Highlights
-
•
Metastases from extrapelvic organs to the uterine cervix are rare.
-
•
Cervical metastases from the stomach are often accompanied by extrauterine metastases.
-
•
We report a case of cervical metastasis of gastric cancer, occurring 10 years after the first surgical treatment.
Keywords: Gastric cancer, Metastasis to the uterine cervix, Late recurrence
Introduction
Late recurrence of gastric cancer after curative resection is rare. Distant metastasis to the uterine cervix is also quite rare, especially from extrapelvic organs (Abrams et al., 1950; Lemoine and Hall, 1986). Despite its rarity, it is important to consider the possibility of metastasis from gastric cancer to the uterine cervix if a female patient experiences genital bleeding or presents with a cervical tumor. Here, we present a case of gastric cancer metastasis to the uterine cervix 10 years after distal gastrectomy.
Case description
A 46-year-old woman, gravida 2 para 2, presented to our hospital with a complaint of irregular genital bleeding and a 27.1 × 20.7 mm uterine cervix tumor, which was detected on pelvic magnetic resonance imaging (MRI). One year prior to the present event, her cervical and endometrial cytology had been negative, and a pelvic MRI examination had shown normal findings (Fig. 1a). Ten years prior, the patient had undergone adjuvant chemotherapy following distal gastrectomy for pathologically diagnosed stage IIIB poorly differentiated gastric adenocarcinoma. The exact pathological stage of disease was pT3N2M0 according to the 5th edition Union for International Cancer Control (UICC) classification, and pT4aN2M0 according to the 7th edition UICC classification.
Fig. 1.
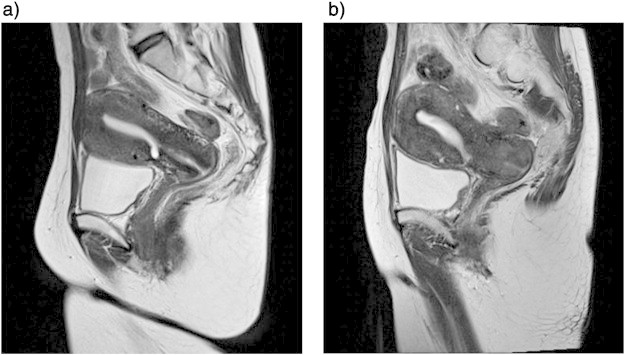
Pelvic magnetic resonance images. (a) No abnormalities are evident 1 year before the patient was referred to our hospital. (b) A mass in the uterine cervix is observed at the time of admission.
The patient's father had a history of prostate cancer. She showed no signs of recurrence during the 10 years following distal gastrectomy. The occurrence of her last menstrual period was unclear because of irregular vaginal bleeding. At the first clinical examination, genital bleeding was present and the patient bled easily from her uterine cervix. A transvaginal sonography revealed a 22 × 20 mm high echoic lesion at the dorsal side of the cervix. Biochemical blood examination results were all within normal limits, but the level of squamous cell carcinoma-related antigen was slightly elevated at 2.4 ng/mL. The result of a high-risk human papillomavirus (HPV)–DNA hybrid capture test was negative. Neither an upper gastrointestinal nor a colon fiberscopy showed any evidence of abnormalities.
A Papanicolaou (Pap) smear of the uterine cervix revealed atypical glandular cells, and a biopsy specimen taken from the uterine cervix indicated poorly differentiated adenocarcinoma, which made us suspect recurrence of the previous gastric cancer. Pelvic MRI showed a high-intensity 27.1 × 20.7 mm irregular mass on T2-weighted images of the cervix (Fig. 1b). Fluorine-18-fluorodeoxyglucose (18F-FDG) positron emission computed tomography did not show increased FDG uptake in the uterine cervix or other organs. Accordingly, the patient was diagnosed as having gastric cancer metastasis to the uterine cervix. Modified radical hysterectomy with bilateral salpingo-oophorectomy was performed with a curative intent and for diagnostic confirmation. Intraoperative examination did not reveal any ascitic fluid, palpable lymph nodes, or peritoneal seeding. The pathological diagnosis was metastatic adenocarcinoma in the uterine cervix, originating from the known gastric cancer. Subsequently, a postoperative Pap smear revealed atypical glandular cells because of residual tumor in the vaginal stump. First, the regimen with S-1 (tegafur, gimeracil and oteracil potassium) and cisplatin, which is one of the standard regimens for a recurrent gastric cancer in Japan, was presented to the patient however she didn't accept it because of the side effect. Therefore, chemotherapy with S-1 was originally planned to be administered daily for the first week of every 3-week cycle. However, the administration schedule was reduced due to side effects. The patient eventually received adjuvant chemotherapy with 100 mg/day S-1 administered daily during the first week of every 6-week cycle for 1 year. Pap smear results became negative for an intraepithelial lesion after chemotherapy. Follow-up computed tomography scans have not indicated any recurring tumor to date. At the time of this report, the patient remains alive and is believed to have achieved complete response 24 months after surgery.
Pathological findings
A mass with a focus on the dorsal side of the uterine cervix was observed. The tumor cells produced mucus and formed clusters, the findings are consistent with a poorly differentiated adenocarcinoma (the same type of adenocarcinoma as the patient's previous gastric cancer). The tumor cells mainly developed in the uterine cervical stroma, rather than in the epithelia of the uterine cervix (Fig. 2). An immunohistochemical analysis of the specimen was performed. Cancer cells stained positive for cytokeratin (CK) 7, CK20, carcinoembryonic antigen (CEA), mucin (MUC) 2, CDX2, p16 and p53, but negative for MUC1, MUC5 and MUC6. There was no evidence of cancer cells in the ovaries or the fallopian tubes. Tumor cells were not observed on peritoneal washing cytology analysis. Therefore, the patient was diagnosed with metastasis from the previous gastric cancer to the uterine cervix, involving the uterine corpus, vagina and uterosacral ligament.
Fig. 2.
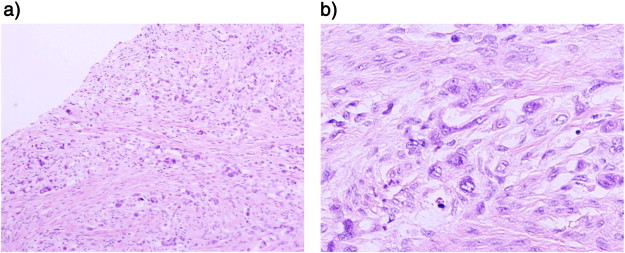
Microscopic findings of metastatic adenocarcinoma at the uterine cervix. (a) Hematoxylin and eosin staining, 100 ×. (b) Hematoxylin and eosin staining, 400 × .
Discussion
Metastasis from extrapelvic organs to the uterine cervix is rare. Abrams et al. reported that metastatic involvement of the uterine cervix occurred in only 3 of 1000 cases of metastatic cancer (Abrams et al., 1950). It has also been reported that, of all metastases to the uterine cervix except for the result of direct extension of a primary tumor, 36.4% originated from ovarian cancer, 30.3% from colorectal cancer, 15.2% from gastric cancer, 12.1% from breast cancer, 3.0% from renal cell cancer, and 3.0% from transitional cell cancer of the renal pelvis (Lemoine and Hall, 1986). The reason why incidence of metastasis to the uterine cervix is low remains unclear. However, Lemoine et al. suggested that this low incidence might result from an abundance of fibrous matrix and a low degree of vascularity (Lemoine and Hall, 1986). To our knowledge, only one case has been previously reported on the diagnosis of an isolated metastasis of gastric cancer to the uterine cervix by surgical specimens (Yokoyama et al., 2000). To identify relevant case reports, we searched PubMed for cases of metastatic gastric cancer to the uterine cervix, and reviewed 39 such cases from 7 references listed in Table 1 (Lemoine and Hall, 1986; Yokoyama et al., 2000; Perez-Montiel et al., 2012; Imachi et al., 1993; Matsuura et al., 1997; Zhang et al., 1983; Kashimura et al., 1983). In the following paragraphs, we present a summary of these cases.
Table 1.
Metastases to the uterine cervix from gastric cancer.
| Number of cases | 39 | Cervical cytology, No (%) | |||
|---|---|---|---|---|---|
| Positive | 19 | (61.3) | |||
| Age Mets (years) | Negative | 12 | (38.7) | ||
| Median | 44 | Not reported | 8 | ||
| Range | 23–75 | ||||
| Histology of metastatic cervical tumor, No (%) | |||||
| Metastatic sites documented (36 cases), No. (%) | SRC | 22 | (64.7) | ||
| Ovary | 27 | (75.0) | PD | 7 | (20.6) |
| Peritoneum | 12 | (33.3) | UD | 1 | (2.9) |
| Douglas mass | 8 | (22.2) | UD + SRC | 3 | (8.8) |
| Cervix alone | 2 | (5.5) | PD + tubular | 1 | (2.9) |
| Not reported | 5 | ||||
| Clinical stage of the primary gastric cancer, No (%) | |||||
| I | 1 | (5.3) | P/M Interval (months) | ||
| II | 0 | Median | 22 | ||
| III | 0 | Range | 1–121 | ||
| IV | 18 | (94.7) | |||
| Not reported | 20 | Survival (months) | |||
| Dead (25 cases) | |||||
| Symptoms, No (%) | Median | 4 | |||
| Atypical genital bleeding | 14 | (35.8) | Range | 0–33 | |
| Abdominal pain | 8 | (20.5) | Alive (8 cases) | ||
| Abdominal mass | 4 | (10.2) | Median | 4 | |
| Other | 8 | (20.5) | Range | 1–8 | |
| No symptom | 2 | (5.1) | Not reported (6 cases) | ||
Metastatic sites included multiple cases. Cervical cytology positive; cancer cells or atypical cells were observed. SRC, signet ring cells; PD, poorly differentiated; UD, undifferentiated. P/M interval; interval between the diagnosis of primary gastric cancer and metastatic cervical tumor in 22 recurrent cases.
Among patients with cervical metastases, the median age at the time of metastasis diagnosis was 44 years (range, 23–75 years), and only 9 cases were diagnosed among patients older than 50 years (Lemoine and Hall, 1986; Yokoyama et al., 2000; Perez-Montiel et al., 2012; Imachi et al., 1993; Matsuura et al., 1997; Zhang et al., 1983; Kashimura et al., 1983). Therefore, we speculate that high blood flow to the uterine cervix could be an important factor in cases of cervical metastasis of gastric cancer.
The major histological types of metastatic gastric cancer are signet ring adenocarcinoma (64.7% of cases) and poorly differentiated adenocarcinoma (20.6% of cases) (Yokoyama et al., 2000; Perez-Montiel et al., 2012; Imachi et al., 1993; Matsuura et al., 1997; Zhang et al., 1983; Kashimura et al., 1983). At the time of presentation, the most common chief complaint was atypical genital bleeding, followed by abdominal pain. Pap smears may be a useful method for diagnosing metastases of gastric cancer to the uterine cervix, because abnormal cytological findings such as cancer cells or atypical glandular cells were observed in 19 of 31 cases (61.3%) (Yokoyama et al., 2000; Perez-Montiel et al., 2012; Imachi et al., 1993; Matsuura et al., 1997; Zhang et al., 1983; Kashimura et al., 1983).
In most cases involving metastasis of gastric cancer to the uterine cervix, the primary gastric cancer was classified as stage IV and had poor prognosis. Median survival from the time of diagnosis of cervical metastasis was only 4 months (range, 0–33 months). Of 36 cases, 34 also involved other extrauterine metastatic sites, including the ovaries (27 cases; 75.0%), the peritoneum (12 cases; 33.3%), and the pouch of Douglas (8 cases; 22.2%) (Lemoine and Hall, 1986; Yokoyama et al., 2000; Perez-Montiel et al., 2012; Imachi et al., 1993; Matsuura et al., 1997; Zhang et al., 1983; Kashimura et al., 1983). Only one case was found to be a cervical metastasis from a gastric cancer without extrauterine metastasis, based on postsurgical pathological findings (Yokoyama et al., 2000). In the other case, the diagnosis was only confirmed via biopsy (Perez-Montiel et al., 2012). Accordingly, metastasis of gastric cancer to the uterine cervix without the involvement of extrauterine metastatic sites is quite rare. Yet, the case we reported here had this feature.
Gastric cancer recurrence usually occurs within 2 years of treatment (75.4%) and the recurrence rates after > 5 years and > 7 years are only 5.7% and 2.1%, respectively (Shiraishi et al., 2000). In 22 recurrent cases, the median interval between the diagnosis of primary gastric cancer and the diagnosis of the cervical metastasis was 22 months (range, 1–121 months) (Lemoine and Hall, 1986; Yokoyama et al., 2000; Perez-Montiel et al., 2012; Imachi et al., 1993; Matsuura et al., 1997; Zhang et al., 1983; Kashimura et al., 1983). Therefore, recurrence 10 years after the diagnosis of primary gastric cancer, as in our case, is extremely rare.
When choosing a therapeutic strategy, it is important to distinguish primary tumors from metastases. In our case, the cervical tumor was a poorly differentiated adenocarcinoma, which has the same histological type as the primary gastric cancer. Immunohistochemical analysis revealed that tumor cells stained positive for CK7, CK20, CDX2 and CEA. These results are much more likely to be observed in gastric cancer than in primary cervical adenocarcinoma, although we couldn't know which the primary gastric adenocarcinoma was stained for CDX2 and the other marker due to a lack of access to the primary specimen for re-staining. In addition, the overall prevalence of high-risk HPV (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) in primary cervical adenocarcinoma is 91.1% (Castellsague et al., 2006). Yet, a high-risk HPV–DNA test was negative in our case. On the other hand, an immunohistochemical analysis showed the tumor cells stained positive for p16, as one of a marker for HPV infection, and negative in the normal cervical glands. The results seem to conflict, however, gastric cancers were also positive for p16 in 75% of cases (Schneider et al., 2000), again suggesting that the cancer was not a primary cervical adenocarcinoma. Based on this finding, we suggest that high-risk HPV–DNA testing could be valuable in diagnosing metastasis of adenocarcinoma to the uterine cervix.
We selected a modified hysterectomy, which resected the parametrium broadly but did not resect the vaginal side. However, unfortunately the margin was slightly positive. Therefore the adjuvant chemotherapy was needed.
In conclusion, metastasis from extrapelvic organs to the uterine cervix should be considered during differential diagnosis of a female patient with genital bleeding or cervical tumors, even in cases of carcinoma treatment many years prior to the presentation of such symptoms.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Contributor Information
Takuro Yamamoto, Email: teku@koto.kpu-m.ac.jp.
Taisuke Mori, Email: moriman@koto.kpu-m.ac.jp.
Jo Kitawaki, Email: kitawaki@koto.kpu-m.ac.jp.
References
- Abrams H.L., Spiro R., Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Castellsague X., Diaz M., de Sanjose S., Munoz N., Herrero R., Franceschi S. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J. Natl. Cancer Inst. 2006;98:303–315. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- Imachi M., Tsukamoto N., Amagase H., Shigematsu T., Amada S., Nakano H. Metastatic adenocarcinoma to the uterine cervix from gastric cancer. A clinicopathologic analysis of 16 cases. Cancer. 1993;71:3472–3477. doi: 10.1002/1097-0142(19930601)71:11<3472::aid-cncr2820711103>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Kashimura M., Kashimura Y., Matsuyama T., Tsukamoto N., Sugimori H., Taki I. Adenocarcinoma of the uterine cervix metastatic from primary stomach cancer. Cytologic findings in six cases. Acta Cytol. 1983;27:54–58. [PubMed] [Google Scholar]
- Lemoine N.R., Hall P.A. Epithelial tumors metastatic to the uterine cervix. A study of 33 cases and review of the literature. Cancer. 1986;57:2002–2005. doi: 10.1002/1097-0142(19860515)57:10<2002::aid-cncr2820571021>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Saito R., Kawagoe T., Toki N., Sugihara K., Kashimura M. Cytologic analysis of primary stomach adenocarcinoma metastatic to the uterine cervix. Acta Cytol. 1997;41:291–294. doi: 10.1159/000332514. [DOI] [PubMed] [Google Scholar]
- Perez-Montiel D., Serrano-Olvera A., Salazar L.C., Cetina-Perez L., Candelaria M., Coronel J. Adenocarcinoma metastatic to the uterine cervix: a case series. J. Obstet. Gynaecol. Res. 2012;38:541–549. doi: 10.1111/j.1447-0756.2011.01747.x. [DOI] [PubMed] [Google Scholar]
- Schneider B.G., Gulley M.L., Eagan P., Bravo J.C., Mera R., Geradts J. Loss of p16/CDKN2A tumor suppressor protein in gastric adenocarcinoma is associated with Epstein-Barr virus and anatomic location in the body of the stomach. Hum Pathol. 2000;31:45–50. doi: 10.1016/s0046-8177(00)80197-5. [DOI] [PubMed] [Google Scholar]
- Shiraishi N., Inomata M., Osawa N., Yasuda K., Adachi Y., Kitano S. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer. 2000;89:255–261. doi: 10.1002/1097-0142(20000715)89:2<255::aid-cncr8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y., Sato S., Futagami M., Saito Y. Solitary metastasis to the uterine cervix from the early gastric cancer: a case report. Eur. J. Gynaecol. Oncol. 2000;21:469–471. [PubMed] [Google Scholar]
- Zhang Y.C., Zhang P.F., Wei Y.H. Metastatic carcinoma of the cervix uteri from the gastrointestinal tract. Gynecol. Oncol. 1983;15:287–290. doi: 10.1016/0090-8258(83)90084-7. [DOI] [PubMed] [Google Scholar]


