Abstract
Asymmetric hydrogenation uses inexpensive, clean hydrogen gas and a very small amount of a chiral molecular catalyst, providing the most powerful way to produce a wide array of enantio-enriched compounds in a large quantity without forming any waste. The recent revolutionary advances in this field have entirely changed the synthetic approach to producing performance chemicals that require a high degree of structural precision. The means of developing efficient asymmetric hydrogenations is discussed from a mechanistic point of view.
Asymmetric catalysis lies outside the realm of traditional organic synthesis. It is a pervasive, global endeavor involving synthetic organic chemistry, catalytic chemistry, structural chemistry, inorganic and coordination chemistry, physical chemistry, and theoretical chemistry, as well as chemical engineering.
Chiral Molecular Catalysts: Beyond the Shape
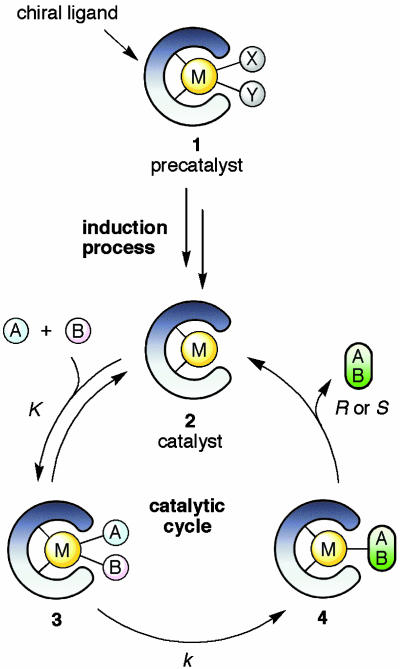
Asymmetric catalysis is four-dimensional chemistry (1). High efficiency can be achieved only by using a combination of both an ideal 3D structure (x, y, z) and suitable kinetics (t). Currently, efficient asymmetric catalysis primarily uses a molecular catalyst that consists of a metallic element and chiral organic ligand(s) (2). Fig. 1 illustrates a typical (but not general) catalytic scheme. Under reaction conditions, the initially used precatalyst 1 is converted to the true catalyst 2 (induction process) that activates achiral molecules A and B and transforms them to the chiral product A–B (catalytic cycle). Although the ligand must create a distinct enantio-differentiating environment in transition metal-based complexes, such an architectural design does not suffice to achieve asymmetric catalysis. Some of the steps in the multistep transformation are reversible, whereas the first irreversible step, for example 3→4, kinetically determines the absolute stereochemistry of A–B. Efficient asymmetric catalysis requires a high turnover number and high turnover frequency, but the best way to generate high catalytic activity is not immediately apparent. First, the induction process of converting 1 to 2 is often not straightforward. Furthermore, to obtain a high turnover frequency, all of the transition metal-based entities 2–4 in this cycle must be neither very unstable nor very stable, avoiding substrate and/or product inhibition. Instead, 2–4 are required to interconvert one another smoothly by means of a low kinetic barrier, and without any destructive side reactions. The reaction conditions strongly influence the stability and reactivity of 1–4. In general, both suitable architectural and functional engineering are crucial for obtaining sufficient catalytic efficiency. “Molecular catalysis” can cope with such requirements, because any molecules, by definition, can be designed and synthesized at will.
Fig. 1.
The principle of asymmetric catalysis with chiral organometallic molecular catalysts. M, metal; A and B, reactant and substrate; X or Y, neutral or anionic ligand.
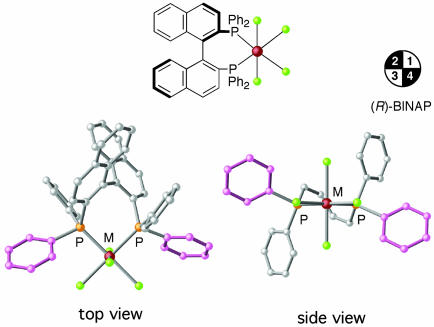
This perspective deals largely with the intrinsic mechanistic aspects of asymmetric hydrogenation developed in our laboratories (3–5). Although H—H bonds are readily cleaved by transition metal complexes, truly useful asymmetric hydrogenations are limited. 2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl (BINAP)–transition metal complexes are shaped in a manner that is beneficial for chiral recognition (Fig. 2) (6, 7), and these complexes can act as hydrogenation catalysts. However, their efficiency highly depends on the structures of unsaturated substrates, the properties of the central metal and the auxiliary anionic or neutral ligands, and the reaction conditions (e.g., hydrogen pressure, temperature, solvent, and additive). No universal catalysts are known to exist, because of the diversity of unsaturated organic compounds.
Fig. 2.
Chiral environment of an (R)-BINAP–transition metal complex. The second and fourth quadrants are more crowded than the first and third quadrants because of shielding by the pink phenyl groups. M, metallic element.
Metallic Elements: Rhodium vs. Ruthenium in Asymmetric Hydrogenation of Enamides
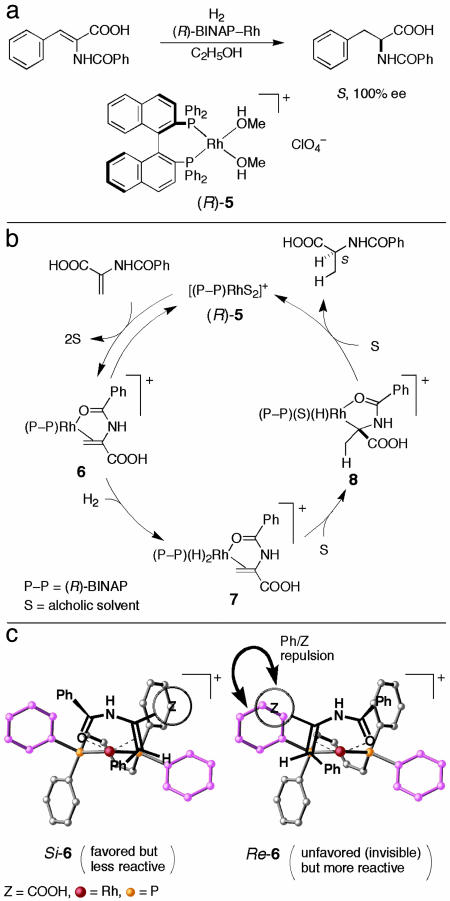
In 1980, we reported that cationic BINAP–Rh(I) complexes catalyze the hydrogenation of α-(acylamino)acrylic acids and esters to give the amino acid derivatives in >90% enantiomeric excess (ee) (8). For example, in the presence of [Rh{(R)-binap}(CH3OH)2]+ [(R)-5], (Z)-α-(benzamido)cinnamic acid is converted to (S)-N-benzoylphenylalanine in near 100% ee and 97% yield [substrate/catalyst molar ratio (S/C) = 100, 4 atm, room temp, 48 h, C2H5OH] (Fig. 3a). This reaction appeared to be excellent in terms of enantioselection, but proved to be less than ideal because of the “unsaturate/dihydride mechanism” that was thoroughly substantiated by Halpern, Brown, and coworkers (Fig. 3b) (9–11). When a C2-chiral diphosphine ligand such as CHIRAPHOS or DIPAMP is used, the Rh complex 5 forms a mixture of two diastereomeric substrate complexes 6, depending on the Re/Si face selection at C(2), which lead to the S or R hydrogenation product. Therefore, the enantioselectivity is determined by the relative equilibrium ratio and reactivity of diastereomeric 6. Most importantly, under these reaction conditions, the more stable, major diastereomer is consumed much more slowly than the less stable, minor isomer because of the lower reactivity toward H2, giving 7, or, if this step is reversible, this leads to the more difficult migratory insertion of the Rh dihydride species, 7→8. This situation can be monitored by 31P NMR. Because of the superior chiral recognition ability of BINAP, the 31P-NMR spectrum of a 6:1 mixture of (Z)-α-(benzamido)cinnamic acid and (R)-BINAP–Rh catalyst 5 in CH3OH showed a single eight-line signal for Si-6, whereas the signal of the minor Re-6 was undetectable (Fig. 3c) (12). Thermodynamically favored Si-6, leading to the R product, is only weakly reactive and, before hydrogenation, is converted to the NMR-invisible but highly reactive diastereomer Re-6, giving the S product, via decoordination and recoordination of the substrate. Therefore, the observed enantioselectivity is a result of the delicate balance of the stability and reactivity of diastereomeric 6. Because of this inherent mechanistic problem, the optimum conditions leading to near 100% enantioselectivity are obtainable only by a very careful choice of reaction parameters. The reaction must be conducted under a low substrate concentration and low hydrogen pressure. The BINAP–Rh-catalyzed reaction occurs very slowly, because the reactive substrate/Rh complex 6 is present in a very low concentration under these hydrogenation conditions. The scope of the olefinic substrates is narrow. Thus, BINAP–Rh-catalyzed hydrogenation suffers from such a serious mechanistic limitation that this particular asymmetric reaction remains far from ideal. Excellent enantioselection is obtainable only with Rh complexes of DuPhos, certain monodentate phosphites, or phosphoamidites (13–16) or by using the dihydride/unsaturate mechanism (17).
Fig. 3.
Hydrogenation of (Z)-α-benzamidocinnamic acid catalyzed by an (R)-BINAP–Rh complex. (a) Reaction scheme. (b) Catalytic cycle, where the β-phenyl group in the substrate is omitted for clarity. (c) Diastereomeric Rh complexes.
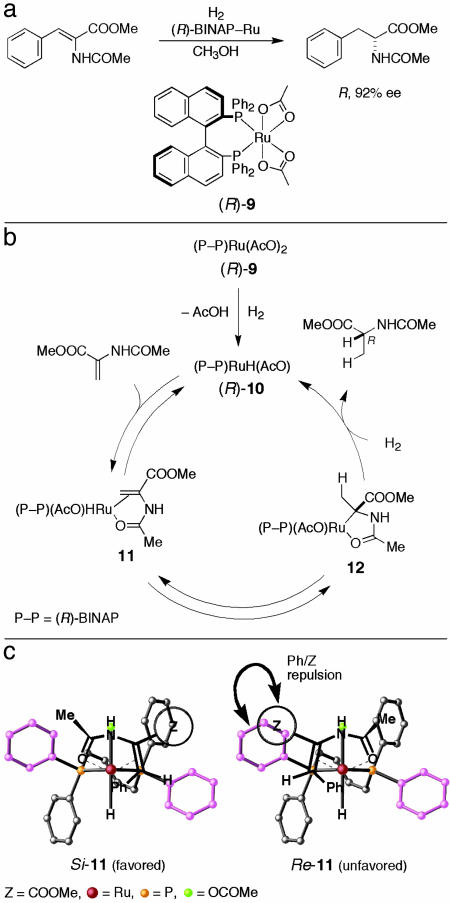
A breakthrough in this respect came when the Rh(I) element was replaced by Ru(II). When methyl (Z)-α-(acetamido)-cinnamate was hydrogenated in the presence of Ru(OCOCH3)2[(R)-binap] [(R)-9] (18, 19) (S/C = 200, 1 atm, 30°C, 24 h, CH3OH), methyl (R)-α-(acetamido)cinnamate was obtained in 92% ee and 100% yield (Fig. 4a) (20). Most notably, the sense of the asymmetric induction was the opposite of that obtained with the (R)-BINAP–Rh catalyst (Fig. 3a), namely, R/R vs. R/S (20, 21). This contrasting behavior was found to be caused by a “monohydride/unsaturate mechanism” (20, 22), in which the Ru monohydride 10, formed by the heterolytic cleavage of H2 by the precatalyst 9, acted as true catalyst (Fig. 4b). Importantly, unlike in the Rh chemistry involving the “unsaturate/dihydride mechanism” (9–12), the metal hydride species in this reaction is generated before the substrate coordination. The stereochemistry of the product is determined by the cleavage of the Ru—C bond in 12 largely by H2. The enantiomer ratio corresponds well to the relative stability of the diastereomeric substrate/RuH complexes Si- and Re-11 (Fig. 4c). Here, the major Si-11 is converted to the R hydrogenation product in a straightforward manner, i.e., the migratory insertion followed by hydrogenolysis. Notably, the two hydrogen atoms incorporated in the product are from two different H2 molecules, as proven by isotope labeling experiments, and this result stands in contrast to the standard Rh-catalyzed reaction, which uses only one H2 molecule per product.
Fig. 4.
Hydrogenation of methyl (Z)-α-acetamidocinnamate catalyzed by an (R)-BINAP–Ru complex. (a) Reaction scheme. (b) Catalytic cycle, where the β-phenyl group in the substrate is omitted. (c) Diastereomeric Ru complexes.
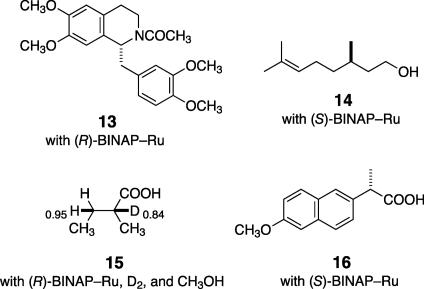
Replacement of Rh by Ru has greatly extended the reaction scope, allowing for the asymmetric hydrogenation of many kinds of olefinic substrates (Fig. 5) (6, 7). For example, the reaction with N-acylated benzylidene-tetrahydroisoquinolines gives chiral benzyl-tetrahydroisoquinolines such as N-protected tetrahydropapaverine (13) in near 100% ee, providing a general method of asymmetric synthesis of isoquinoline alkaloids (23). Hydrogenation of geraniol possessing two olefinic bonds occurs only at the allylic alcohol part, thereby affording citronellol (14) in >95% ee (24).
Fig. 5.
Examples of BINAP–Ru-catalyzed asymmetric hydrogenation of olefins.
Hydrogen Source
In the hydrogenation of enamides, the Ru—C bond of 12 is cleaved mainly by H2 (Fig. 4b) but to some extent (≈14%) also by protic CH3OH (20). The protonolysis pathway becomes conspicuous when α,β-unsaturated carboxylic acids are used as a substrate because of a change in the mechanism or a subtle difference in the properties of the Ru—C bond (25, 26). Reaction of (Z)-2-methyl-2-butenoic acid with D2 and (R)-9 in CH3OH (S/C = 100, 4 atm, room temp) afforded (R)-2-methylbutanoic acid-2d (15) in 91% ee and 100% yield (Fig. 5). The hydrogen at C(3) comes mainly from hydrogen gas and the C(2) hydrogen is from CH3OH. The reaction is net hydrogenation, whereby both H2 and protic agents can serve as the hydrogen source. This hydrogenation reaction can be performed in supercritical CO2 as well (27). In fact, the sense and degree of the asymmetric hydrogenation of α,β-unsaturated carboxylic acids highly depends on the structure of the substrates and the hydrogen pressure, indicating the existence of various catalytic pathways. High-pressure (>100 atm) hydrogenation of an α-arylacrylic acid in CH3OH affords (S)-naproxen (16), an important antiinflammatory agent, in 97% ee (Fig. 5) (28).
Significance of Anionic Ligands: Asymmetric Hydrogenation of Functionalized Ketones
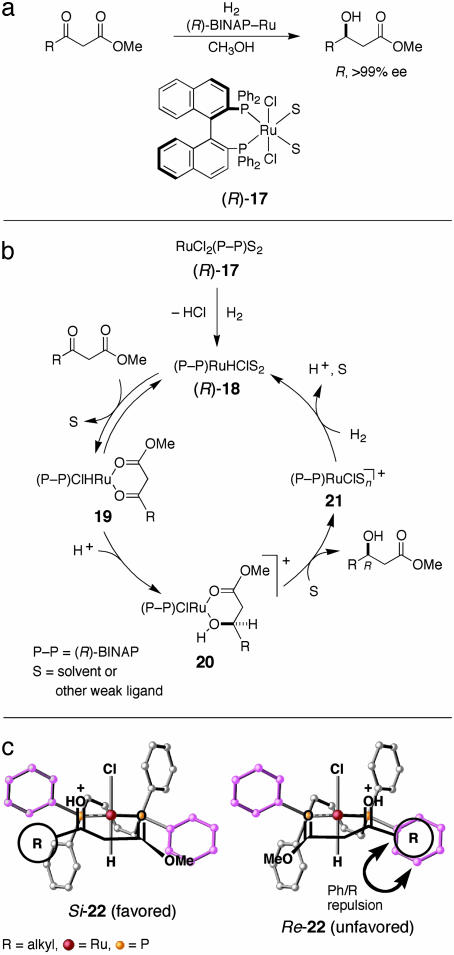
Ru(OCOCH3)2(binap) (9) (18, 19), although excellent for the asymmetric hydrogenation of functionalized olefins, is totally ineffective in reactions of structurally similar ketones such as β-keto esters. This failure is caused by the properties of the anionic ligands, in particular, the carboxylates. On the other hand, when the carboxylates are simply replaced by halides, very high catalytic activity can be generated for functionalized ketones. Thus, the useful chiral precatalysts include RuX2(binap) (17) (X = Cl, Br, I; polymeric form) (29, 30), [RuX(binap)(arene)]Y (X = halogen, Y = halogen or BF4) (31), [NH2(C2H5)2][{RuCl(binap)}2-(μ-Cl)3] (32, 33), and other in situ-formed halogen-containing BINAP–Ru complexes (34, 35). Various β-keto esters are hydrogenated in alcoholic solvent with an S/C of up to 10,000 to give chiral β-hydroxy esters in high ee (Fig. 6a).
Fig. 6.
Hydrogenation of β-keto esters catalyzed by an (R)-BINAP–Ru complex. (a) Reaction scheme. (b) Catalytic cycle. (c) Diastereomeric transition states.
Fig. 6b presents a mechanistic model, in which the actual catalyst is RuHCl 18, formed by the reaction between 17 and H2 (36). First, 18 interacts reversibly with the β-keto ester to form the σ-type chelate complex 19, in which metal-to-carbonyl hydride transfer is geometrically difficult. Then, protonation occurs at the oxygen to increase the electrophilicity of the carbon and convert the geometry from σ to π, thereby facilitating the hydride migration. The hydroxy ester ligand in the resulting product 20 is liberated by solvent molecules. The cationic Ru complex 21 once again cleaves H2, thus regenerating 18. This catalytic cycle takes place repeatedly. Enantioselection of a ratio of >99:1 is achieved in the hydride transfer step 19→20. Here, the key is the carbonyl protonation in 19, which is caused by HCl (37, 38) generated in the induction step 17→18. When Ru(OCOCH3)2(binap) (9) is used, acetic acid is produced in place of HCl. The weak carboxylic acid is unable to generate high catalytic activity. Thus, even achiral anionic ligands in the precatalyst are not innocent in catalytic hydrogenation (39).
Ligation of the ester C—O to the Ru center stabilizes the transition states (TSs) in the hydride transfer step. Thanks to the shape of (R)-BINAP, diastereomeric Si- and Re-22 are clearly differentiated (Fig. 6c). The R-generating Si structure is highly favored over the S-generating Re isomer, which suffers from the phenyl/R repulsive interaction. In addition, the oxygen/Ru interaction in 22 is crucial for catalytic activity as well. Notably, the BINAP–Ru-catalyzed hydrogenation of β-keto esters can be performed even in acetone containing a small amount of water. Acetone, the simplest ketone used as a solvent, is almost inert to the hydrogenation, whereas the structurally more complex β-keto ester substrates are easily hydrogenated. This difference proves to be an important distinction.
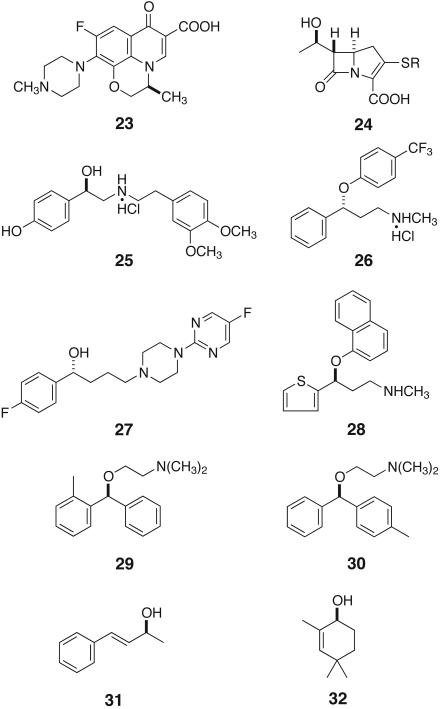
The list of potential substrates can be extended to include various ketones possessing a directive functional group such as a dialkylamino, hydroxyl, alkoxyl, siloxyl, keto, halogeno, alkoxycarbonyl, alkylthiocarbonyl, dialkylaminocarbonyl, phosphoryl, and sulfoxyl group, among other possibilities (3–7). This asymmetric hydrogenation has been used for the synthesis of a large variety of biologically or pharmaceutically important chiral compounds (3–5). For example, the simple asymmetric hydrogenation of acetol to (R)-propanediol (40) is used for industrial synthesis of levofloxacin (23) (Fig. 7), a quinolone antibacterial agent.
Fig. 7.
Chiral compounds obtained by the asymmetric hydrogenation of ketones.
Solvent Effects on Stereoselectivity in Dynamic Kinetic Resolution
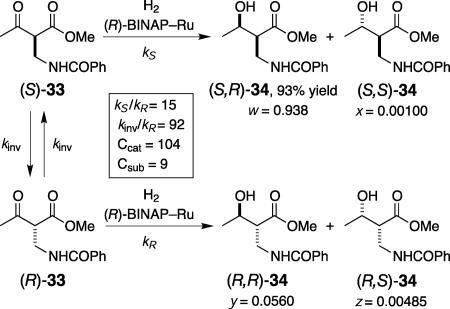
Although BINAP–Ru-catalyzed hydrogenation is normally best conducted in alcoholic solvents, sometimes different solvents must be used to achieve a high degree of stereoselectivity. In a chiral environment, enantiomers react at different rates. Therefore, if one chooses the appropriate chiral catalyst and reaction conditions, racemic β-keto esters possessing a configurationally labile α-stereogenic center can be selectively transformed into one stereoisomer of the four possible stereoisomers (41). As illustrated in Fig. 8, (R)-BINAP–Ru-catalyzed the hydrogenation of racemic methyl α-(benzamidomethyl)-acetoacetate (33) in dichloromethane (not CH3OH) ([33] = 0.2 M, [(R)-17] = 1.3 mM, 100 atm, 50°C, 40 h), which gives the 2S,3R hydroxy ester (S,R)-34 with 94:6 syn/anti diastereoselectivity and 99.5:0.5 enantioselectivity (42). This asymmetric synthesis via a dynamic kinetic resolution is used for the industrial production of chiral 4-acetoxyazetidinone, a synthetic intermediate for carbapenem antibiotics (24) (Fig. 7).
Fig. 8.
Stereoselective hydrogenation of racemic methyl α-(benzamidomethyl) acetoacetate (33) with (R)-17 under dynamic kinetic resolution.
Computer-aided quantitative analysis has revealed the following results under these catalytic conditions: (i) the inherent, highest S,R/S,S/R,R/R,S selectivity at time t = 0 (w/x/y/z partition parameter, where the starting S and R ketones are assumed to exist in equal amounts) is 93.8:0.1:5.6:0.49; (ii) (S)-33 is hydrogenated 15 times faster than (R)-33; (iii) the inversion of the slow-reacting (R)-33 to (S)-33 takes place 92 times faster than hydrogenation; (iv) the extent of the (R)-BINAP catalyst-based asymmetric induction (Ccat) is calculated to be 104:1 in favor of the 3R isomer; and (v) the substrate-based asymmetric induction (Csub) is 9:1 in favor of syn C(2)/C(3) stereochemistry (43). Thus, various steric and kinetic parameters can be beneficially combined to selectively produce the desired 2S,3R stereoisomer. When the reaction is conducted in CH3OH ([33] = 0.2 M, [(R)-17] = 1.3 mM, 100 atm, 25°C, 20 h), the diastereoselectivity is drastically reduced to form a 1:1 mixture of (S,R)-34 in 93% ee and (R,R)-34 in 97% ee. Under such conditions, both (S)- and (R)-33 have comparable reactivity, leading to hydrogenation.
Metal–Ligand Bifunctional Catalysis: The NH Effect in the Asymmetric Hydrogenation of Simple Ketones
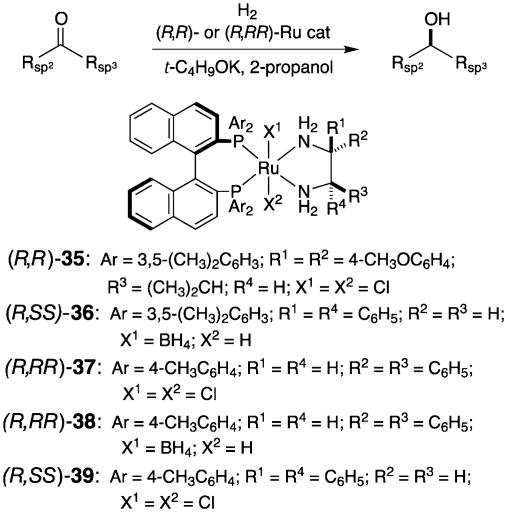
For the mechanistic reasons given in Fig. 6, simple unfunctionalized ketones are inert to hydrogenation in the presence of RuX2(diphophine) (X = halogen). On the other hand, the newly devised RuCl2(diphosphine)(1,2-diamine) complexes including 35, 37, and 39 are excellent precatalysts for the asymmetric hydrogenation of simple ketones that lack heteroatom functionality capable of producing any interaction with the transition metal center (Fig. 9) (36). The use of BINAP and a suitably shaped chiral 1,2-diamine allows for the practical asymmetric hydrogenation of various ketones in 2-propanol containing an alkaline base cocatalyst such as KOH, KOCH(CH3)2, and KO-t-C4H9. Moreover, when RuH-(η1-BH4)(binap)(1,2-diamine), 36 and 38, is used as precatalyst, such a strong base is unnecessary (44). In these complexes, the presence of an NH2 moiety in the diamine ligand is crucial for obtaining high catalytic activity. This type of hydrogenation tolerates many substituents, including halogen, CF3, alkoxyl, ester, amide, NO2, and NH2, as well as various electron-rich and -deficient heterocycles (45, 46). In addition, this reaction is rapid and productive. The hydrogenation of acetophenone with RuCl2[(R)-tolbinap][(R,R)-dpen] [(R,RR)-37] (DPEN=1,2-diphenylethylenediamine) proceeds with a high turnover number of 2,400,000 and a high turnover frequency of 63 s–1 (47). This asymmetric hydrogenation has been applied to the synthesis of various pharmaceutically important chiral compounds such as denopamine hydrochloride (25) (β1-receptor agonist), fluoxetine hydrochloride (26) (antidepressant), BMS 181100 (27) (antipsychotic), duloxetine (serotonin and norepinephrine inhibitor) (28), orphenadrine (29) (antihistaminic and anticholinergic), and neobenodine (30) (antihistaminic) (Fig. 7) (48, 49).
Fig. 9.
Asymmetric hydrogenation of simple ketones catalyzed by BINAP/1,2-diamine–Ru complexes.
Furthermore, cyclic and acyclic α,β-unsaturated ketones can be selectively converted into allyllic alcohols of high enantiomeric purity (45, 46). This selectivity is in contrast with that of diamine-free RuX2(binap) complexes (X = carboxylate or halide) (23) converting geraniol or nerol to 14 in high ee (Fig. 5). The chiral alcohol 32 (Fig. 7), a key intermediate leading to various bioactive compounds, is obtainable by the hydrogenation of the corresponding cyclohexenone in the presence of (R,SS)-39. In addition, various cyclic and acyclic ketones are hydrogenated with high diastereoselectivity by simply using RuCl2(PAr3)2(en) and an alkaline base (50).
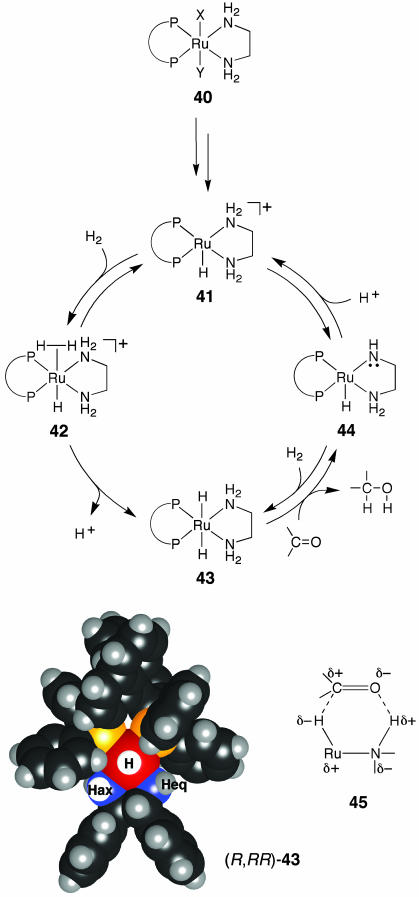
The high rate of the reaction and carbonyl selectivity are based on nonclassical metal–ligand bifunctional catalysis, which uses an “NH effect,” as illustrated in Fig. 10 (51). Under the present hydrogenation conditions, the catalyst precursor 40 generates 41 equilibrating with 44. The major cationic 16e species 41 is converted to 42 and then 43, which in turn reacts with a ketonic substrate to form 44 and an alcohol product. The 16e Ru amide 44 returns largely to 41 by protonation, but gives some 43 caused by the reaction with H2. The reducing 18e Ru dihydride 43 possesses the hydride and two nitrogen atoms with a fac relationship. When (R)-BINAP and (R,R)-DPEN are used as chiral ligands, two hydrogen atoms are asymmetrically activated, as shown by hydridic RuH and protic NHax in (R,RR)-43. This 18e complex reacts with a simple ketone in its outer coordination sphere, where neither a ketone/Ru nor an alkoxy/Ru complex is involved. This mechanistic scheme is distinct from the conventional model shown in Fig. 1. The six-membered, pericyclic transition state is schematically shown by 45.
Fig. 10.
Metal–ligand bifunctional mechanism in the Ru-catalyzed hydrogenation of ketones.
Kinetic Resolution of Simple Ketones Under Basic and Neutral Conditions
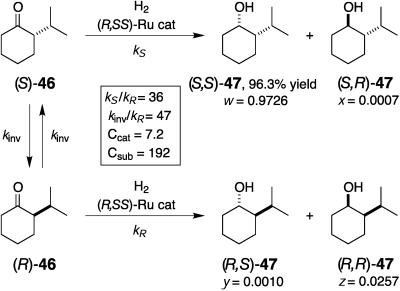
The hydrogenation of racemic 2-isopropyl-cyclohexanone (46) in 2-propanol containing (R)-17, (S,S)-DPEN, and KOH ([46] = 0.8 M, [17] = [DPEN] = 1.6 mM, [KOH] = 32 mM, 28°C, 11 h) quantitatively gives the 1S,2S alcohol (S,S)-47 in 93% ee with 99.8:0.2 cis selectivity (Fig. 11) (50). This dynamic kinetic resolution is based on the facile configurational inversion of the substrate caused by the alkaline base and a protic solvent. These conditions are appropriate for producing alcoholic products of high ee (50, 52) but remain unsuitable for obtaining the configurationally labile starting ketones. However, when racemic 46 was hydrogenated in 2-propanol with (R,SS)-36, but without any base (S/C = 2,000, 8 atm, 25°C, 2 h), at 53% conversion, unreacted (R)-46 with 91% ee was recovered together with the 1S,2S alcohol (S,S)-47 in 85% ee (44).
Fig. 11.
Dynamic kinetic resolution of a racemic ketone by hydrogenation with (R)-17,(S,S)-DPEN, and KOH in 2-propanol.
Conclusion
Asymmetric hydrogenation is a core technology in modern chemical synthesis. High rates and selectivities are attainable only by the combination of structurally well designed catalysts and suitable kinetic parameters (2, 36). All of our Ru-based molecular catalysts commonly involve an octahedral BINAP–RuH species; however, the reactivity of these catalysts is remarkably influenced by the anionic or neutral auxiliaries present in the other three coordination sites. Deep mechanistic insight is indispensable for developing truly efficient asymmetric hydrogenation reactions. A diverse array of chiral compounds are accessible by this purely chemical means. The recent substantial expansion of this subject has given rise to enormous economic potential in the chemical manufacture of pharmaceuticals, animal health products, agrochemicals, fungicides, pheromones, flavors, and fragrances (53).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BINAP, 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl; ee, enantiomeric excess; S/C, substrate/catalyst molar ratio; DPEN, 1,2-diphenylethylenediamine.
References
- 1.Noyori, R. (1994) Asymmetric Catalysis in Organic Synthesis (Wiley, New York), pp. 2–3.
- 2.Noyori, R. (2002) Angew. Chem. Int. Ed. 41, 2008–2022. [PubMed] [Google Scholar]
- 3.Ohkuma, T. & Noyori, R. (1998) in Transition Metals for Organic Synthesis, eds. Beller, M. & Bolm, C. (Wiley, Weinheim, Germany), Vol. 2, pp. 25–69. [Google Scholar]
- 4.Ohkuma, T. & Noyori, R. (1999) in Comprehensive Asymmetric Catalysis, eds. Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (Springer, Berlin), Vol. 1, pp. 199–246. [Google Scholar]
- 5.Ohkuma, T., Kitamura, M. & Noyori, R. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley, New York), 2nd Ed., pp. 1–110.
- 6.Noyori, R. (1990) Science 248, 1194–1199. [DOI] [PubMed] [Google Scholar]
- 7.Noyori, R. & Takaya, H. (1990) Acc. Chem. Res. 23, 345–350. [Google Scholar]
- 8.Miyashita, A., Yasuda, A., Takaya, H., Toriumi, K., Ito, T., Souchi, T. & Noyori, R. (1980) J. Am. Chem. Soc. 102, 7932–7934. [Google Scholar]
- 9.Chan, A. S. C. & Halpern, J. (1980) J. Am. Chem. Soc. 102, 838–840. [Google Scholar]
- 10.Landis, C. R. & Halpern, J. (1987) J. Am. Chem. Soc. 109, 1746–1754. [Google Scholar]
- 11.Brown, J. M. & Chaloner, P. A. (1980) J. Am. Chem. Soc. 102, 3040–3048. [Google Scholar]
- 12.Miyashita, A., Takaya, H., Souchi, T. & Noyori, R. (1984) Tetrahedron 40, 1245–1253. [Google Scholar]
- 13.Burk, M. J. (2000) Acc. Chem. Res. 33, 363–372. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg, M., Minnaard, A. J., Schudde, E. P., van Esch, J., de Vries, A. H. M., de Vries, J. G. & Feringa, B. L. (2000) J. Am. Chem. Soc. 122, 11539–11540. [Google Scholar]
- 15.Claver, C., Fernandez, E., Gillon, A., Heslop, K., Hyett, D. J., Martorell, A., Orpen, A. G. & Pringle, P. G. (2000) Chem. Commun. 961–962.
- 16.Reetz, M. T. & Mehler, G. (2000) Angew. Chem. Int. Ed. 39, 3889–3890. [DOI] [PubMed] [Google Scholar]
- 17.Gridnev, I. D., Higashi, N., Asakura, K. & Imamoto, T. (2000) J. Am. Chem. Soc. 122, 7183–7194. [Google Scholar]
- 18.Ohta, T., Takaya, H. & Noyori, R. (1988) Inorg. Chem. 27, 566–569. [Google Scholar]
- 19.Kitamura, M., Tokunaga, M. & Noyori, R. (1992) J. Org. Chem. 57, 4053–4054. [Google Scholar]
- 20.Kitamura, M., Tsukamoto, M., Bessho, Y., Yoshimura, M., Kobs, U., Widhalm, M. & Noyori, R. (2002) J. Am. Chem. Soc. 124, 6649–6667. [DOI] [PubMed] [Google Scholar]
- 21.Ikariya, T., Ishii, Y., Kawano, H., Arai, T., Saburi, M., Yoshikawa, S. & Akutagawa, S. (1985) J. Chem. Soc. Chem. Commun. 922–924.
- 22.Wiles, J. A., Bergens, S. H. & Young, Y. G. (1997) J. Am. Chem. Soc. 119, 2940–2941. [Google Scholar]
- 23.Kitamura, M., Hsiao, Y., Ohta, M., Tsukamoto, M., Ohta, T., Takaya, H. & Noyori, R. (1994) J. Org. Chem. 59, 297–310. [Google Scholar]
- 24.Takaya, H., Ohta, T., Sayo, N., Kumobayashi, H., Akutagawa, S., Inoue, S., Kasahara, I. & Noyori, R. (1987) J. Am. Chem. Soc. 109, 1596–1597, and correction (1987) 109, 4129. [Google Scholar]
- 25.Ohta, T., Takaya, H. & Noyori, R. (1990) Tetrahedron Lett. 31, 7189–7192. [Google Scholar]
- 26.Ashby, M. T. & Halpern, J. (1991) J. Am. Chem. Soc. 113, 589–594. [Google Scholar]
- 27.Xiao, J., Nefkens, S. C. A., Jessop, P. G., Ikariya, T. & Noyori, R. (1996) Tetrahedron Lett. 37, 2813–2816. [Google Scholar]
- 28.Ohta, T., Takaya, H., Kitamura, M., Nagai, K. & Noyori, R. (1987) J. Org. Chem. 52, 3174–3176. [Google Scholar]
- 29.Noyori, R., Ohkuma, T., Kitamura, M., Takaya, H., Sayo, N., Kumobayashi, H. & Akutagawa, S. (1987) J. Am. Chem. Soc. 109, 5856–5858. [Google Scholar]
- 30.Kitamura, M., Ohkuma, T., Inoue, S., Sayo, N., Kumobayashi, H., Akutagawa, S., Ohta, T., Takaya, H. & Noyori, R. (1988) J. Am. Chem. Soc. 110, 629–631. [Google Scholar]
- 31.Mashima, K., Kusano, K., Ohta, T., Noyori, R. & Takaya, H. (1989) J. Chem. Soc. Chem. Commun. 1208–1210.
- 32.King, S. A. & DiMichele, L. (1995) in Catalysis of Organic Reactions, eds. Scaros, M. G. & Prunier, M. L. (Dekker, New York), pp. 157–166.
- 33.Ohta, T., Tonomura, Y., Nozaki, K., Takaya, H. & Mashima, K. (1996) Organometallics 15, 1521–1523. [Google Scholar]
- 34.Genet, J. P. (1996) in Reduction in Organic Synthesis, ed. Abdel-Magit, A. F. (Am. Chem. Soc., Washington, DC), pp. 31–51.
- 35.Kumobayashi, H., Miura, T., Sayo, N., Saito, T. & Zhang, X. (2001) Synlett, 1055–1064.
- 36.Noyori, R. & Ohkuma, T. (2001) Angew. Chem. Int. Ed. 40, 40–73. [PubMed] [Google Scholar]
- 37.King, S. A., Thompson, A. S., King, A. O. & Verhoeven, T. R. (1992) J. Org. Chem. 57, 6689–6691. [Google Scholar]
- 38.Kitamura, M., Yoshimura, M., Kanda, N. & Noyori, R. (1999) Tetrahedron 55, 8769–8785. [Google Scholar]
- 39.Fagnou, K. & Lautens, M. (2002) Angew. Chem. Int. Ed. 41, 26–47. [DOI] [PubMed] [Google Scholar]
- 40.Kitamura, M., Tokunaga, M., Ohkuma, T. & Noyori, R. (1991) Tetrahedron Lett. 32, 4163–4166. [Google Scholar]
- 41.Noyori, R., Tokunaga, M. & Kitamura, M. (1995) Bull. Chem. Soc. Jpn. 68, 36–56. [Google Scholar]
- 42.Noyori, R., Ikeda, T., Ohkuma, T., Widhalm, M., Kitamura, M., Takaya, H., Akutagawa, S., Sayo, N., Saito, T., Taketomi, T. & Kumobayashi, H. (1989) J. Am. Chem. Soc. 111, 9134–9135. [Google Scholar]
- 43.Kitamura, M., Tokunaga, M. & Noyori, R. (1993) J. Am. Chem. Soc. 115, 144–152. [Google Scholar]
- 44.Ohkuma, T., Koizumi, M., Muñiz, K., Gerhard, H., Kabuto, C. & Noyori, R. (2002) J. Am. Chem. Soc. 124, 6508–6509. [DOI] [PubMed] [Google Scholar]
- 45.Ohkuma, T., Koizumi, M., Doucet, H., Pham, T., Kozawa, M., Murata, K., Katayama, E., Yokozawa, T., Ikariya, T. & Noyori, R. (1998) J. Am. Chem. Soc. 120, 13529–13530. [Google Scholar]
- 46.Ohkuma, T., Koizumi, M., Yoshida, M. & Noyori, R. (2000) Org. Lett. 2, 1749–1751. [DOI] [PubMed] [Google Scholar]
- 47.Doucet, H., Ohkuma, T., Murata, K., Yokozawa, T., Kozawa, M., Katayama, E., England, A. F., Ikariya, T. & Noyori, R. (1998) Angew. Chem. Int. Ed. 37, 1703–1707. [DOI] [PubMed] [Google Scholar]
- 48.Ohkuma, T., Koizumi, M., Ikehira, H., Yokozawa, T. & Noyori, R. (2000) Org. Lett. 2, 659–662. [DOI] [PubMed] [Google Scholar]
- 49.Ohkuma, T., Ishii, D., Takeno, H. & Noyori, R. (2000) J. Am. Chem. Soc. 122, 6510–6511. [Google Scholar]
- 50.Ohkuma, T., Ooka, H., Yamakawa, M., Ikariya, T. & Noyori, R. (1996) J. Org. Chem. 61, 4872–4873. [Google Scholar]
- 51.Sandoval, C. A., Ohkuma, T., Muñiz, K. & Noyori, R. (2003) J. Am. Chem. Soc. 125, 13490–13503. [DOI] [PubMed] [Google Scholar]
- 52.Murata, K., Okano, K., Miyagi, M., Iwane, H., Noyori, R. & Ikariya, T. (1999) Org. Lett. 1, 1119–1121. [Google Scholar]
- 53.Blaser, H. U. & Schmidt, E., eds. (2003) Asymmetric Catalysis in Industrial Scale: Challenges, Approaches and Solutions (Wiley, Weinheim, Germany).