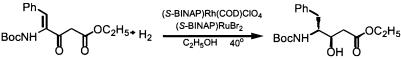Abstract
Chirality of organic molecules plays an enormous role in areas ranging from medicine to material science, yet the synthesis of such entities in one enantiomeric form is one of the most difficult challenges. The advances being made stem from the convergence of a broader understanding of theory and how structure begets function, the developments in the interface between organic and inorganic chemistry and, most notably, the organic chemistry of the transition metals, and the continuing advancements in the tools to help define structure, especially in solution. General themes for designing catalysts to effect asymmetric induction are helping to make this strategy more useful, in general, with the resultant effect of a marked enhancement of synthetic efficiency.
Even before the understanding that carbon was tetravalent, the French physicist Biot established that certain organic compounds rotated the plane of polarization of light (1, 2). However, it was Pasteur who correlated this phenomenon with an asymmetric grouping of atoms within molecules (3). Kekulé establishing that carbon has four valences (4) and van't Hoff (5) and Le Bel (6) arranging these valences in a tetrahedral fashion set the stage for one of the most profound features of organic molecules—their ability to exist in mirror-image forms. The implications of this fundamental feature of organic molecules is immense. Undoubtedly, the richness of the biological world would not exist without this structural feature. Indeed, the very existence of the biological world is likely to have become possible only because of its exquisite use of this phenomenon.
Properties of molecules and molecular arrays depend on chirality. Molecular communication in biological systems emanates from this intrinsic structural feature. Optical and electronic materials exhibit their effects by highly ordered assemblies whose sense of ordering frequently derives from this feature. Bulk properties also derive from this phenomenon, and it is amply seen in the frequently enhanced properties of stereodefined macromolecules compared with stereorandom polymers (7–12). Despite this importance, the ability to obtain chiral molecules in enantiopure form remained extremely limited until recently.
Nature provided the richest source of such compounds, providing a limited catalog of enantiomerically pure compounds referred to as the “chiral pool” (13–16). Separating racemates (18–20), a procedure introduced by Pasteur (21–24), represents the most widespread industrially important method for production of enantiomerically pure products. Because any racemate contains only 50% of the desired enantiomer, the theoretical yield of this strategy is limited to 50%, unless it is possible to convert the opposite enantiomer into the desired one either by a dynamic kinetic resolution or by an alternative synthetic scheme.
The fact that nature already used such methods led to exploitation of nature's asymmetric synthetic machinery (25) in the early part of the 20th century (26–28). Purified enzymes like hydrolases and lipases, which do not require cofactors, have proven to be the most versatile (29–31). Enzymes requiring more complex ancillary biological machinery like cofactors are also succumbing to use in the test tube by developing ancillary cofactor regeneration schemes with multicomponent enzyme systems (32). Selective introduction of oxygen by mono- or dioxygenases have particular merit (33), as illustrated by the commercial synthesis of corticosteroids. A particularly intriguing example is the dihydroxylation of arenes as illustrated in Scheme 1 (34, 35). Not only is the enantioselectivity virtually perfect, but the chemoselectivity is extraordinary (36). Catalytic antibodies competent to bind transition-state mimics represent another dimension of biological methods for potential chemical synthesis (37). To obviate the yield limitations of kinetic resolutions require combination with a racemization event. The most common approach involves substrates in which racemization can be accomplished simply by adjusting pH. A more intriguing strategy is the combination of a biological and a nonbiological catalyst as illustrated in Scheme 2 (38). Thus, the racemate of alcohol 1 undergoes equilibration by means of the ketone 2 with a Ru catalyst that is compatible with the enzymatic system that selectively transesterifies the R enantiomer (ent-1) (39), which increases the yield of the desired product to 91%.
Scheme 1.
Scheme 2.
Asymmetric chemical catalysts have the greatest potential for general asymmetric synthesis since virtually no constraints exist in terms of molecular design, except those imposed by the human designing them, or in terms of what reactions are potentially capable of being performed asymmetrically (40, 41). Perhaps the earliest example is the utilization of cinchona alkaloids as catalysts for cyanohydrin formation in 1912 (42), a type of catalysis recently dubbed “organocatalysis.” The use of metal complexes for asymmetric catalysis perhaps dates from efforts in the 1950s to effect asymmetric hydrogenation (42–45). Several developments accelerated the growth of defined transition metal complexes for asymmetric catalysis. First, the ability to synthesize and characterize well defined transition metal complexes improved dramatically. Second, access to defined complexes set the stage for understanding the implication of structure for function, which begot the development of defined transition metal complexes, typically hybrids of organic entities and transition metals, for chemical catalysis. Third, the ability for individuals to wed the understanding that arises by integrating theoretical and physical, organic, and inorganic chemistry with solving complex problems becomes enabling.
Asymmetric Hydrogenation
Probably, the most important strategy to introduce chirality involves the ability of a catalyst to differentiate the enantiotopic faces of a prochiral functional group, notably a π-unsaturation like a carbon–carbon or carbon–oxygen double bond. Catalytic hydrogenation represents the archtypical example involving such a mechanism (46, 47). In addition, such reactions are among the most important synthetic methods because of their broad scope and efficiency (i.e., selectivity and atom economy). The discovery of tris(triphenylphosphine)rhodium chloride as a catalyst for hydrogenation by Wilkinson and colleagues in 1966 (48) set the stage for the development of asymmetric catalysts. Replacing triphenylphosphine with chiral phosphines is a straightforward extrapolation. The key question is what type of phosphine. In 1972, Knowles et al. (49) reported excellent results with a monodentate phosphine CAMP (structure 3). The minimal success with monodentate phosphines induced the design of bidentate ligands to limit the degrees of freedom and, by so doing, enhance the enantiomeric excess (ee). Most notably, the development of DIOP (structure 4) by Kagan, first reported in 1971, proved the validity of the approach (50, 51). In 1975, Knowles (52) reported the bis-phosphine analog DI-PAMP (structure 5) of his CAMP series of monodentate ligands. This ligand became the key to an asymmetric synthesis of β-arylalanines such as the anti-Parkinson drug (S)-DOPA and (S)-phenylalanine (Scheme 4), one of the two amino acids that constitutes the artificial sweetener aspartame. Indeed, this reaction is practiced commercially for these applications (53–55).
Scheme 4.
Despite the commercial success of DIPAMP, the real broad potential of asymmetric hydrogenation was not realized until the introduction of 2,2′-bis-(diphenylphosphino)-1,1′-binaphthyl (BINAP) (Scheme 3, structure 6) by Noyori and colleagues in 1980 (56, 57). For example, hydrogenation of the benzamide (Scheme 4, structure 5b) with a Rh complex of BINAP produced the corresponding phenylalanine derivative with near perfect enantioselectivity. The production of the chiral piperazine unit 7 that is one of the components of the clinically important HIV protease inhibitor indinavir (Scheme 5) is also accessed with this catalyst in high yield and ee (58). Some 10 years later, Burk and colleagues introduced a novel structural motif, which they termed DU-PHOS (Scheme 3, structure 8) (59–61). This ligand has one of the broadest substrate scopes of any of the hundreds of chiral ligands introduced for Rh complexes. Replacing Rh by Ru increased the scope of such reductions dramatically, notably by embracing carbonyl compounds as substrates (62–64). By using a combination of rhodium and ruthenium BINAP complexes, a one-pot asymmetric reduction of both a carbon–carbon and carbon–oxygen double bond has been performed (Scheme 6) in an approach to statine analogs, which constitute fragments of HIV protease inhibitors (65). The power of BINAP transcends well beyond asymmetric hydrogenation.
Scheme 3.
Scheme 5.
Scheme 6.
The corresponding imines constitute an important class of substrates since they provide access to chiral amines and amides (46). The success of iridium for such reductions ultimately led to a commercially successful synthesis of the herbicide (S)-metolachor, which is produced on the scale of >10,000 tons per year (66). This success validated the development of a class of ligands based on ferrocenes represented by the so-called JOSIPHOS ligands (i.e., structure 9 termed XYLIPHOS in Scheme 3) (67). Thus, a structural curiosity, the sandwich type of organometallics whose founding member is ferrocene (68–70), became a very useful scaffold onto which are placed metal-binding sites and heralded the onset of numerous novel systems for many other asymmetric catalytic processes.
Although the efforts of the past several decades highlight the utility of bidentate ligands for asymmetric hydrogenation, such design should not be viewed as mandatory. The development of catalysts based on axial chirality evolved from the concepts of Cram, who demonstrated the ability of such systems embedded in crown compounds to provide extraordinary levels of differential chiral recognition between enantiomers (71, 72). This motif, embodied in the standard 1,1′-bi-2-naphthol (BINOL) (73–75) and, of course, BINAP led to the development of monodentate phosphites (76) and phosphoramidites that exhibit excellent enantioselectivity (77).
Asymmetric Oxidation
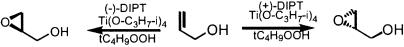
The discovery of metal-catalyzed epoxidation methods set the stage for the development of an asymmetric version. The first successful asymmetric epoxidation combined a simple tartrate ligand with titanium (Sharpless asymmetric epoxidation) (78) and has led to early commercialization for the synthesis of both enantiomers of glycidol (Scheme 7), an important chiral building block (79, 80). The requirement of this method for a hydroxyl group proximal to the double bond, preferably allylic, was relieved to some extent by the development of epoxidations based on manganese embraced by salen ligands 10 pioneered by Jacobsen and colleagues (81) and Katsuki and colleagues (82) as illustrated in Scheme 7 (83, 84). This example highlights the significance of these new methodologies by facilitating the development of novel and selective Iks-channel blockers for control of cardiac arrhythmias, such as HMR 1556 (Scheme 8) (85). The salen motif has also proven to have applicability across a spectrum of reactions. Whereas metals are at the heart of most catalytic processes, simple organic catalysts, the classical strategy, still constitute an important avenue of endeavor. Epoxidations involving dioxirane intermediates, i.e., structure 12, formed in situ from chiral ketone, structure 11 (Scheme 9), exemplifies the utility of such concepts, because it leads to a process with the least number of constraints on the types of alkenes that are suitable substrates (86, 87).
Scheme 7.
Scheme 8.
Scheme 9.
Epoxidation followed by ring opening constitutes the equivalent of trans-dihydroxylation if the nucleophile is based on oxygen. The stereochemical complement is cis-dihydroxylation. The invention of a dihydroxylation by using a catalytic amount of osmium tetraoxide and a stoichiometric amount of a reoxidant such as an amine oxide at the Upjohn Pharmaceutical Company (88, 89), combined with the early observation of ligand rate acceleration with pyridine among other amines by Criegee et al. (90), provided the basis for the development of asymmetric dihydroxylation by Sharpless and colleagues (91). Once again, the Cinchona alkaloids dihydroquinidine and dihydroquinine become the chiral inducing elements. A facile route to the side chain of the clinically important anticancer agent taxol resulted (Scheme 10, path a) (92). A related reaction, aminohydroxylation (93), shares the same characteristics and provides an even shorter route to this important appendage (Scheme 10, path b) (94).
Scheme 10.
Asymmetric C—C Bond Formation
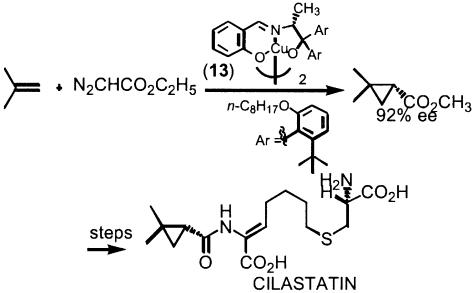
Historically, asymmetric cyclopropanation was a logical early choice because of the well known ability of copper to catalyze cyclopropanations with diazo complexes (95). Salen ligand (Scheme 11, structure 13) proved effective and led to an early success, a practical synthesis of cilastatin (96), a dipeptidase inhibitor used in a combination therapy with imipenem as an antibiotic adjunct (Scheme 11) (97, 98). It was a decade later that significant studies occurred as a result of the introduction of new families of ligands, most of which were c2 symmetric represented by semicorrins (e.g., structure 14 in Scheme 12) (99, 100) and bis-oxazoline ligands (e.g., structure 15 in Scheme 12) (101–103). Indeed, this latter class has proved to be quite useful for numerous reactions catalyzed by higher oxidation state metals beyond cyclopropanation.
Scheme 11.
Scheme 12.
In 1973, a paradigm shift occurred with the introduction of rhodium carboxylates as catalysts for cyclopropanation by Teyssié and coworkers (104, 105) Chiral carboxylates and their surrogates become ligand possibilities (106). Application of McKervey's proline-derived rhodium complex (Scheme 13, structure 16) (107) to cyclopropanation by Davies proved their success (108, 109). The carboxamides illustrated by structures 17–19 (Scheme 13) developed by Doyle and Forbes demonstrated their best selectivities in intramolecular cyclopropanations (106). The ability of rhodium carboxylates to promote C—H insertion reactions of the intermediate carbene complexes (110) demonstrated by Teyssié can convert these processes into excellent asymmetric ones with similar chiral versions (111), as demonstrated in a synthesis of ritalin (112, 113).
Scheme 13.
Chiral Lewis acids have emerged strongly for numerous C—C bond-forming reactions. Reactions that involve highly ordered transition states like Diels–Alder reactions are natural candidates. Indeed, complexes of BINAP, BINOL, salen (structure 10), and bis-oxazolines (structure 15), which all share the same feature of being c2 symmetric ligands, have been effective for numerous [4 + 2] cycloadditions (114). However, such a motif is not mandatory. A particularly simple class of non-c2 symmetric ligands derived from α-hydroxy or α-amino acids and boron, first introduced by Yamamoto and coworkers (115, 116) and Helmchen and coworkers (117) and further developed by Corey et al. (118).
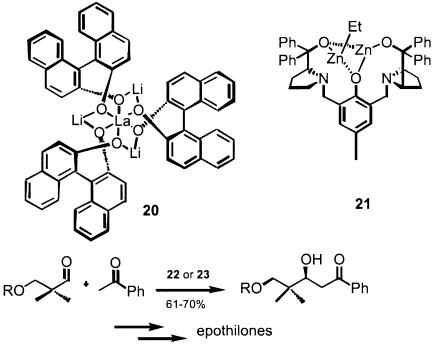
Next to the Diels–Alder reaction, the aldol addition stands out because of its potency for the synthesis of bioactive natural products (in part, because that is also nature's route to these substances) but also because of the frequently highly ordered transition states (119, 120). Indeed, the same chiral ligands, including the boron-derived ones, function well (121–123). However, in all these cases, a preformed enol or enolate equivalent is required. Effecting the aldol reaction in its simplest iteration, i.e., simply adding an active methylene compound to a carbonyl group in the presence of a catalyst, provides the most atom economic strategy. Shibasaki revealed the first such catalysts based on a novel class of BINOL complexes (Scheme 14, structure 20) formed by self-assembly of BINOL in the presence of a lanthanide and base (74, 124, 125). Thus, acetophenone adds directly to aldehydes to give the aldol adducts. The immediate utility of this methodology was demonstrated by the synthesis of an intermediate for the synthesis of epothilones, promising candidates as clinically useful anticancer agents (Scheme 14 Lower).
Scheme 14.
A new motif for ligand design was based on the notion that a chiral hemi-crown might be more useful as a catalyst than chiral crown compounds, because it would bind neither substrates nor products too tightly but yet might retain sufficient enantiodiscrimination to be synthetically useful. Using such a notion, Trost designed the ligands represented by structure 21 as a potential catalyst for the aldol process (126, 127) to give results nearly identical with those of Shibasaki for the reaction of Scheme 14. This ligand spontaneously self-assembles as a dinuclear zinc complex, which is key for its high enantioselectivity. The systems of both Shibasaki and colleagues (128) and Trost and Yeh (129) also perform well for the so-called nitoaldol reaction, wherein the active methylene partners are nitroalkanes. Furthermore, the nitrogen analog of the carbonyl partner, i.e., imines (a Mannich-type process), also serves as a suitable partner with these same catalysts (130, 131). These ligands create proximal multisite catalysts in contrast to the more common single-site catalysts. For bimolecular addition reactions, such a design appears to be ideal since it helps assemble and orient the two reaction partners, thereby reducing entropies of activation and imprinting chirality.
Although metal-based methods constitute the bulk of the attention for such processes, simple organic structures also have promise. The key discovery reported in 1971 by Eder et al. (132) and in 1974 by Hajos and Parrish (ref. 133; see also ref. 134) demonstrated that amino acids, notably proline, effected an intramolecular aldol reaction in high yields and enantioselectivity (Scheme 15). More than 20 years later, the implications of this finding began to be realized by the demonstration that such a simple amino acid can promote intermolecular aldol additions with remarkable chemo-, diastereo-, and enantioselectivity (135, 136). These types of catalysts also serve in Mannich-type processes (137).
Scheme 15.
Carbonyl additions of nonstabilized nucleophiles have led to more explicit consideration of some of the most interesting general philosophical concepts. Oguni et al. reported the key discovery in 1983 (138), subsequently largely developed in the Noyori laboratory (139, 140), that simple chiral amino alcohols like ephedrine catalyze the addition of dialkylzinc reagents to aldehydes (Scheme 16) (141). In violation of traditional practice, chiral amino alcohols of low enantiomeric purity still provided the resultant product with excellent enantioselectivity. Indeed, the alcohol (structure 22) of 5 × 10–5 % ee reduced itself with >99.5% ee (142). The concept of chiral amplification, an example of a nonlinear effect, which previously was largely ignored, now makes consideration of nonlinear effects important for all asymmetric reactions (143, 144). Since the example of Scheme 16 is indeed an amino alcohol, the question of whether this enantiomerically pure product can serve as its own catalyst, i.e., the concept of autocatalysis, arises (145, 146). Furthermore, combining autocatalysis with chiral amplification raises the question of whether a small amount of product of low ee can catalyze its own formation with high ee. In 1932, Mills (147) had already proposed that statistics suggest that random small fluctuations in the exact ratio of enantiomers in forming racemates would always occur, ≈200 of 100,000 molecules. In accord with this proposal, performing the reaction illustrated in Scheme 16 with no chiral catalysts gave ee's as high as 91% in a statistically random fashion, i.e., in 37 runs, the S enantiomer was favored 19 times and the R enantiomer was favored 18 times (148). Such “spontaneous” generation of chirality stemming from the combination of statistics, autocatalysis, and chiral amplification provides some insight into the creation of the chiral world of biology.
Scheme 16.
Allyl organometallics may be considered the all-carbon analog of an enolate. Allyl stannanes and silanes represent the most common allyl transfer group with BINOL and its analogs typically serving as the enantio-discriminating agent (149). A more efficient approach uses alkenes as the nucleophilic partner wherein an allylic C—H bond becomes transferred during the process, i.e., a carbonyl-ene reaction (150) as in Scheme 17 (151).
Scheme 17.
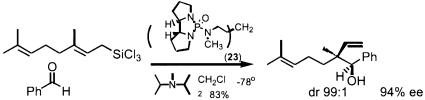
An ancillary approach for the asymmetric carbonyl additions derives from activating an otherwise unreactive “organometallic” by coordinating a chiral electron-donating ligand, a phenomenon termed nucleophilic catalysis. Such an effect is particularly highlighted by the addition of allyl trichlorosilanes as shown in Scheme 18 (152) by using a chiral Lewis base (structure 23) as catalyst. Since allylmetals can be considered all-carbon analogs of enolates, the same concept extends to the reactions of enolates, notably the aldol addition.
Scheme 18.
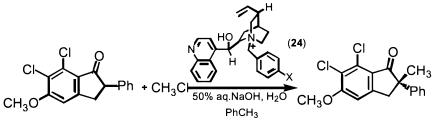
For alkylation of enolates, induction of chirality also typically requires interaction of the stereochemical inducing agent with the nucleophile rather than the electrophile. Designing chiral cations to engage in ion pairing with the nucleophile becomes a rational mechanism in phase-transfer catalysis. In 1984, a Merck group reported the first successful realization of this concept in an asymmetric methylation (Scheme 19) for the synthesis of the uricosuric agent (S)-indacrinone (153, 154) by using the Cinchona alkaloids as the core of the enantio-discriminating agent (structure 24). About a decade later, this design resurfaced to create a host of phase-transfer catalysts to effect asymmetric alkylations whereby both tertiary and quaternary centers are formed (155).
Scheme 19.
The related 1,4-addition of nucleophiles to α,β-unsaturated carbonyl substrates normally requires ligand designs based on chiral Lewis acids that complex the carbonyl group to create a chiral environment around the double bond, such as those discussed for cyclo- and aldol additions, including bis-oxazoline (156, 157) and self-assembled BINOL-type systems (158). A different mechanism derives from the proline-catalyzed aldol chemistry in which the catalyst (structure 25 in Scheme 20) in situ forms a chiral iminium ion such as structure 26, which becomes the reactive electrophile rather than leading to a reactive nucleophile (Scheme 20) (159). With nonstabilized nucleophiles, the requirement of a metal counterion to promote 1,4 (i.e., Michael or conjugate) additions rather than 1,2 additions defines the nature of the chiral-inducing element. Traditionally, the use of copper for such chemoselectivity led to the design of amine-based ligands, such as the bis-oxazolines, or of phosphorus-based ligands, such as the phosphoramidites previously mentioned in the context of catalytic hydrogenation. In this scenario, dialkylzinc reagents, which are unreactive with typical Michael acceptors but are competent to transmetalate to copper, participate in copper-catalyzed additions with high enantiomeric excess (160).
Scheme 20.
An important development arose from the discovery that transmetallation from boron to rhodium occurs readily: the ability to perform conjugate additions of organoboron compounds in the presence of rhodium complexes as catalysts. Borrowing again from catalytic hydrogenation, BINAP complexes of rhodium impart excellent enantioselectivity in such additions (Scheme 21) (161–163). Since the initial adduct is an enol boron species, besides protonation, it can be captured in a highly diastereo-selective aldol process, thus creating three stereogenic centers in one asymmetric catalytic event.
Scheme 21.
Whereas introduction of chirality into Michael acceptors represents the more common strategy, the Michael donor may also be a prostereogenic reaction partner. Mechanisms that influence the stereochemistry of the nucleophilic partner that have been discussed with respect to the reaction of enolates such as the aldol addition and alkylations, may apply here. Most notably, asymmetric phase-transfer catalysis stands out as a promising strategy (155).
Asymmetric carbon–carbon bond-forming reactions with unactivated alkenes have emerged as the feasibility of such methodologies increases. Based on the “classical” asymmetric hydrogenation that involves asymmetric hydrometallation as the first step, replacing the second stage of such processes by C—C bond formation becomes a logical promising direction. Asymmetric hydroformylation (164) and hydrocyanation have been early areas of endeavor with only modest success (165). Reactions involving carbametallation represent an alternative. Among these, the asymmetric Heck reaction using chiral ligands, like BINAP and its analogs (166), stands out as shown in Scheme 22 (167).
Scheme 22.
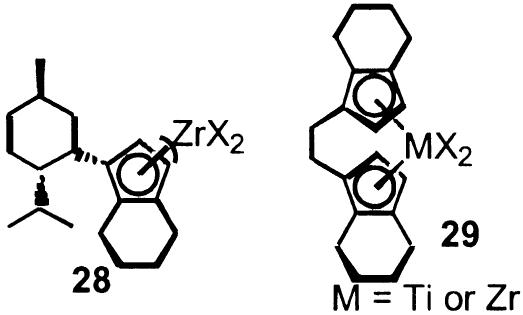
A more atom economical approach to perform the equivalent of a Heck vinylation emerged from the Trost laboratories which exchanges a vinyl halide for an alkyne in which the vinyl metal intermediate is formed by a hydrometallation (Scheme 23) (168). An asymmetric version from the Mikami laboratories utilizes an axially chiral non-c2 symmetric ligand (structure 27) (169). As in the Heck reaction, the enantio-discriminating step involves the preferential carbapalladation of one the enantiotopic faces of the alkene. Titanium and zirconium complexes such as sandwich complexes 28 and 29 (see Scheme 24) also promote asymmetric carbametallation of alkenes but with simple alkyl moieties, as illustrated by syntheses of vitamins E and K (170) and hydrosilylations of carbony and imine groups (171, 172), among others. Related sandwich complexes have been important in asymmetric polymerizations of propylene involving a similar asymmetric carbametallation as the chain propagation step (9–11).
Scheme 23.
Scheme 24.
Multiple Bond-Type Asymmetric Processes
Each of the asymmetric processes discussed so far involve formation of only one bond type, a C—H, C—O, or C—C bond. However, a few processes permit formation of all of the bond types above but also many additional types of bonds, such as C—N, C—S, C—P, etc. These bimolecular reactions typically involve attack of a broad range of nucleophiles on a suitable electrophile. The most obvious classical example is the Michael addition. Most attention has focused on formation of C—C bonds in such processes with very little being done with respect to noncarbon nucleophiles (173).
Asymmetric allylic alkylation has proven to be a process in which such multiple bond formation can be achieved asymmetrically with the same catalyst (174). The problem of asymmetric induction in this case is aggravated by the most common mechanism—the bond-forming and bond-breaking events occur outside the coordination sphere of the metal and therefore distal to the asymmetric inducing elements. This issue led to the development of new types of ligand design represented by ligands 30–32 (see Scheme 25), each operating by a different design concept (175–177). As shown in Scheme 26, C—C (178), C—N (178), C—O (179), and C—S (180) bonds have all been formed with high ee. This process also differentiates itself from other asymmetric catalytic processes by the number of mechanisms for enantiodiscrimination. Thus, inducing stereochemistry in the attacking nucleophile which is even more distant from the chiral environment, can be achieved with the same ligands (181, 182), a key reaction for the asymmetric synthesis of benzomorphans possessing an enhanced clinical profile with diminished addictive properties (Scheme 27) (183).
Scheme 25.
Scheme 26.
Assymetric allylic alkylation with ligands of type 32.
Scheme 27.
Conclusions
Asymmetric catalysis has begun to demonstrate its potential. Within the context of a few reactions, most notably asymmetric hydrogenation, significant efforts have been expended on developing an ever broadening catalog of asymmetric catalysts involving variation of both metal and ligand environment. By doing so, the broadest range of potential suitable substrates for practical levels of selectivity has become possible; nevertheless, numerous potential substrates still remain. Furthermore, new concepts that provide important insights continue to emerge. For example, mixtures of different ligands (heterocombinations) can give higher enantioselectivities than the use of a single ligand (homocombination) in certain circumstances (184). Such results dramatically expand the permutations that now need to be considered. Thus, in a field that many would claim is well understood and developed, such pronouncements are clearly premature. In most every other type of reaction, we are much earlier in our stage of development.
In this issue are assembled a group of perspectives and research reports from leading laboratories that touch on all the themes noted herein and many others. Although the developments are impressive and are making slow but steady inroads into being embedded in the dogma of synthesis, the successes to date should be kept in perspective. Opportunities for major developments are enormous. To attempt to grasp the magnitude of the opportunity, one can estimate that the number of possible structures that can function as catalysts with and without being bound to metals and multiply that figure by the number of reaction variables that may influence reactivity and selectivity. Since the number of compounds possessing only C, H, N, O, S, P, F, Cl, and Br of molecular weight ≤500 exceeds 1062, the number of permutations becomes truly unimaginable. Opportunity for major discovery will remain vibrant for a very long time indeed.
Acknowledgments
I thank the General Medical Sciences Institute of the National Institutes of Health and the National Science Foundation for financial support.
Abbreviations: BINOL, 1,1′-bi-2-naphthol; BINAP, 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl; ee, enantiomeric excess.
References
- 1.Biot, J. B. (1815) Bull. Soc. Philomath. Paris 190.
- 2.Biot, J. B. (1816) Bull. Soc. Philomath. Paris 125.
- 3.Richardson, G. M., ed. (1901) The Foundation of Stereochemistry (Am. Book Co., New York).
- 4.Kekulé, A. (1858) Annals 106, 154. [Google Scholar]
- 5.van't Hoff, J. H. (1875) Bull. Soc. Chim. France 23, 295. [Google Scholar]
- 6.Le Bel, J. A. (1874) Bull. Soc. Chim. France 22, 337. [Google Scholar]
- 7.Natta, G. (1961) Makromol. Chem. 43, 68–71. [Google Scholar]
- 8.Natta, G., Porri, L. & Valenti, S. (1963)) Makromol. Chem. 67, 225–228. [Google Scholar]
- 9.Coates, G. W. & Waymouth, R. M. (1993) J. Am. Chem. Soc. 115, 91–98. [Google Scholar]
- 10.Natta, G. (1966) Pure. Appl. Chem. 12, 165–182. [Google Scholar]
- 11.Natta, G. (1965) Science 147, 261–272. [DOI] [PubMed] [Google Scholar]
- 12.Brintzinger, H. H., Fischer, D., Muelhaupt, R., Rieger, B. & Waymouth, R. M. (1995) Angew. Chem. Int. Ed. Engl. 34, 1143–1170. [Google Scholar]
- 13.Hanessian, S. (1993) Pure Appl. Chem. 65, 1189–1204. [Google Scholar]
- 14.Hanessian, S., Franco, J. & Larouche, B. (1990) Pure Appl. Chem. 62, 1887–1910. [Google Scholar]
- 15.Hanessian, S., Franco, J., Gagnon, G., Laramee, D. & Larouche, B. (1990) J. Chem. Inf. Comput. Sci. 30, 413–425. [Google Scholar]
- 16.Blaser, H.-U. (1992) Chem. Rev. 92, 935–952. [Google Scholar]
- 17.Casiraghi, G., Zanardi, F., Rassu, G. & Span, P. (1995) Chem. Rev. 95, 1677–1716. [Google Scholar]
- 18.Wilen, S. H. (1971) Top. Stereochem. 6, 107–176. [Google Scholar]
- 19.Wilen, S. H. (1972) Tables of Resolving Agents and Optical Resolutions (Univ. Notre Dame Press, Notre Dame, IN).
- 20.Jacques, J., Collet, A. & Wilen, S. H. (1981) Enantiomers, Racemates and Resolutions (Wiley, New York).
- 21.Pasteur, L. (1853) Ann. Chim. Phys. 38, 437. [Google Scholar]
- 22.Pasteur, L. (1853) C. R. Acad. Sci. 37, 162. [Google Scholar]
- 23.Pasteur, L. (1858) C. R. Acad. Sci. 46, 615. [Google Scholar]
- 24.Ladenburg, A. (1886) Chem. Ber. 19, 2578. [Google Scholar]
- 25.Drauz, K. & Waldmann, H. (2002) Enzyme Catalysis in Organic Synthesis (Wiley-VCH, Weinheim, Germany), 2nd Ed.
- 26.Rosenthaler, L. (1909) Biochem. Z. 14b, 238–253. [Google Scholar]
- 27.Rosenthaler, L. (1912) Biochem. Z. 17, 257–269. [Google Scholar]
- 28.Hayashi, S. (1929) Biochem. Z. 206, 223–227. [Google Scholar]
- 29.Schmid, R. D. & Verger, R. (1998) Angew. Chem. Int. Ed. Engl. 37, 1609–1633. [Google Scholar]
- 30.Boichem, Z. & Faber, K. (2000) Biotransformations in Organic Chemistry (Springer, Berlin), 4th Ed.
- 31.Kazlauskas, R. J. & Bornscheuer, U. T. (1999) Hydrolases in Organic Chemistry (Wiley-VCH, Weinheim, Germany).
- 32.Wong, C.-H. & Whitesides, G. M. (1994) Enzymes in Synthetic Chemistry (Pergamon, Oxford).
- 33.Holland, H. L. (1992) Organic Synthesis with Oxidative Enzymes (VCH, New York).
- 34.Chen, K. C. & Wey, H. C. (1990) Enzyme Microb. Technol. 12, 305–308. [Google Scholar]
- 35.Gibson, D. T., Gschwendt, B., Yeh, W. K. & Kobal, V. M. (1973) Biochemistry 12, 1520–1528. [DOI] [PubMed] [Google Scholar]
- 36.Bestetti, G., Galli, E., Benigni, C., Orsini, F. & Pelizzoni, F. (1989) Appl. Microbiol. Biotechnol. 30, 252–256. [Google Scholar]
- 37.Schultz, P. G., Yin, J. & Lerner, R. A. (2002) Angew. Chem. Int. Ed. Engl. 41, 4427–4437. [DOI] [PubMed] [Google Scholar]
- 38.Pamies, O. & Bäckvall, J.-E. (2003) Chem. Rev. 103, 3247–3261. [DOI] [PubMed] [Google Scholar]
- 39.Kim, M. J., Choi, Y. K., Choi, M. Y. & Park, J. (2001) J. Org. Chem. 66, 4736–4738. [DOI] [PubMed] [Google Scholar]
- 40.Jacobsen, E. N., Pflatz, A. & Yamamoto, H., eds. (1999) Comprehensive Asymmetric Catalysis (Springer, Berlin).
- 41.Ojima, I. ed. (2000) Catalytic Asymmetric Synthesis (Wiley-VCH, New York).
- 42.Bredig, G. & Fiske, P. S. (1913) Biochem. Z. 46, 7–23. [Google Scholar]
- 43.Akabori, S., Sakurai, S. & Izumi, Y. (1956) Nature 178, 323–324.13358737 [Google Scholar]
- 44.Izumi, Y. (1983) Adv. Catal. 32, 215. [Google Scholar]
- 45.Tai, A. & Harada, T. (1986) in Thailand Metal Catalysts, ed. Iwasawa, Y. D. (Reidel, Dordrecht, The Netherlands), p. 265–285.
- 46.Ohkuma, T., Kitamura, M. & Noyori, R. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley-VCH, New York), pp. 1–110.
- 47.Tang, W. & Zhang, X. (2003) Chem. Rev. 103, 3029–3069. [DOI] [PubMed] [Google Scholar]
- 48.Osborn, J. A., Jardine, F. S., Young, J. F. & Wilkinson, G. (1966) J. Chem. Soc. 1711–1732.
- 49.Knowles, W. S., Sabacky, M. J. & Vineyard, B. D. (1972) J. Chem. Soc. Chem. Commun. 10–11.
- 50.Dang, T. P. & Kagan, H. B. (1971) J. Chem. Soc. Chem. Commun. 481–482.
- 51.Kagan, H. B. & Dang, T. P. (1972) J. Am. Chem. Soc. 94, 6429–6433. [Google Scholar]
- 52.Knowles, W. S., Sabacky, M. J., Vineyard, B. D. & Weinkauff, D. J. (1975) J. Am. Chem. Soc. 97, 2567–2568. [Google Scholar]
- 53.Knowles, W. S. (2003) Adv. Synth. Catal. 345, 3–13. [Google Scholar]
- 54.Nugent, W. A., Rajan Babu, T. V. & Burk, M. J. (1993) Science 259, 479–483. [DOI] [PubMed] [Google Scholar]
- 55.Blaser, H.-U., Malan, C., Pugin, B., Spindler, F., Steiner, H. & Studer, M. (2003) Adv. Synth. Catal. 345, 103–151. [Google Scholar]
- 56.Miyashita, A., Yasuda, A., Takaya, H., Toriumi, K., Ito, T, Souchi, T. & Noyori, R. (1980) J. Am. Chem. Soc. 102, 7932–7934. [Google Scholar]
- 57.Noyori, R. (2003) Adv. Synth. Catal. 345, 15–32. [Google Scholar]
- 58.Askin, D. (1998) Curr. Opin. Drug Discovery Dev. 1, 338–348. [PubMed] [Google Scholar]
- 59.Burk, M. J. (1991) J. Am. Chem. Soc. 113, 8518–8519. [Google Scholar]
- 60.Burk, M. J., Feaster, J. E., Nugent, W. A. & Harlow, R. L. (1993) J. Am. Chem. Soc. 115, 10125–10138. [Google Scholar]
- 61.Burk, M. J., Bienewald, F., Challenger, S., Derrick, A. & Ramsden, J. A. (1999) J. Org. Chem. 64, 3290–3298. [DOI] [PubMed] [Google Scholar]
- 62.Noyori, R. (1994) in Asymmetric Catalysis in Organic Synthesis (Wiley-Interscience, New York), pp. 16–94.
- 63.Noyori, R. (1990) Science 248, 1194–1199. [DOI] [PubMed] [Google Scholar]
- 64.Noyori, R. (1990) Acc. Chem. Res. 23, 345–350. [Google Scholar]
- 65.Doi, T., Kokubo, M., Yamamoto, K. & Takahashi, T. (1998) J. Org. Chem. 63, 428–429. [DOI] [PubMed] [Google Scholar]
- 66.Blaser, H. U., Buser, H. P., Loers, K., Hanreich, R., Jalett, H. P., Jelsch, E., Pugin, B., Schneider, H. D., Spindler, F. & Wagmann, A. (1999) Chimica 53, 275–280. [Google Scholar]
- 67.Calacot, T. J. (2003) Chem. Rev. 103, 3101–3118. [DOI] [PubMed] [Google Scholar]
- 68.Kelly, T. J. & Pauson, P. L. (1951) Nature 168, 1039–1040. [Google Scholar]
- 69.Irvine, J. W. & Wilkinson, G. (1961) Science 113, 742. [DOI] [PubMed] [Google Scholar]
- 70.Wilkinson, G., Pauson, P. L., Birmingham, J. M. & Cotton, F. A. (1953) J. Am. Chem. Soc. 75, 1011–1012. [Google Scholar]
- 71.Cram, D. J. & Cram, J. M. (1998) Container Molecules and Their Guests (Royal Soc. Chem., Cambridge).
- 72.Cram, D. J. (1992) Nature 356, 29–36. [Google Scholar]
- 73.Chen, Y., Yekta, S. & Yudin, A. K. (2003) Chem. Rev. 103, 3155–3212. [DOI] [PubMed] [Google Scholar]
- 74.Shibasaki, M., Sasai, H. & Arai, T. (1997) Angew. Chem. Int. Ed. Engl. 36, 1236–1256. [Google Scholar]
- 75.Shibasaki, M. & Yoshikawa, N. (2002) Chem. Rev. 102, 2187–2209. [DOI] [PubMed] [Google Scholar]
- 76.Reetz, M. T. & Mehler, G. (2000) Angew. Chem. Int. Ed. Engl. 39, 3889–3890. [DOI] [PubMed] [Google Scholar]
- 77.van den Berg, M., Minnaard, A. J., Haak, R. M., Leeman, M., Schudde, E. P., Meetsma, A., Feringa, B. L., de Vries, A. H. M., Maljaars, C. E. P., Willans, C. E., et al. (2003) Adv. Synth. Catal. 345, 308–323. [Google Scholar]
- 78.Katsuki, T. & Sharpless, K. B. (1980) J. Am. Chem. Soc. 102, 5974–5976. [Google Scholar]
- 79.Klunder, J. M., Onami, T. & Sharpless, K. B. (1989) J. Org. Chem. 54, 1295–1304. [Google Scholar]
- 80.Shum, W. P. & Cannarsa, M. J. (1997) Chirality Ind. 2, 363–380. [Google Scholar]
- 81.Zhang, W., Loebach, J. L., Wilson, S. R. & Jacobsen, E. N. (1990) J. Am. Chem. Soc. 112, 2801–2803. [Google Scholar]
- 82.Irie, R., Noda, K., Ito, Y., Matsumoto, N. & Katsuki, T. (1990) Tetrahedron Lett. 31, 7345–7348. [Google Scholar]
- 83.Jacobsen, E. N., Zhang, W., Muci, L. C., Ecker, J. R. & Deng, L. (1991) J. Am. Chem. Soc. 113, 7063–7064. [Google Scholar]
- 84.Sasaki, H., Irie, R., Hamada, T., Suzuki, K. & Katsuki, T. (1994) Tetrahedron 50, 11827–11838. [Google Scholar]
- 85.Gerlach, U., Brendel, J., Lang, H.-J., Paulus, E. F., Weidmann, K., Brüggemann, A., Busch, A., Suessbrich, H., Bleich, M. & Greger, R. (2001) J. Med. Chem. 44, 3831–3837. [DOI] [PubMed] [Google Scholar]
- 86.Tu, Y., Wang, Z.-X. & Shi, Y. (1996) J. Am. Chem. Soc. 118, 9806–9807. [Google Scholar]
- 87.Yang, D., Yip, Y. C., Tang, M. W., Wong, M. K., Zheng, J. H. & Cheung, K. K. (1996) J. Am. Chem. Soc. 118, 491–492. [Google Scholar]
- 88.Van Rheenan, V., Kelly, R. C. & Cha, D. Y. (1976) Tetrahedron Lett. 17, 1973–1977. [Google Scholar]
- 89.Minato, M., Yamamoto, K. & Tsuji, J. (1990) J. Org. Chem. 55, 766–768. [Google Scholar]
- 90.Criegee, R., Marchand B. & Wannowius, H. (1942) Justus Liebigs Ann. Chem. 550, 99–133. [Google Scholar]
- 91.Jacobsen, E. N., Marko, I., Mungall, W. S., Schröder, G. & Sharpless, K. B. (1988) J. Am. Chem. Soc. 110, 1968–1970. [Google Scholar]
- 92.Wang. Z. M., Kolb, K. C. & Sharpless, K. B. (1994) J. Org. Chem. 59, 5104–5105. [Google Scholar]
- 93.Li, G., Chang, H.-T. & Sharpless, K. B. (1996) Angew. Chem. Int. Ed. Engl. 35, 451–454. [Google Scholar]
- 94.Li. G. & Sharpless, K. B. (1996) Acta Chem. Scand. 50, 649–651. [DOI] [PubMed] [Google Scholar]
- 95.Nozaki, H., Moruiti, S., Takaya, H. & Noyori, R. (1966) Tetrahedron Lett. 22, 5239–5243. [Google Scholar]
- 96.Aratani, T., Yoneyoshi, Y. & Nagase, T. (1975) Tetrahedron Lett. 31, 1707–1711. [Google Scholar]
- 97.Kahan, F. M., Kropp, H., Sundelof, J. G. & Birnbaum, J. (1975) J. Antimicrob. Chemother. 12, Suppl. D, 1–35. [DOI] [PubMed] [Google Scholar]
- 98.Birnbaum, J., Kahan, F. M., Kropp, H. & Mac Donald, J. S. (1985) Am. J. Med. 78, 3–21. [DOI] [PubMed] [Google Scholar]
- 99.Fritschi, H., Leuteneggar, U. & Pfaltz, A. (1986) Angew. Chem. Int. Ed. Engl. 25, 1005–1006. [Google Scholar]
- 100.Fritschi, H., Leuteneggar, U. & Pfaltz, A. (1988) Helv. Chim. Acta 71, 1553–1565. [Google Scholar]
- 101.Lowenthal, R. E., Abiko, A. & Masamune, S. (1990) Tetrahedron Lett. 31, 6005–6008. [Google Scholar]
- 102.Lowenthal, R. E. & Masamune, S. (1991) Tetrahedron Lett. 32, 7373–7376. [Google Scholar]
- 103.Evans, D. A., Woerpel, K. A. & Hinman, M. M. (1991) J. Am. Chem. Soc. 113, 726–728. [Google Scholar]
- 104.Hubert, A. J., Noels, A. F., Anciaux, A. J. & Teyssié, P. (1976) Synthesis 600–602.
- 105.Anciaux, A. J., Hubert, A. J., Noels, A. F., Petiniot, N. & Teyssié, P. (1980) J. Org. Chem. 45, 695–702. [Google Scholar]
- 106.Doyle, M. P. & Forbes, D. C. (1998) Chem. Rev. 98, 911–935. [DOI] [PubMed] [Google Scholar]
- 107.Kennedy, M., McKervey, M. A., Maguire, A. R. & Roos, G. H. P. (1990) Chem. Commun. 361–362.
- 108.Davies, H. M. L. & Hutcheson, D. K. (1993) Tetrahedron Lett. 34, 7243–7246. [Google Scholar]
- 109.Davies, H. M. L., Bruzinski, P. R., Lake, O. H., Kong, N. & Fall, M. J. (1996) J. Am. Chem. Soc. 118, 6897–6907. [Google Scholar]
- 110.Demonceau, A., Noels, A. F., Hubert, A. J. & Teyssié, P. (1981) Chem. Commun. 46, 688–689. [Google Scholar]
- 111.Davies, H. M. L. & Beckwith, R. E. J. (2003) Chem. Rev. 103, 2861–2903. [DOI] [PubMed] [Google Scholar]
- 112.Davies, H. M. L., Hansen, T., Hopper, D. W. & Panaro, S. A. (1999) J. Am. Chem. Soc. 121, 6509–6510. [Google Scholar]
- 113.Axten, J. M., Ivy, R., Krim, L. & Winkler, J. D. (1999) J. Am. Chem. Soc. 121, 6511–6512. [Google Scholar]
- 114.Maruoka, K. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley-VCH, New York), pp. 467–492.
- 115.Furuta, K., Miwa, Y., Iwanaga, K. & Yamamoto, H. (1988) J. Am. Chem. Soc. 110, 6254–6255. [DOI] [PubMed] [Google Scholar]
- 116.Ishihara, K., Gao, Q. & Yamamoto, H. (1993) J. Am. Chem. Soc. 115, 10412–10413. [Google Scholar]
- 117.Sartor, D., Saffrich, J. & Helmchen, G. (1990) Synlett 197–198.
- 118.Corey, E. J., Guzman-Perez, A. & Loh, T.-P. (1994) J. Am. Chem. Soc. 116, 3611–3612. [Google Scholar]
- 119.Wong, C.-H. & Machajewski, T. D. (2000) Angew. Chem. Int. Ed. Engl. 39, 1352–1374. [DOI] [PubMed] [Google Scholar]
- 120.Carreira, E. M. (1999) in Comprehensive Asymmetric Catalysis, eds. Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (Springer, Heidelberg), Vol. 3, Chap. 29.1.
- 121.Corey, E. J., Cywin, C. L. & Roper, T. D. (1992) Tetrahedron Lett. 33, 6907–6910. [Google Scholar]
- 122.Parmee, E. R., Tempkin, O., Masumune, S. & Abiko, A. (1991) J. Am. Chem. Soc. 113, 9365–9366. [Google Scholar]
- 123.Furuta, K., Maruyama, T. & Yamamoto, H. (1991) J. Am. Chem. Soc. 113, 11041–11042. [Google Scholar]
- 124.Yoshikawa, N., Yamada, Y. M. A., Das, J., Sasai, H. & Shibasaki, M. (1999) J. Am. Chem. Soc. 121, 4168–4178. [Google Scholar]
- 125.Kumagai, N., Matsunaga, S., Kinoshita, T., Harada, S., Okada, S., Sakamoto, S., Yamaguchi, K. & Shibasaki, M. (2003) J. Am. Chem. Soc. 125, 2169–2178. [DOI] [PubMed] [Google Scholar]
- 126.Trost, B. M. & Ito, H. (2000) J. Am. Chem. Soc. 122, 12003–12004. [Google Scholar]
- 127.Trost, B. M., Ito, H. & Silcoff, E. R. (2001) J. Am. Chem. Soc. 123, 3367–3368. [DOI] [PubMed] [Google Scholar]
- 128.Sasai, H., Suzuki, T., Arai, S. & Shibasaki, M. (1992) J. Am. Chem. Soc. 114, 4418–4420. [Google Scholar]
- 129.Trost, B. M. & Yeh, V. S. C. (2002) Angew. Chem. Int. Ed. Engl. 41, 861–863. [DOI] [PubMed] [Google Scholar]
- 130.Yamasaki, S., Iida, T. & Shibasaki, M. (1999) Tetrahedron 55, 8857–8867. [Google Scholar]
- 131.Trost, B. M. & Terrell, L. R. (2003) J. Am. Chem. Soc. 125, 338–339. [DOI] [PubMed] [Google Scholar]
- 132.Eder, U., Sauer, G. & Wiechert, R. (1971) Angew. Chem. Int. Ed. Engl. 10, 496–497. [Google Scholar]
- 133.Hajos, Z. G. & Parrish, D. R. (1974) J. Org. Chem. 39, 1615–1621. [Google Scholar]
- 134.Cohen, N. (1976) Acc. Chem. Res. 9, 412–417. [Google Scholar]
- 135.List, B., Lerner, R. A. & Barbas, C. F., III. (2000) J. Am. Chem. Soc. 122, 2395–2396. [Google Scholar]
- 136.List, B. (2002) Tetrahedron 58, 5573–5590. [Google Scholar]
- 137.List, B., Pojarliev, P., Biller, W. T. & Martin, H. J. (2002) J. Am. Chem. Soc. 124, 827–833. [DOI] [PubMed] [Google Scholar]
- 138.Oguni, N., Omi, T., Yamamoto, Y. & Nakamura, A. (1983) Chem. Lett. 841–844.
- 139.Kitamura, M., Suza, S., Kawai, K. & Noyori, R. (1986) J. Am. Chem. Soc. 108, 6071–6072. [DOI] [PubMed] [Google Scholar]
- 140.Kitamura, M., Okada, S., Suga, S. & Noyori, R. (1989) J. Am. Chem. Soc. 111, 4028–4036. [Google Scholar]
- 141.Pu, L. & Yu, H.-B. (2001) Chem. Rev. 101, 757–824. [DOI] [PubMed] [Google Scholar]
- 142.Sato, I., Urabe, H., Ishiguro, S., Shibata, T. & Soai, K. (2003) Angew. Chem. Int. Ed. Engl. 42, 315–317. [DOI] [PubMed] [Google Scholar]
- 143.Puchot, C., Samuel, O., Dunach, E., Zha, S., Agami, C. & Kagan, H. B. (1986) J. Am. Chem. Soc. 108, 2353–2357. [DOI] [PubMed] [Google Scholar]
- 144.Girard, C. & Kagan, H. B. (1998) Angew. Chem. Int. Ed. Engl. 37, 2923–2959. [Google Scholar]
- 145.Soai, K. & Shibaka, T. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley-VCH, New York), 2nd Ed., pp. 699–725.
- 146.Mikami, K. & Yamanaka, M. (2003) Chem. Rev. 103, 3369–3400. [DOI] [PubMed] [Google Scholar]
- 147.Mills, W. H. (1932) Chem. Ind. 750–759.
- 148.Soai, K., Sato, I., Shibata, T., Komiya, S., Hayashi, M., Matsueda, Y., Imamura, H., Hayase, T., Morioka, H., Tabira, H., et al. (2003) Tetrahedron Asymmetry 14, 185–188. [Google Scholar]
- 149.Denmark, S. & Fu, J. (2003) Chem. Rev. 103, 2763–2793. [DOI] [PubMed] [Google Scholar]
- 150.Mikami, K. & Nakai, T. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley-VCH, New York), 2nd Ed., pp. 543–568.
- 151.Mikami, K., Terada, M. & Nakai, T. (1990) J. Am. Chem. Soc. 112, 3949–3954. [Google Scholar]
- 152.Denmark, S. E. & Fu, J. (2001) J. Am. Chem. Soc. 123, 9488–9489. [DOI] [PubMed] [Google Scholar]
- 153.Dolling, U.-H., Davis, P. & Grabowski, E. J. (1984) J. Am. Chem. Soc. 106, 446–447. [Google Scholar]
- 154.Hughes, D. L., Dolling, U.-H., Ryan, K. M., Schoenewaldt, E. F. & Grabowski, E. J. J. (1987) J. Org. Chem. 52, 4745–4752. [Google Scholar]
- 155.O'Donnell, M. J. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley-VCH, New York), 2nd Ed., pp. 727–755.
- 156.Evans, D. A., Willis, M. C. & Johnston, J. N. (1999) Org. Lett. 1, 865–868. [DOI] [PubMed] [Google Scholar]
- 157.Johnson, J. S. & Evans, D. A. (2000) Acc. Chem. Res. 33, 325–335. [DOI] [PubMed] [Google Scholar]
- 158.Yamada, K., Arai, T., Sasai, H. & Shibasaki, M. (1998) J. Org. Chem. 63, 3666–3672. [DOI] [PubMed] [Google Scholar]
- 159.Austin, J. F. & Mac Millan, D. W. C. (2002) J. Am. Chem. Soc. 124, 1172–1173. [DOI] [PubMed] [Google Scholar]
- 160.Naasz, R., Arnold, L. A., Pineschi, M., Keller, E. & Feringa, B. (1999) J. Am. Chem. Soc. 121, 1104–1105. [Google Scholar]
- 161.Yoshida, K., Ogasawara, M. & Hayashi, T. (2003) J. Org. Chem. 68, 1901–1905. [DOI] [PubMed] [Google Scholar]
- 162.Yoshida, K., Ogasawara, M. & Hayashi, T. (2002) J. Am. Chem. Soc. 124, 10984–10985. [DOI] [PubMed] [Google Scholar]
- 163.Hayashi, T. & Yamasaki, K. (2003) Chem. Rev. 103, 2829–2844. [DOI] [PubMed] [Google Scholar]
- 164.Agbossou, F., Carpentier, J.-F. & Mortreux, A. (1995) Chem. Rev. 95, 2485–2506. [Google Scholar]
- 165.Casalnuovo, A. L. & Rajan Babu, T. V. (1997) Chirality Chem. 2, 309–333. [Google Scholar]
- 166.Dounay, A. B. & Overmann, L. E. (2003) Chem. Rev. 103, 2945–2963. [DOI] [PubMed] [Google Scholar]
- 167.Matsuura, T., Overman, L. E. & Poon, D. J. (1998) J. Am. Chem. Soc. 120, 6500–6503. [Google Scholar]
- 168.Trost, B. M. & Krische, M. J. (1998) Synlett 1–16.
- 169.Hatano, M., Yamazaka, M. & Mikami, K. (2003) Eur. J. Org. Chem. 2552–2555.
- 170.Huo, S., Shi, J.-C. & Negishi, E. (2002) Angew. Chem. Int. Ed. Engl. 41, 2141–2143. [PubMed] [Google Scholar]
- 171.Carter, M. B., Schiott, B., Gutiérrez, A. & Buchwald, S. L. (1994) J. Am. Chem. Soc. 116, 11667–11670. [Google Scholar]
- 172.Verdauger, X., Lange, U. E. W. & Buchwald, S. L. (1998) Angew. Chem. Int. Ed. Engl. 37, 1103–1107. [DOI] [PubMed] [Google Scholar]
- 173.Wynberg, H. (1986) Top. Stereochem. 16, 87–129. [Google Scholar]
- 174.Trost, B. M. & Crawley, M. L. (2003) Chem. Rev. 103, 2921–2944. [DOI] [PubMed] [Google Scholar]
- 175.Hayashi, T. (1988) Pure Appl. Chem. 60, 7–12. [Google Scholar]
- 176.Helmchen, G. & Pfaltz, A. (2000) Acc. Chem. Res. 33, 336–345. [DOI] [PubMed] [Google Scholar]
- 177.Trost, B. M. (1996) Acc. Chem. Res. 29, 355–364. [Google Scholar]
- 178.Trost, B. M. & Bunt, R. C. (1994) J. Am. Chem. Soc. 116, 4089–4090. [Google Scholar]
- 179.Trost, B. M. & Organ, M. G. (1994) J. Am. Chem. Soc. 116, 10320–10321. [Google Scholar]
- 180.Trost, B. M., Organ, M. G. & O'Doherty, G. A. (1995) J. Am. Chem. Soc. 117, 9662–9663. [Google Scholar]
- 181.Trost, B. M. & Schoroeder, G. M. (1999) J. Am. Chem. Soc. 121, 6759–6760. [Google Scholar]
- 182.Trost, B. M., Schroeder, G. M. & Kristensen, J. (2002) Angew. Chem. Int. Ed. Engl. 41, 3492–3495. [DOI] [PubMed] [Google Scholar]
- 183.Trost, B. M. & Tang, W. (2003) J. Am. Chem. Soc. 125, 8744–8745. [DOI] [PubMed] [Google Scholar]
- 184.Reetz, M. T., Sell, T., Meiswinkel, A. & Mehler, G. (2003) Angew. Chem. Int. Ed. Engl. 42, 790–793. [DOI] [PubMed] [Google Scholar]