Abstract
Only a few catalytic asymmetric C—C bond-forming reactions have been shown to be useful for constructing all-carbon quaternary stereocenters. This Perspective examines the current state of such methods.
Carbon atoms bonded to four carbon substituents (all-carbon quaternary centers) pose a particular challenge for synthesis because creation of such centers is complicated by steric repulsion between the carbon substituents. When the four substituents differ, quaternary stereocenters become a singular challenge for achieving efficient asymmetric syntheses of chiral organic molecules (1–4). The invention of catalytic methods for asymmetric synthesis is one of the foremost recent achievements of chemistry (5), with the 2001 Nobel Prize in Chemistry recognizing William S. Knowles, Ryoji Noyori, and K. Barry Sharpless for their pioneering development of catalytic asymmetric hydrogenation and oxidation reactions. At present, many broadly useful methods for catalytic asymmetric oxidation and reduction exist; however, far fewer catalytic asymmetric methods for forming C—C bonds have been invented to date (5). As disclosures in this special feature will attest, this latter area of catalytic asymmetric synthesis is a current focus of intense investigation worldwide.
At present only a few catalytic asymmetric C—C bond-forming reactions have been shown to be useful for constructing quaternary carbons, undoubtedly reflecting the additional steric challenge involved in forming all-carbon quaternary centers. This Perspective will examine the current state of such methods. The focus will be on reactions for which some generality has been documented, and several examples exist of reactions that proceed with enantiomeric selectivities of at least 9:1 [enantiomeric excesses (ees) >80%]. The discussion is organized by general reaction types that have proven useful, not by the more commonly used organizations that focus on one specific transformation or one family of asymmetric catalysts. It is hoped that this organization will highlight the many opportunities that exist for future discoveries in this area. The less general approach for forming all-carbon quaternary stereocenters in which a group-selective catalytic asymmetric reaction is used to desymmetrize a prochiral intermediate containing a quaternary carbon will be briefly mentioned also. No attempt to summarize this latter field will be made, as any catalytic asymmetric reaction could in principle be used in this approach. In four areas, catalytic methods for directly forming all-carbon stereocenters are most developed: Diels–Alder reactions, the combination of chiral carbon nucleophiles with carbon electrophiles, reactions of allylmetal intermediates with carbon nucleophiles, and intramolecular Heck reactions. Our discussion will begin with these reaction types.
Asymmetric Diels–Alder Reactions
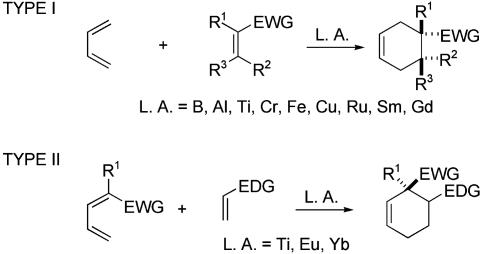
The Diels–Alder reaction provides two approaches for forming quaternary stereocenters contained within the cyclohexene framework (Scheme 1). In reactions of type I, electron-rich dienes condense with electron-poor prochiral dienophiles containing a 1,1-disubstituted or trisubstituted double bond to generate a quaternary carbon. The dienophile is activated, and one prochiral face selected, by complexation of the electron-withdrawing group (EWG; EDG indicates electron-donating group) with a Lewis acid (L. A.) possessing chiral and nonracemic ligands.
Scheme 1.
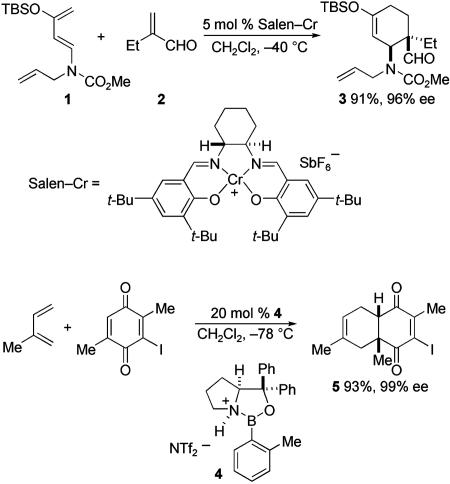
Numerous chiral Lewis acids have been used to catalyze Diels–Alder reactions (6–9). Although many reactions of this type employ cyclopentadiene as the diene and 2-alkyl acrolein derivatives as the dienophile, several examples involve more intricate dienes or dienophiles. For example, a salen–Cr-catalyzed type I Diels–Alder reaction of siloxydiene carbamate 1 and 2-ethylpropenal (2) to form 3 was the key step in a recent total synthesis of (+)-aspidospermidine (Scheme 2; TBS, tert-butyldimethylsilyl) (10). Other examples of noteworthy enantio- and regioselectivity are found in oxazaborolidine-catalyzed cycloadditions of acyclic dienes with substituted quinones, such as the synthesis of dienedione 5 by using the Lewis acid catalyst 4 (ref. 11; Tf, trifluoromethanesulfonyl). Sm and Gd complexes of chiral pyridyl-bis(oxazoline) ligands have also been used with much success in asymmetric Diels–Alder reactions employing 2-acylquinones (12).
Scheme 2.
In a less developed strategy, the geminal substituents are incorporated in the diene rather than the dienophile (type II). To our knowledge, this strategy has been implemented only in inverse electron-demand Diels–Alder reactions. For example, high enantioselectivity has been reported for the cycloaddition of 3-carboxymethyl-2-pyrone with thiophenylethylene catalyzed by a 2,2′-dihydroxy-1,1′-binaphthyl (BINOL)–Yb complex (13). This catalytic approach to all-carbon quaternary stereocenters remains largely undeveloped.
Combination of Chiral Carbon Nucleophiles with Carbon Electrophiles
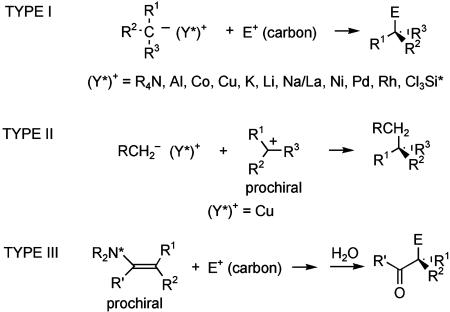
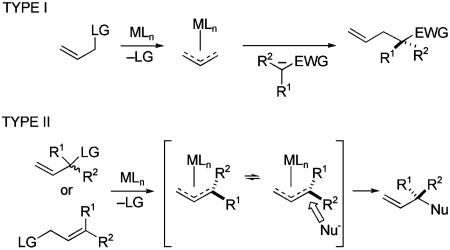
The C—C bond-forming step of three approaches for forming quaternary stereocenters by the union of catalytically generated chiral carbon nucleophiles with carbon electrophiles are depicted in Scheme 3. In reactions of type I, a carbon with three disparate carbon substituents is activated for bond formation by conversion to a carbanion or organometallic intermediate; diastereoselection in the catalytic asymmetric step arises when the counter ions, or ligands on the metal, are chiral and nonracemic. As summarized in Scheme 3, various metals and counter-ions have been used in these reactions.
Scheme 3.
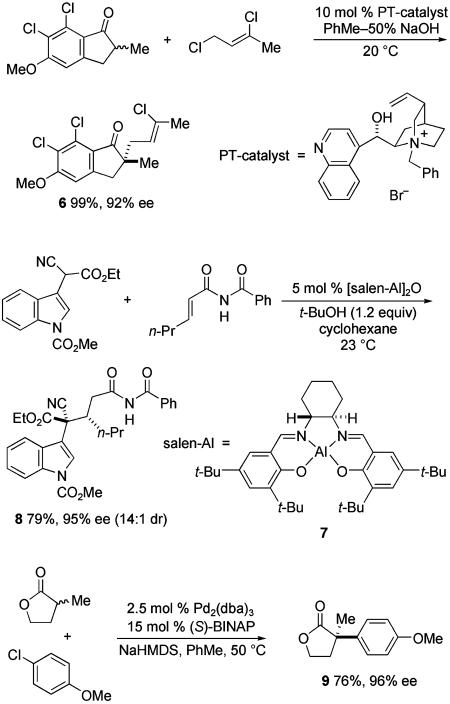
Many useful catalytic asymmetric phase-transfer alkylation reactions have been developed where the chiral counter-cation (Y*)+ is a quaternary ammonium salt (14, 15). The synthesis of 6 is exemplary (Scheme 4) (16). In most transformations of this type disclosed to date, two activating groups flank the acidic carbon; however, this example shows that a single carbonyl group can suffice. The chiral counter-cation also can be a complex containing an electropositive metal, although useful enantioselection in forming quaternary stereocenters has been achieved only rarely (17). High enantioselectivity was reported recently for the reaction of cyanoacetates with α,β-unsaturated imides catalyzed by salen–Al complex 7, as illustrated in the synthesis of indole derivative 8 (18). Palladium- and nickel-catalyzed arylation/vinylation reactions of carbonyl compounds and related species have been shown to have fairly broad scope (19, 20). These processes have now been documented for anions generated from several carbon acids: lactones [e.g., synthesis of 9; dba, dibenzylideneacetone; BINAP, 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl; HMDS, hexamethyldisilazane], ketones, and oxindoles (19).
Scheme 4.
Much less developed is the related strategy in which the electrophile is prochiral (Scheme 3, type II). For example, notably absent are useful procedures involving conjugate addition to α,β-unsaturated carbonyl compounds (or related electrophiles) in which the β carbon is disubstituted (21). Copper-catalyzed SN2′ displacement of prochiral allylic phosphates with achiral organozinc nucleophiles is one example of a useful type II process (22). The stoichiometric reaction of enamines derived from chiral amines with carbon electrophiles has notable utility for preparing all-carbon quaternary centers (cf. ref. 23). Nonetheless, few catalytic reactions of type III have been reported (24) and none of the related reaction in which R1 and R2 are hydrogen and the carbon electrophile is prochiral.
Reactions of Chiral Allylmetal Electrophiles with Carbon Nucleophiles
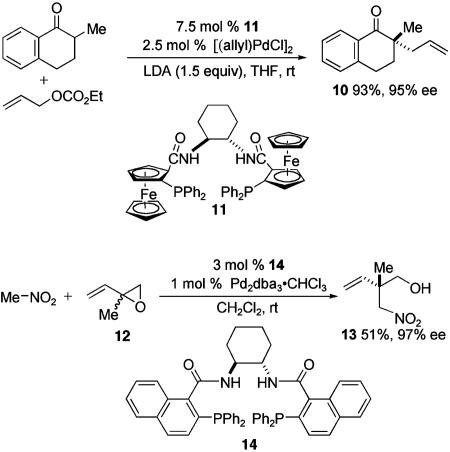
Two approaches for generating all-carbon quaternary stereocenters by reaction of catalytically generated allylmetal complexes with carbon nucleophiles are shown in Scheme 5 (25–27). In reactions of type I, a carbon nucleophile containing three diversified carbon substituents is alkylated by an allylmetal intermediate in which the ancillary ligands (L) on the metal (M) are chiral and nonracemic. Many type I reactions have been developed wherein the ligands are chiral bidentate phosphines; an example is synthesis of tetralin 10 catalyzed by the Pd complex of diphosphine 11 (Scheme 6; LDA, lithium diisopropylamide; rt, room temperature) (28). Although most examples of reactions of this type employ nucleophiles having two electron-withdrawing groups attached to the acidic carbon, the synthesis of 10 demonstrates that one carbonyl group can be sufficient. This example also illustrates a current limitation in alkylations of this type: the carbon nucleophile must possess only a single acidic α-hydrogen to prevent reaction at other acidic sites.
Scheme 5.
Scheme 6.
In reactions of type II, diastereomeric chiral and nonracemic allylmetal intermediates containing two different substituents at one allyl terminus are generated from either an achiral or a racemic chiral allylic precursor. Although two diastereomeric allylmetal intermediates can be formed, enantioselection is achieved by preferential reaction of one of these intermediates at its disubstituted terminus. In an example of dynamic kinetic resolution of a racemic precursor, high enantioselection is obtained in joining isoprene oxide (12) with nitromethane to form nitroalkene 13 by using diphosphine 14 (Scheme 6) (29). Other nucleophiles (Nu) such as carbonyl compounds containing two activating groups have been used in similar reactions; however, little is known regarding the types of allylic leaving groups (LGs) that can be used. Type II reactions of allyl metal intermediates have received less attention than type I reactions for the synthesis of all-carbon quaternary stereocenters.
Intramolecular Heck Reactions
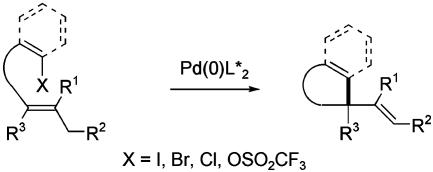
Intramolecular Pd(0)-catalyzed coupling of an aryl or vinyl halide [or triflate (Tf)] with a tethered alkene containing an additional substituent on the proximal alkene terminus is a broadly applicable method for simultaneously forming rings and all-carbon quaternary stereocenters (Scheme 7). Since the first reports of the catalytic asymmetric variant of this chemistry in 1989 (30, 31), this method has been used widely for the asymmetric construction of quaternary stereocenters. Numerous examples of fashioning five- and six-membered rings in this way have been documented (32–34). No other method for catalytic asymmetric synthesis of all-carbon quaternary stereocenters has been verified with as broad a range of substrates.
Scheme 7.
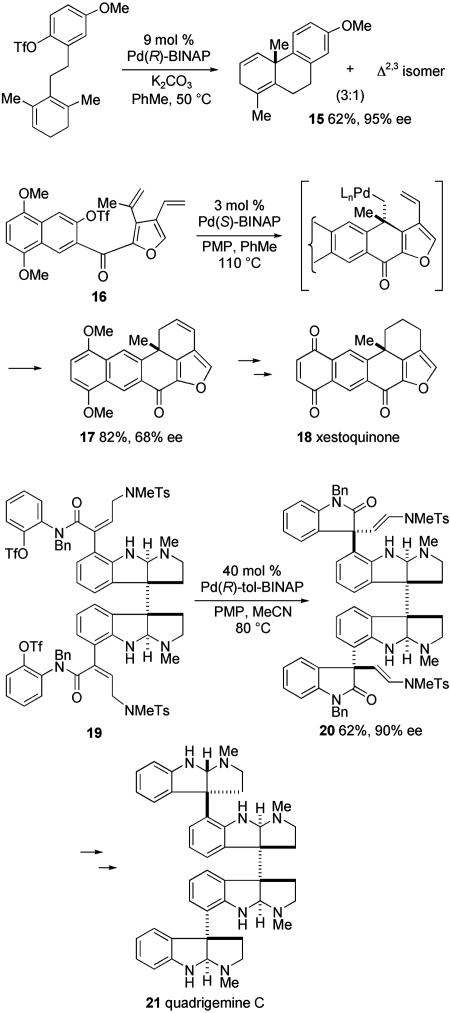
Several illustrative applications of the use of catalytic asymmetric intramolecular Heck reactions in the synthesis of complex polycyclic molecules containing all-carbon quaternary stereocenters are shown in Scheme 8; PMP, 1,2,2,6,6-pentamethylpiperidine; Bn, benzyl. The preparation of hydrophenanthrene 15 illustrates an asymmetric 6-exo Heck cyclization (35). The use of sequenced 6-exo and 6-endo Heck cyclizations is exemplified in the formation of oxapentacyclic ketone 17 from tricyclic triflate 16 (36). In this example, catalyst control in the initial 6-exo cyclization delivers 17 in moderate enantiopurity. Two additional steps convert 17 to the anthraquinone antibiotic xestoquinone (18). The use of catalytic asymmetric Heck cyclizations to desymmetrize a structurally elaborate meso intermediate is illustrated by double Heck cyclization of octacyclic ditriflate 19 to provide the C1-symmetric bisoxindole 20 in 62% yield and 90% ee (37). This transformation highlights the structural complexity that can be tolerated, as cyclization substrate 19 possesses both secondary and tertiary amine functionalities as well as a labile σ-bond linking its contiguous benzylic quaternary centers. However, the presence of the amines is likely responsible for the need for high catalyst loadings to achieve useful cyclization rates. The first total synthesis of the dodecacyclic polyindoline alkaloid quadrigemine C (21) was realized in two additional steps from Heck product 20 (37).
Scheme 8.
Combination of Chiral Carbon Electrophiles with Carbon Nucleophiles
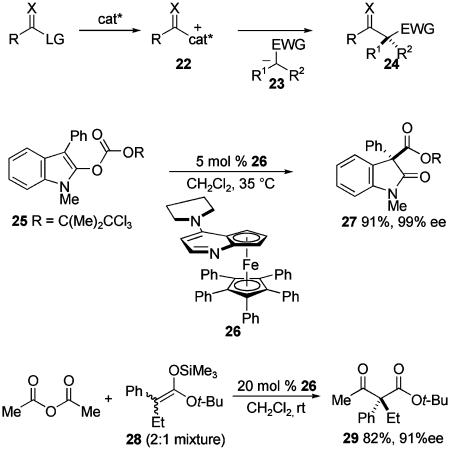
Asymmetric nucleophilic catalysts can be used to generate chiral electrophiles. The union of these with prochiral carbon nucleophiles represents a promising catalytic approach to the synthesis of all-carbon quaternary stereocenters (Scheme 9). In reactions of this type, a chiral electrophile 22 is generated and then alkylated in a C—C bond-forming event with a nucleophile 23 containing three disparate carbon substituents to form quaternary carbon stereocenters as exemplified in 24.
Scheme 9.
Although many different types of catalytically generated chiral electrophiles are possible, acyl electrophiles have received the most attention to date (X = O, Scheme 9). Several catalytic acyltransfer reactions have been developed wherein the chiral nucleophilic catalyst is a nonracemic amine nucleophile (38, 39). For example, oxindole 27 was formed in high enantiopurity from 2-acyloxindole precursor 25 by using planar chiral 4-aminopyridine catalyst 26 (40). The related reaction of silylketene acetal 28 with acetic anhydride, catalyzed by 26, is reported to give β-ketoester 29 in good yield and enantiopurity (41). The use of chiral phosphines as nucleophilic catalysts in similar reactions to generate all-carbon quaternary stereocenters has been less successful to date (42, 43).
Cyclopropanations
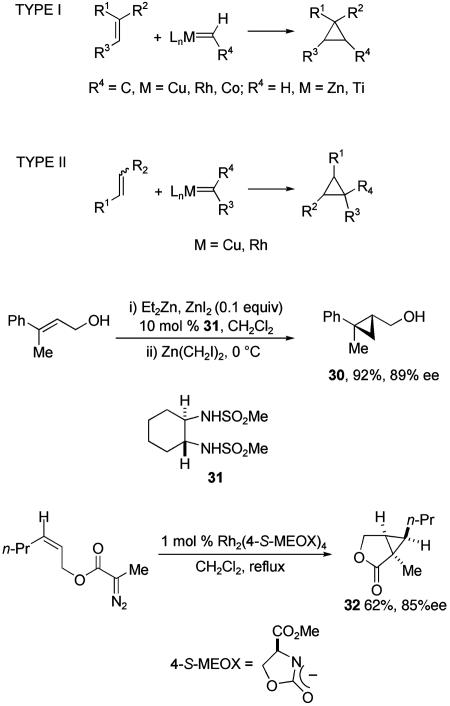
Cyclopropanation of alkenes offers two distinct approaches for the synthesis of all-carbon quaternary stereocenters (Scheme 10) (44). In one approach (type I), a methylene unit is added to a prochiral alkene containing two different geminal substituents. Alternatively, all-carbon stereocenters can arise from addition of a carbenoid containing two different carbon substituents to any prochiral alkene (type II). Diastereoselectivity in the transition state of both processes is achieved by employing a metal carbenoid (Cu, Rh, or Co) possessing chiral nonracemic ligands coordinated to the metal center.
Scheme 10.
Both of these approaches have been used successfully. High enantioselectivity in type I processes has been achieved with alkenes containing Lewis basic allylic substituents and carbenoids derived from diiodomethane (45). The synthesis of cyclopropyl carbinol 30 by methylene transfer from a zinc carbenoid containing the nonracemic disulfonamide ligand 31 illustrates such a process (46). In type II processes, diazo compounds are commonly used (47–49). High levels of enantioselectivity and high diastereoselectivity are often observed in intramolecular reactions, but rarely if the alkene is not tethered to the diazo precursor. Rhodium-catalyzed synthesis of bicyclic lactone 32 by using the 4-(S)-MEOX ligand illustrates the high enantioselectivity and diastereoselectivity that can be achieved in such intramolecular reactions (50).
Metal-Catalyzed Cyclizations
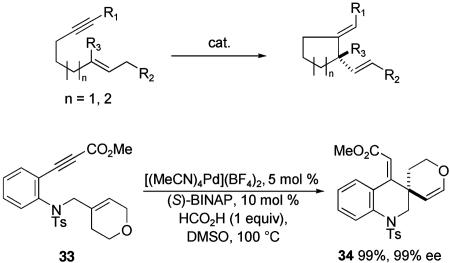
Metal-catalyzed cyclizations of enynes or dienes provide another approach for catalytic asymmetric synthesis of all-carbon quaternary stereocenters (51). In reactions of these types, an alkene that is either 1,1-disubstituted or trisubstituted undergoes metal-catalyzed cyclization with a tethered alkyne or alkene when a metal catalyst having chiral nonracemic ligands is used. In these reactions, the catalyst is regenerated by further transformation of a metal-containing intermediate. Many reaction types can be classified in this general category; one of these, metal-catalyzed ene cyclization, is depicted in Scheme 11.
Scheme 11.
Several palladium-catalyzed enyne cyclizations using BINAP as a chiral ligand have been reported to proceed with high enantioselection to form five- and six-membered rings (52, 53). In cases where double bond migration of the first formed product is possible, it is observed to some degree; typically a blocking group such as a heteroatom is required in the substrate to prevent alkene migration. The cyclization of ynoate 33 to form the spirocyclic product 34 is representative of this strategy. Although catalytic asymmetric procedures for Pauson–Khand cyclizations (54, 55) as well as asymmetric zirconium-catalyzed diene cyclizations (56, 57) have been reported, these potentially powerful methods have rarely proven useful for the catalytic asymmetric synthesis of quaternary carbon stereocenters.
Desymmetrization of Molecules Having Prochiral Quaternary Centers
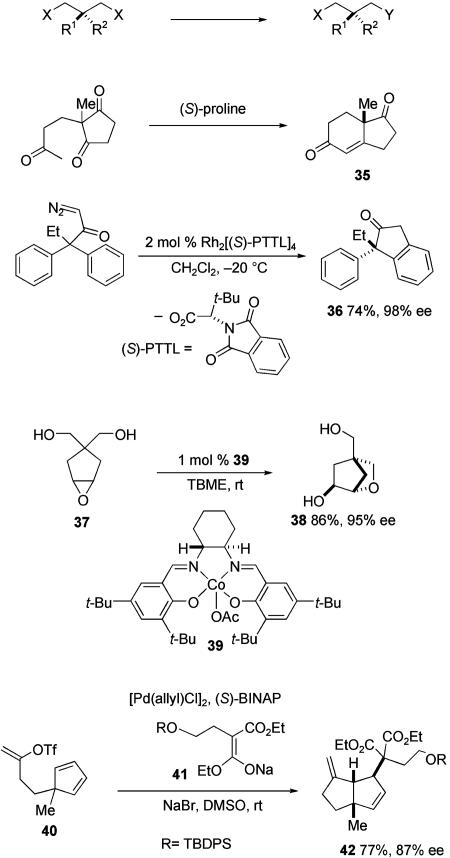
Desymmetrization of a prochiral intermediate containing a quaternary carbon is a less general, but extremely useful approach for synthesis of all-carbon quaternary stereocenters (Scheme 12; TBME, tert-butyl methyl ether; TBDPS, tert-butyldiphenylsilyl) (58). Although theoretically any catalytic asymmetric reaction could be used in the synthesis of quaternary centers by using this strategy, several reactions have been recognized as particularly useful. An outstanding early example is the proline-catalyzed intramolecular aldol reaction to form the Hajos–Parrish ketone 35 (59). Catalytic asymmetric C—H bond insertion reactions have also found notable use in the formation of quaternary carbon stereocenters by desymmetrization, as exemplified in the synthesis of tetralone 36 (60, 61). An additional useful method is illustrated by the formation of oxabicycloheptane 38 by intramolecular opening of prochiral epoxide 37 catalyzed by salen-Co complex 39 (62). Intramolecular Heck reactions have proven to be particularly useful in this approach to all-carbon quaternary centers (32, 33). An impressive example is found in a total synthesis of (–)-capenellene; the synthesis of bicyclooctene 42 was achieved by catalytic asymmetric Heck cyclization of 40 followed by capture of the resulting η3-allylpalladium intermediate with malonate anion 41 (63).
Scheme 12.
Conclusions and Future Directions
After initially lagging far behind developments in catalytic asymmetric reduction and oxidation, a number of useful methods for the catalytic asymmetric formation of carbon–carbon bonds have been developed during the last decade. Nonetheless, at present, only a few of these reactions have been shown (typically within the last 2–3 years) to be useful for constructing all-carbon quaternary stereocenters. Even in the most developed of these methods, Diels–Alder reactions, coupling reactions of allylmetal intermediates, and intramolecular Heck reactions, the demonstrated scope is quite limited. For example, practical catalytic asymmetric Diels–Alder reactions of α,β-unsaturated ketones, esters, or nitriles have yet to be developed. Similarly, of the three described methods for forming all-carbon quaternary stereocenters from catalytically generated chiral nucleophiles, only the type I process (Scheme 3) has been developed extensively. Likewise, catalytic asymmetric formation of quaternary stereocenters by using allylmetal electrophiles is currently documented only for η3-allylpalladium species. Even for catalytic asymmetric Heck reactions, which have shown the broadest scope to date, structural features required to achieve high ee are poorly understood.
There are bountiful opportunities for further discoveries. Large gaps in the known methods, some of which are noted in this brief survey, remain to be filled. In addition, many catalytic asymmetric C—C bond-forming reactions have yet to be used to forge quaternary carbons. For example, practical examples of forming quaternary carbons by catalytic asymmetric hydrocarbonylation, hydrocyanation, or hydrovinylation of carbon-carbon double bonds have yet to be described. Likewise, Heck and other carbometallation reactions have not yet been engineered to form all-carbon quaternary stereocenters in bimolecular reactions. Moreover, as only a few metals other than palladium have been used in catalytic asymmetric reactions to form all-carbon stereocenters, the reactivity of many additional metals remains to be exploited. Also certain to play a role in future developments in this area are organocatalytic reactions in which catalytically generated chiral nonracemic iminium ion and enamine intermediates are used to construct stereogenic quaternary carbons (38, 64).
References
- 1.Christoffers, J. & Mann, A. (2001) Angew. Chem. Int. Ed. 40, 4591–4597. [DOI] [PubMed] [Google Scholar]
- 2.Corey, E. J. & Guzman-Perez, A. (1998) Angew. Chem. Int. Ed. 37, 388–401. [DOI] [PubMed] [Google Scholar]
- 3.Fuji, K. (1993) Chem. Rev. 93, 2037–2066. [Google Scholar]
- 4.Martin, S. F. (1980) Tetrahedron 36, 419–460. [Google Scholar]
- 5.Jacobsen, E. N., Pfaltz, A. & Yamamoto, H., eds. (1999) Comprehensive Asymmetric Catalysis (Springer, Berlin), Vols. 1–3.
- 6.Hayashi, Y. (2002) in Cycloaddition Reactions in Organic Synthesis, eds. Kobayashi, S. & Jorgensen, K. A. (Wiley-VCH, New York), pp. 5–56.
- 7.Maruoka, K. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley-VCH, New York), 2nd Ed., pp. 467–491.
- 8.Corey, E. J. (2002) Angew. Chem. Int. Ed. 41, 1650–1667. [DOI] [PubMed] [Google Scholar]
- 9.Evans, D. A. & Johnson, J. S. (1999) in Comprehensive Asymmetric Catalysis, eds. Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (Springer, Berlin), Vol. 3, pp. 1177–1235. [Google Scholar]
- 10.Kozmin, S. A., Iwama, T., Huang, Y. & Rawal, V. H. (2002) J. Am. Chem. Soc. 124, 4628–4641. [DOI] [PubMed] [Google Scholar]
- 11.Ryu, D. U. & Corey, E. J. (2003) J. Am. Chem. Soc. 125, 6388–6390. [DOI] [PubMed] [Google Scholar]
- 12.Evans, D. A. & Wu, J. (2003) J. Am. Chem. Soc. 125, 10162–10163. [DOI] [PubMed] [Google Scholar]
- 13.Markó, I. E. & Evans, G. R. (1994) Tetrahedron Lett. 35, 2771–2774. [Google Scholar]
- 14.O'Donnell, M. J. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley-VCH, New York), 2nd Ed., pp. 727–755.
- 15.Nelson, A. (1999) Angew. Chem. Int. Ed. 38, 1583–1585. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharya, A., Dolling, U.-H., Grabowski, E. J. J., Karady, S., Ryan, K. M. & Weinstock, L. (1986) Angew. Chem. Int. Ed. Engl. 25, 476–477. [Google Scholar]
- 17.Shibasaki, M. & Yoshikawa, N. (2002) Chem. Rev. 102, 2187–2209. [DOI] [PubMed] [Google Scholar]
- 18.Taylor, M. S. & Jacobsen, E. N. (2003) J. Am. Chem. Soc. 125, 11204–11205. [DOI] [PubMed] [Google Scholar]
- 19.Culkin, D. A. & Hartwig, J. F. (2003) Acc. Chem. Res. 36, 234–245. [DOI] [PubMed] [Google Scholar]
- 20.Spielvogel, D. J. & Buchwald, S. L. (2002) J. Am. Chem. Soc. 124, 3500–3501. [DOI] [PubMed] [Google Scholar]
- 21.Krause, N. & Hoffmann-Roeder, A. (2001) Synthesis 171–196.
- 22.Luchaco-Cullis, C. A., Mizutani, H., Murphy, K. E. & Hoveyda, A. H. (2001) Angew. Chem. Int. Ed. 40, 1456–1460. [DOI] [PubMed] [Google Scholar]
- 23.d'Angelo, J., Desmaele, D., Dumas, F. & Guingant, A. (1992) Tetrahedron: Asymmetry 3, 459–505. [Google Scholar]
- 24.Hodous, B. L. & Fu, G. C. (2002) J. Am. Chem. Soc. 124, 1578–1579. [DOI] [PubMed] [Google Scholar]
- 25.Trost, B. M. & Crawley, M. L. (2003) Chem. Rev. 103, 2921–2943. [DOI] [PubMed] [Google Scholar]
- 26.Lee, C. & Trost, B. M. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley-VCH, New York), 2nd Ed., pp. 593–649.
- 27.Trost, B. M. & Van Vranken, D. L. (1996) Chem. Rev. 96, 395–422. [DOI] [PubMed] [Google Scholar]
- 28.You, S. L., Hou, X. L., Dai, L. X. & Zhu, X. Z. (2001) Org. Lett. 3, 149–151. [DOI] [PubMed] [Google Scholar]
- 29.Trost, B. M. & Jiang, C. (2001) J. Am. Chem. Soc. 123, 12907–12908. [DOI] [PubMed] [Google Scholar]
- 30.Sato, Y., Sodeoka, M. & Shibasaki, M. (1989) J. Org. Chem. 54, 4738–4739. [Google Scholar]
- 31.Carpenter, N. E., Kucera, D. J. & Overman, L. E. (1989) J. Org. Chem. 54, 5845–5848. [Google Scholar]
- 32.Dounay, A. B. & Overman, L. E. (2003) Chem. Rev. 103, 2945–2963. [DOI] [PubMed] [Google Scholar]
- 33.Shibasaki, M. & Vogl, E. M. (1999) J. Organomet. Chem. 576, 1–15. [Google Scholar]
- 34.Donde, Y. & Overman, L. E. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley-VCH, New York), 2nd Ed., pp. 675–697.
- 35.Kondo, K., Sodeoka, M. & Shibasaki, M. (1995) J. Org. Chem. 60, 4322–4323. [Google Scholar]
- 36.Maddaford, S. P., Andersen, N. G., Cristofoli, W. A. & Keay, B. A. (1996) J. Am. Chem. Soc. 118, 10766–10773. [Google Scholar]
- 37.Lebsack, A. D., Link, J. T., Overman, L. E. & Stearns, B. A. (2002) J. Am. Chem. Soc. 124, 9008–9010. [DOI] [PubMed] [Google Scholar]
- 38.France, S., Guerin, D. J., Miller, S. J. & Lectka, T. (2003) Chem. Rev. 103, 2985–3012. [DOI] [PubMed] [Google Scholar]
- 39.Shaw, S. A., Aleman, P. & Vedejs, E. (2003) J. Am. Chem. Soc. 125, 13368–13369. [DOI] [PubMed] [Google Scholar]
- 40.Hills, I. D. & Fu, G. C. (2003) Angew. Chem. Int. Ed. 42, 3921–3924. [DOI] [PubMed] [Google Scholar]
- 41.Mermerian, A. H. & Fu, G. C. (2003) J. Am. Chem. Soc. 125, 4050–4051. [DOI] [PubMed] [Google Scholar]
- 42.Chen, Z., Zhu, G., Jiang, Q., Xiao, D., Cao, P. & Zhang, X. (1998) J. Org. Chem. 63, 5631–5635. [Google Scholar]
- 43.Buono, G., Chiodi, O. & Wills, M. (1999) Synlett, 377–388.
- 44.Forbes, D. C. & McMills, M. C. (2001) Curr. Org. Chem. 5, 1091–1105. [Google Scholar]
- 45.Charette, A. B. & Lebel, H. (1999) in Comprehensive Asymmetric Catalysis, eds. Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (Springer, Berlin), Vol. 2, pp. 581–603. [Google Scholar]
- 46.Denmark, S. E. & O'Connor, S. P. (1997) J. Org. Chem. 62, 584–594. [DOI] [PubMed] [Google Scholar]
- 47.Doyle, M. P. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley-VCH, New York), 2nd Ed., pp. 191–228.
- 48.Pfaltz, A. (1999) in Comprehensive Asymmetric Catalysis, eds. Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (Springer, Berlin), Vol. 2, pp. 513–538. [Google Scholar]
- 49.Lydon, K. M. & McKervey, M. A. (1999) in Comprehensive Asymmetric Catalysis, eds. Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (Springer, Berlin), Vol. 2, pp. 539–580. [Google Scholar]
- 50.Doyle, M. P. & Zhou, Q. L. (1995) Tetrahedron: Asymmetry 6, 2157–2160. [Google Scholar]
- 51.Negishi, E. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley-VCH, New York), 2nd Ed., pp. 165–189.
- 52.Hatano, M., Terada, M. & Mikami, K. (2001) Angew. Chem. Int. Ed. 40, 249–253. [PubMed] [Google Scholar]
- 53.Hatano, M. & Koichi, M. (2003) J. Am. Chem. Soc. 125, 4704–4705. [DOI] [PubMed] [Google Scholar]
- 54.Gibson, S. E. & Stevenazzi, A. (2003) Angew. Chem. Int. Ed. 42, 1800–1810. [DOI] [PubMed] [Google Scholar]
- 55.Hicks, F. A. & Buchwald, S. L. (1996) J. Am. Chem. Soc. 118, 11688–11689. [Google Scholar]
- 56.Degrado, S. J., Adams, J. A. & Hoveyda, A. H. (2003) Inorg. Chim. Acta 345, 261–267. [Google Scholar]
- 57.Yamaura, Y., Hyakutake, M. & Mori, M. (1997) J. Am. Chem. Soc. 119, 7615–7616. [Google Scholar]
- 58.Willis, M. C. (1999) J. Chem. Soc. Perkin Trans. 1, 1765–1784.
- 59.Hajos, Z. G. & Parrish, D. R. (1974) J. Org. Chem. 39, 1615–1621. [Google Scholar]
- 60.Davies, H. M. L. & Beckwith, R. E. J. (2003) Chem. Rev. 103, 2861–2903. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe, N., Ogawa, T., Ohtake, Y., Ikegami, S. & Hashimoto, S. (1996) Synlett, 85–86.
- 62.Wu, M. H., Hansen, K. B. & Jacobsen, E. N. (1999) Angew. Chem. Int. Ed. 38, 2012–2014. [DOI] [PubMed] [Google Scholar]
- 63.Ohshima, T., Kagechika, K., Adachi, M., Sodeoka, M. & Shibasaki, M. (1996) J. Am. Chem. Soc. 118, 7108–7116. [Google Scholar]
- 64.Dalko, P. I. & Moisan, L. (2001) Angew. Chem. Int. Ed. 40, 3726–3748. [DOI] [PubMed] [Google Scholar]