predominant pattern in histology and were not identified as a common ovarian CCA because of a lack of hobnail cells and papillary architecture with eosinophilic stromal core. The transcription factor hepatocyte nuclear factor-1β (HNF1β), known to overexpress in CCA, was negative in this case. The cellular cytoplasm was immunohistochemically positive for AFP. These cells were also positive for SALL4 and GPC3, which are known as oncofetal proteins expressed in germ cell tumors. Although AFP producing hepatoid ovarian cancers were known to mimic clear cells, the combination of morphological findings and immunohistochemical expression profile confirmed the diagnosis of AFP-producing ovarian CCA partially simulating YST of fetal gut type, not hepatoid ovarian cancers.
The appropriate adjuvant therapy and biological behavior of this type of tumor at recurrence need to be considered. This is because this AFP-producing ovarian tumor appears to have two propensities: an epithelial tumor and a germ cell tumor. Physicians need to be aware that AFP-producing propensity may disappear in metastases, even if the primary tumor is originally provided with AFP production (Mostofi, 1985). This kind of tumor might be a case with discrepancy between function and morphology at recurrence because of latent multipotentiality and heterogeneity.
In our case, in the therapeutic course, paclitaxel and carboplatin were introduced as first-line chemotherapy for six cycles. We selected this treatment regimen because we focused on epithelial tumor, as conventional CCA is the predominant pattern in this patient, in terms of the prognosis rather than germ cell differentiation. Assuming that this tumor relapses, we would like to attempt to remove tissue and examine its morphology in detail.
In our hospital, serum AFP level in ovarian tumors is not routinely checked preoperatively, especially for elderly women. Yasuda and Meguro reported a AFP-producing ovarian tumor in postmenopausal women and reviewed the similar cases (Meguro and Yasuda, 2011). According to them, most cases were a mixed-type tumor, including epithelial malignancies or YST. The category of these rare tumors remains controversial, and it is unknown which cases should be investigated for the elevation of preoperative serum AFP level and immunohistochemical expression for AFP. We suggest that serum AFP levels should be measured in elderly or postmenopausal women if their ovarian tumor shows multilocular or heterogeneous imaging in an ultrasonographic examination.
The following are the Supplementary data related to this article.
Serum AFP level: preoperative:postperative.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.gynor.2014.03.001.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
We thank Ms Ushio and Ms Kinoshita for their excellent technical assistance.
References
- Abe A., Furumoto H., Yoshida K., Nishimura M., Irahara M., Kudo E., Sano T. A case of ovarian endometrioid adenocarcinoma with a yolk sac tumor component. Int. J. Gynecol. Cancer. 2008;18:168–172. doi: 10.1111/j.1525-1438.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- Bergstrand C.G., Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand. J. Clin. Lab. Invest. 1956;8:174. doi: 10.3109/00365515609049266. [DOI] [PubMed] [Google Scholar]
- Cetin A., Bahat Z., Cilesiz P., Demirbag N., Yavuz E. Ovarian clear cell adenocarcinoma producing alpha-fetoprotein: case report. Eur. J. Gynaecol. Oncol. 2007;28:241–244. [PubMed] [Google Scholar]
- Esheba G.E., Pate L.L., Longacre T.A. Oncofetal protein glypican-3 distinguishes yolk sac tumor from clear cell carcinoma of the ovary. Am. J. Surg. Pathol. 2008;32:600–607. doi: 10.1097/PAS.0b013e31815a565a. [DOI] [PubMed] [Google Scholar]
- Kamoi S., Ohaki Y., Mori O., Okada S., Seto M., Matsushita N., Kawamura T., Araki T. A case of ovarian endometrioid adenocarcinoma with yolk sac tumor component in a postmenopausal woman. APMIS. 2002;110:508–514. doi: 10.1034/j.1600-0463.2002.100609.x. [DOI] [PubMed] [Google Scholar]
- Maida Y., Kyo S., Takakura M., Kanaya T., Inoue M. Ovarian endometrioid adenocarcinoma with ectopic production of alpha-fetoprotein. Gynecol. Oncol. 1998;71:133–136. doi: 10.1006/gyno.1998.5119. [DOI] [PubMed] [Google Scholar]
- Meguro S., Yasuda M. Alpha-fetoprotein-producing ovarian tumor in a postmenopausal woman with germ cell differentiation. Ann. Diagn. Pathol. 2011;17:140–144. doi: 10.1016/j.anndiagpath.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Mostofi F.K. Histological change ostensibly induced by therapy in the metastasis of germ cell tumors of testis. Prog. Clin. Biol. Res. 1985;203:47–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum AFP level: preoperative:postperative.


