Abstract
This article describes three distinct strategies by which stereochemically complex molecules are synthesized and the ways asymmetric catalysis can impact on all three. The development of general methods to prepare synthetically useful building blocks leads to an expanded “chiral pool” of potential starting materials for asymmetric synthesis. The possibility of discovering new reactions to access new types of building blocks is particularly attractive and serves to help define the frontiers of the field. Asymmetric catalysis can also be applied to diastereoselective synthesis such that the stereochemistry of the catalyst, and not that of the substrate, determines the relative configuration of the product. Finally, in reactions where multiple stereocenters are generated simultaneously or in tandem, catalyst and substrate control can operate in a complementary manner to achieve one of many possible stereochemical outcomes selectively.
The synthesis of both natural and unnatural organic compounds in optically active form is a central challenge in chemistry, especially in relation to the study of biologically active compounds. Natural products and synthetic pharmaceutical agents display a rich diversity of molecular structures that range from very simple to astonishingly intricate. A feature common to many such compounds is the presence of multiple stereocenters, giving rise to the possibility of large numbers of stereoisomers (2n, where n is the number of stereocenters). The control of the relationship between stereocenters is therefore an essential element to the synthesis of many complex targets of interest.
There are several ways to generate stereochemical complexity in a selective manner (Fig. 1). Chiral building block assembly, for which peptide synthesis represents the prototype, relies on the availability of the appropriate optically pure starting materials along with the reactions to elaborate and couple them. An alternative is diastereoselective synthesis, wherein new stereocenters are introduced into compounds bearing preexisting ones. The selectivity may arise either from the properties inherent to the substrate (substrate control) or from an external agent (reagent or catalyst control). A third strategy involves the use of transformations that simultaneously introduce multiple stereocenters in a single operation. For example, the coupling of two prochiral substrates may result in four (or more) stereochemical outcomes; enantio- and diastereoselective variants of such processes usually incorporate elements of catalyst and substrate control. Other methods for rapidly achieving stereochemical complexity under catalyst control are possible, including the desymmetrization of meso compounds and stereospecific reactions of geometrically defined precursors.
Fig. 1.
Strategies for the construction of stereochemically complex targets. Asterisks denote the presence of defined stereocenters.
Before the advent of efficient asymmetric synthetic methods, application of the chiral building block approach was constrained by the very limited pool of optically active compounds provided by nature in abundance (the “chiral pool”) (1). Because of this constraint, strategies in target-oriented synthesis were largely premised on the fact that optically active starting materials were particularly precious, and the application of substrate-controlled diastereoselective processes was emphasized. Although this now-classic approach has proven enormously powerful and will surely always play a critical role in complex molecule construction, it is also inherently limited because it requires a specific design concept for every new target (including diastereomers of the same target).
With the discovery of the first general asymmetric synthetic reactions in the 1980s, the way that stereochemically complex targets could be accessed was expanded in two crucial ways. Asymmetric synthesis began to provide access to a greatly expanded pool of readily available chiral starting materials (2), and synthetic chemists could now devise strategies to prepare target structures based on chiral starting materials unavailable from nature. In addition, “powerful” chiral reagents and catalysts were identified that can induce highly diastereoselective reactions through external stereocontrol, overcoming or complementing the inherent substrate bias (3).
Masamune and Sharpless provided a seminal and brilliant illustration of the potential of catalyst-induced double stereodifferentiation in natural product synthesis in their stereocontrolled synthesis of the l-hexoses. The then-recently developed titanium tartrate (Sharpless) epoxidation catalyst was used to generate the key chiral starting material and was subsequently applied in the diastereoselective elaboration of a late-stage intermediate (Scheme 1) (4). Ultimately, all four contiguous stereocenters were controlled independently, providing access to each of the eight hexose diastereomers by essentially the same route.
Scheme 1.
Masamune–Sharpless synthesis of the l-hexoses. Asterisks denote stereocenters under absolute control.
The strategy underlying the synthesis of the hexoses is so compelling that one might ask why asymmetric catalysis has not become the approach by which all complex target synthesis is undertaken. One answer is that certain targets are best accessed from nature's chiral pool or are well suited to substrate-controlled diastereoselective synthesis strategies, and asymmetric catalytic methods do not offer any advantage. However, another reason is that the hexose synthesis relied on a reaction that was far ahead of its time, and that there are still too few reactions as effective and as general as the Sharpless epoxidation. As a result, it has not been possible to devise attractive synthetic routes to target structures based on available methods.
The latter consideration has motivated several research groups throughout the world to overcome this limitation, by expanding the availability of powerful asymmetric catalytic reactions. The fruit of this effort has been the recent discovery of a variety of remarkably general catalytic oxidation, reduction, and C–C bond-forming reactions (5). The impact of these methods on the manner in which complex targets can be synthesized is just beginning to be felt (6) and is discussed here in several representative examples.
An Expanded Chiral Pool
The chiral pool approach to synthesis of optically active targets can be extremely attractive if nature happens to provide an abundant supply of a starting material appropriate for the synthetic target. Accordingly, considerable effort and creativity has been directed toward using inexpensive chiral pool elements in target-oriented synthesis. In the best cases, some of the most practical and brilliant syntheses ever devised have used this approach. The Stork syntheses of prostaglandin A2 and F2α are classic examples (7, 8). However, nature provides only a limited number of chiral starting materials, and usually only one enantiomer is produced naturally. In many cases, this has forced synthetic chemists to develop lengthy, inefficient syntheses to make use of available compounds.
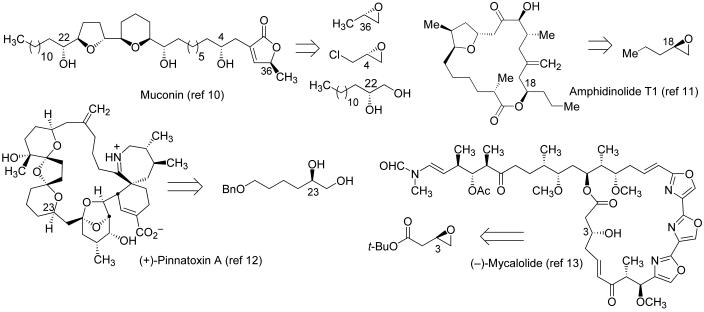
Asymmetric catalysis can provide a powerful solution to this problem by rendering accessible chiral materials not readily provided by nature. In principle, small molecule synthetic catalysts can be applied to the synthesis of building blocks as either enantiomer and with nearly complete generality. For example, whereas nature's chiral pool is not a useful source of optically active terminal epoxides, these very useful compounds are now readily accessible in one step from the corresponding racemates by the (salen)Co-catalyzed hydrolytic kinetic resolution (9). As a result, it is now possible to evaluate complex targets strategically with the knowledge that enantiopure terminal epoxides represent viable starting points. Various groups have incorporated this recently developed transformation into synthetic routes to natural products of varying complexity (Fig. 2) (10–13).
Fig. 2.
Selected applications of the HKR reaction in natural product synthesis.
In many cases, retrosynthetic analysis of a complex target leads to chiral starting materials that are not readily accessible by available methods. In these situations, natural products can provide the inspiration for the discovery of new asymmetric catalytic methods. For example, consideration of synthetic approaches to the structurally interesting antitumor agent FR901464 revealed an intriguing asymmetric catalytic route to the central fragment (Scheme 2). However, asymmetric cycloadditions involving weakly nucleophilic diene partners such as 2 and simple aldehydes were unknown, and as such this target provided the impetus to develop new methodology. In this context, it was discovered that novel (Schiff base)Cr(III) complexes such as 1 are remarkably reactive chiral Lewis acid catalysts, promoting cycloaddition between diene 2 and ynal 3 to establish the complete carbon framework of the central fragment while setting three of the four stereocenters simultaneously (Scheme 3) (14).
Scheme 2.
Synthesis of the central fragment of FR901464.
Scheme 3.
Catalyst-controlled synthesis of iridoid natural products.
Catalyst 1 was also applied successfully to the enantioselective synthesis of the right-hand fragment of FR901464. The total synthesis was completed in a convergent manner by using high yielding Pd-cross coupling and amide bond-forming reactions (15, 16). Subsequently, the hetero-Diels–Alder (HDA) chemistry developed specifically for this synthesis proved applicable to the synthesis of a number of other, structurally distinct natural products (17–21).
Thus, advances in asymmetric catalysis have lifted many of the restrictions on the chiral pool approach to the synthesis of stereochemically complex targets, as synthetic chemists no longer must work only with what nature provides directly. In several cases, there is now general access to entire classes of synthetically useful chiral building blocks. Ultimately, it should be possible to apply existing asymmetric catalytic reactions to afford a practical route to any building block of interest or to devise new processes when the need arises. Although the latter, rather ambitious goal remains far from met, examples such as the HDA reaction developed for the FR901464 synthesis suggest the feasibility of attaining it.
Catalyst-Controlled Stereochemical Elaboration of Chiral Intermediates
The classic approach to generating stereochemical complexity in target-oriented synthesis has involved reliance on the conformational properties of the substrate to direct the diastereoselective generation of new stereocenters (substrate control). To access the specific stereochemical relationship in complex molecules, this has often required extraordinary levels of creativity in synthetic design, and this is the basis for much of the “art” of organic synthesis. Indeed, the history of modern organic synthesis can be tied closely to the question of how stereocontrol has been achieved in complex target synthesis (22, 23). Early successes relied principally on cyclic stereocontrol on conformationally restricted substrates. Woodward's (24–26) work in total synthesis is appreciated widely in this regard, with his synthesis of reserpine being a dramatic, albeit representative example. The development of more sophisticated principles in conformational analysis opened the door to successes in acylic stereocontrol, as demonstrated in Kishi's (27–29) pioneering work in the synthesis of monensin.
The alternative possibility of controlling relative stereochemistry predictably by using an external controlling element (catalyst control) holds significant appeal. The synthetic plan is no longer contingent on the properties of the substrate, but rather on the ability of the catalyst to exert stereoselectivity on a complex substrate. The value of catalyst-controlled transformations is particularly evident when selective access to multiple diastereomers is required in the course of a synthetic effort. Catalyst control introduces the possibility of generating stereoisomeric products by the same route, changing only the enantiomer of catalyst or of substrate to access diastereomeric products selectively. For example, the inverse electron-demand HDA reaction of chiral aldehyde 5 with ethyl vinyl ether was applied toward the preparation of the fused cyclopenta[c]pyran bicyclic ring system characteristic of the iridoid family of natural products (30). The requisite aldehyde 5 was prepared in two steps from citronellal (4), a chiral building block that is produced commercially in either enantiomeric form by a highly efficient catalytic asymmetric isomerization reaction (31). Selective cycloaddition with either enantiomer of 5 was achieved by using chiral chromium catalyst 6. The diastereomeric products were thus generated selectively, and readily elaborated to the natural products boschnialactone and iridomyrmecin.
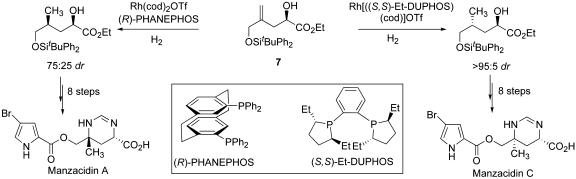
The catalyst-controlled hydrogenation of homoallylic alcohol 7 was a key transformation in the synthesis of the bromopyrrole alkaloids manzacidin A and C (Scheme 4) (32). The requisite building block was prepared by a highly enantioselective catalytic ene reaction. Stereoselective hydrogenation of 7 could not be achieved by using substrate control, but either diastereoisomer could be accessed efficiently by choice of the appropriate chiral rhodium catalyst.
Scheme 4.
Catalyst-controlled syntheses of manzacidin A and C.
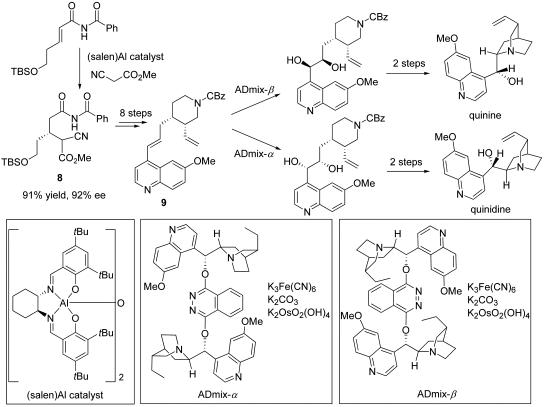
The combined application of asymmetric catalysis to building block preparation and elaboration in the synthesis of diastereomeric natural products is also illustrated in the synthesis of the cinchona alkaloids quinine and quinidine (Scheme 5) (33). Aluminum-catalyzed asymmetric Michael addition methodology provided ready access to enantio-enriched adduct 8, which was converted in several steps to the highly functionalized alkene 9. Asymmetric dihydroxylation using the pseudoenantiomeric catalyst systems ADmix-α and ADmix-β afforded diastereomeric diols 10 and 11 with high selectivity, and these were converted stereospecifically to quinidine and quinine.
Scheme 5.
Asymmetric catalytic syntheses of quinine and quinidine.
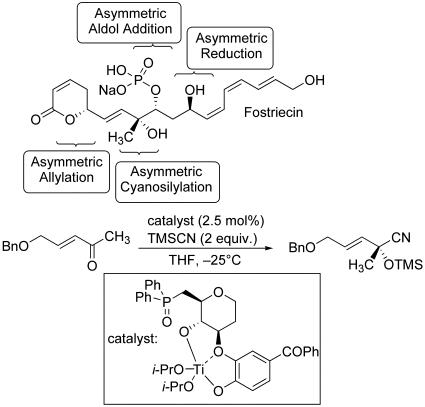
The formal synthesis of fostriecin reported by Shibasaki and coworkers features the use of four catalytic asymmetric processes, of which three are C–C bond-forming reactions, to generate each of the four stereocenters present in this promising anticancer agent (Scheme 6) (34). The configuration of the C8 tertiary alcohol, one of the challenging stereochemical features of the natural product, was established at an early stage by titanium-catalyzed enantioselective ketone cyanation. The resulting chiral building block was elaborated by catalyst-controlled diastereoselective transformations, including a direct aldol fragment coupling catalyzed by a chiral lanthanum complex and a late-stage enantioselective ketone reduction under transfer hydrogenation conditions using Noyori's ruthenium catalyst.
Scheme 6.
Asymmetric catalytic synthesis of fostriecin.
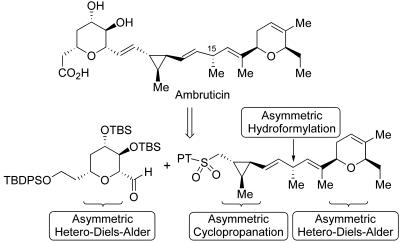
The synthesis of the antifungal agent ambruticin further demonstrates how modern catalyst- and reagent-controlled asymmetric reactions can serve as a guiding strategy in complex target synthesis (Scheme 7) (35). Two new applications of HDA methodology served to establish the left- and right-hand pyran units, and the first application of asymmetric hydroformylation to target-oriented synthesis allowed effective construction of the C15 stereocenter under complete catalyst control. Asymmetric cyclopropanation was applied in a doubly diastereoselective context to introduce the central, trisubstituted cyclopropane ring. The overall sequence was exceptionally efficient (16 steps and 12% yield in the longest linear sequence), and each of the stereochemical challenges was met in a highly selective manner, with recently developed enantioselective C–C bond-forming reactions applied to the direct introduction of 8 of the 10 stereocenters.
Scheme 7.
Retrosynthetic analysis applied to the asymmetric catalytic synthesis of ambruticin.
In general, the implementation of a catalyst-controlled diastereoselective synthesis strategy places the most stringent of demands on the catalytic reaction in question. When elaborate synthetic intermediates are used as substrates, the catalyst must be tolerant of potentially reactive functional groups, and may need to overcome inherent substrate bias to set the required stereochemical relationships. Furthermore, catalyst performance must be highly predictable, because it may be difficult to devise meaningful model systems, and the cost of failure at the late stages of a synthetic effort is high. Nonetheless, the range of reactions that has been applied successfully in catalyst-controlled diastereoselective synthesis is expanding rapidly, as illustrated in the preceding examples.
Setting Multiple Stereocenters Simultaneously
Transformations of simple starting materials to stereochemically complex products lie at the heart of many of the most elegant total syntheses accomplished to date. Asymmetric catalysis may be used to dramatic effect in this context, with both the relative and absolute configuration of multiple stereocenters controlled simultaneously.
The desymmetrization of meso substrates is a powerful approach to the enantioselective synthesis of compounds bearing multiple stereocenters. This strategy forms the basis for a highly efficient approach to the cellular signaling agent myo-insotol-1-phosphate, wherein a simple synthetic pentapeptide catalyzed the phosphorylation of the protected inositol derivative 10, generating a product bearing six stereocenters in one step from an achiral starting material (Scheme 8) (36).
Scheme 8.
Asymmetric catalytic synthesis of myo-inositol-1-phosphate.
The synthesis of the pyrrolidinoindoline alkaloid quadrigemine C illustrates catalyst-controlled desymmetrization of meso compounds in total synthesis in a spectacular way (37). Symmetrical dimeric substrate 11 underwent palladium-catalyzed enantioselective double Heck cyclization, generating a product bearing four carbon-substituted quaternary stereocenters and six of the eight stereocenters present in the natural product (Scheme 9). Compound 11 is one the most complex substrates ever subjected successfully to an enantioselective catalytic reaction, and the tolerance of the palladium catalyst to the numerous functional groups present in this advanced intermediate underlies this success.
Scheme 9.
Asymmetric catalytic synthesis of quadrigemine.
Multiple stereocenters may also be generated by tandem or serial transformations in which a number of bonds are created sequentially in a single operation. The synthesis of the oxasqualenoid glabrescol highlights an innovative implementation of an asymmetric catalytic reaction to generate a highly stereochemically complex structure rapidly from a relatively simple starting material (Scheme 10) (38). Eight of the 10 stereocenters present in the natural product were introduced in a single transformation. Reaction of tetraene 12 with Shi's catalyst led to oxidation of all four double bonds and generation of the indicated tetraepoxide in ≈80% diastereomeric purity. Taking into account that the starting tetraene was enantiomerically pure, each of the four epoxidation events involved a catalyst-controlled diastereoselective process. Seven chemically distinct orderings of the four epoxidations are possible, and it is likely that all sequences occur to some extent given the similar steric and electronic properties of each double bond. Per-epoxidation of tetraene 12 was thus achieved with an estimated selectivity of >20:1 for each reaction. This is a dramatic manifestation of catalyst generality given that the process most likely involved diastereoselective reactions on eight different substrates. The resulting tetraepoxide was elaborated to glabrescol in a short sequence featuring acid-catalyzed cascade cyclization to form four tetrahydrofuran rings in a single step.
Scheme 10.
Asymmetric catalytic synthesis of glabrescol.
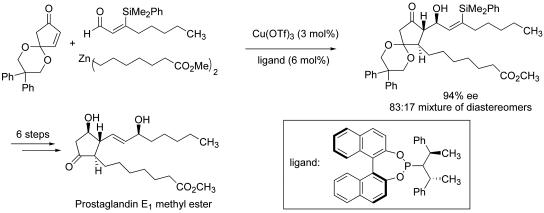
Coupling between prochiral partners can lead to stereochemically complex products in a single reaction, and chiral catalysts introduce the possibility of effecting such transformations enantioselectively. This strategy was used to establish the complete carbon skeleton and three of the four stereocenters of prostaglandin E1, in a copper-catalyzed three-component coupling of a diorganozinc reagent, cyclopenten-3,5-dione monoacetal, and an aldehyde (Scheme 11) (39). The stereochemical outcome of this reaction reflected an interplay of catalyst and substrate control: the chiral copper catalyst dictated the facial selectivity of organozinc conjugate addition to the unsaturated ketone, and the configurations of the two other stereocenters resulted from an intrinsic substrate bias in the subsequent aldol addition.
Scheme 11.
Asymmetric catalytic synthesis of prostaglandin E1 methyl ester.
The combination of substrate and catalyst control also lies at the heart of the selective and simultaneous generation of multiple stereocenters in enantioselective catalytic Diels–Alder reactions. In the synthesis of FR901464 discussed above (Scheme 2), the asymmetric catalytic HDA reaction was used to set three of the four stereocenters of the central ring. This result reflects three different stereochemical control elements: the chiral catalyst (dictating which enantioface of the aldehyde reacts selectively), the stereospecific nature of the cycloaddition reaction (translating the diene configuration to the relative stereochemistry of the two methyl-substituted carbons), and the endo diastereoselectivity (dictating the relative approach of aldehyde to diene).
Whereas the absolute stereochemistry of each of these complexity-generating reactions is under catalyst control, the relative stereochemistry is dictated by the substrate and the reaction mechanism. The successful application of these reactions to natural product targets required that those stereochemical elements controlled by intrinsic reaction bias could be successfully mapped onto the relative stereochemistry present in the natural products. Much greater freedom in choice of synthetic targets would be possible if introduction of all stereochemical elements were under catalyst control, such that any diastereomer could be accessed by choice of catalyst(s). This presents a daunting challenge for reaction development, particularly in cases wherein the intrinsic substrate or reaction bias is high. Devising solutions to this problem will likely require new concepts in catalyst design; independent activation of each reaction partner in cooperative multicatalyst systems represents a particularly intriguing approach (40, 41).
The total syntheses described here provide an indication of how stereochemically complex targets have become more readily accessible with the discovery and reduction to practice of truly general and powerful asymmetric catalytic methods. With continuing progress in catalyst design and development, ultimately one might imagine that practical routes to any complex target of interest may be realized by using selective catalytic reactions to introduce all stereochemical elements independently and efficiently. Although this goal remains quite distant, it is increasingly clear what sorts of advances in asymmetric catalysis will be required to attain it.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: HDA, hetero-Diels–Alder.
References
- 1.Hanessian, S. (1983) in Total Synthesis of Natural Products: The “Chiron” Approach, ed. Baldwin, J. E. (Pergamon, New York).
- 2.Nugent, W. A., RajanBabu, T. V. & Burk, M. J. (1993) Science 259, 479–483. [DOI] [PubMed] [Google Scholar]
- 3.Masamune, S., Choy, W., Petersen, J. S. & Sita, L. R. (1985) Angew. Chem. Int. Ed. Engl. 24, 1–30. [Google Scholar]
- 4.Ko, S. Y., Lee, A. W. M., Masamune, S., Reed, L. A., III, Sharpless, K. B. & Walker, F. J. (1983) Science 220, 949–951. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen, E. N., Pfaltz, A. & Yamamoto, H., eds. (1999) Comprehensive Asymmetric Catalysis (Springer, New York), Vols. 1–3.
- 6.Hoveyda, A. H. (2000) in Stimulating Concepts in Chemistry, eds. Stoddart, F. J., Shibasaki, M. & Vogtle, F. (VCH, Weinheim, Germany), pp. 145–160.
- 7.Stork, G. & Raucher, S. (1976) J. Am. Chem. Soc. 98, 1583–1584. [DOI] [PubMed] [Google Scholar]
- 8.Stork, G., Takahashi, T., Kawamoto, I. & Suzuki, T. (1978) J. Am. Chem. Soc. 100, 8272–8273. [Google Scholar]
- 9.Schaus, S. E., Brandes, B. D., Larrow, J. F., Tokunaga, M., Hansen, K. B., Gould, A. E., Furrow, M. E. & Jacobsen, E. N. (2002) J. Am. Chem. Soc. 124, 1307–1315. [DOI] [PubMed] [Google Scholar]
- 10.Schaus, S. E., Brånalt, J. E. & Jacobsen, E. N. (1998) J. Org. Chem. 63, 4876–4877. [DOI] [PubMed] [Google Scholar]
- 11.Colby, E. A., O'Brien, K. C. & Jamison, T. F. (2004) J. Am. Chem. Soc. 126, 998–999. [DOI] [PubMed] [Google Scholar]
- 12.Nagasawa, K. (2000) J. Synth. Org. Chem. Jpn. 58, 877–886. [Google Scholar]
- 13.Panek, J. S. & Liu, P. (2000) J. Am. Chem. Soc. 122, 11090–11097. [Google Scholar]
- 14.Dossetter, A. G., Jamison, T. F. & Jacobsen, E. N. (1999) Angew. Chem. Int. Ed. Engl. 38, 2398–2400. [DOI] [PubMed] [Google Scholar]
- 15.Thompson, C. F., Jamison, T. F. & Jacobsen, E. N. (2000) J. Am. Chem. Soc. 122, 10482–10483. [Google Scholar]
- 16.Thompson, C. F., Jamison, T. F. & Jacobsen, E. N. (2001) J. Am. Chem. Soc. 123, 9974–9983. [DOI] [PubMed] [Google Scholar]
- 17.Chavez, D. E. & Jacobsen, E. N. (2001) Angew. Chem. Int. Ed. Engl. 40, 3667–3670. [DOI] [PubMed] [Google Scholar]
- 18.Paterson, I., De Savi, C. & Tudge, M. (2001) Org. Lett. 3, 3149–3152. [DOI] [PubMed] [Google Scholar]
- 19.Wender, P. A., Baryza, J. L., Bennett, C. E., Bi, C., Brenner, S. E., Clarke, M. O., Horan, J. C., Kan, C., Lacôte, E., Lippa, B., et al. (2002) J. Am. Chem. Soc. 124, 13648–13649. [DOI] [PubMed] [Google Scholar]
- 20.Paterson, I. & Tudge, M. (2003) Angew. Chem. Int. Ed. Engl. 42, 343–347. [DOI] [PubMed] [Google Scholar]
- 21.Paterson, I. & Luckhurst, C. A. (2003) Tetrahedron Lett. 44, 3749–3754. [Google Scholar]
- 22.Corey, E. J. & Cheng, X.-M. (1995) The Logic of Chemical Synthesis (Wiley, New York).
- 23.Nicolaou, K. C. & Sorensen, E. J. (1996) Classics in Total Synthesis (VCH, New York).
- 24.Woodward, R. B., Bader, F. E., Bickel, H., Frey, A. J. & Kierstead, R. W. (1956) J. Am. Chem. Soc. 78, 2023–2025. [Google Scholar]
- 25.Woodward, R. B., Bader, F. E., Bickel, H., Frey, A. J. & Kierstead, R. W. (1956) J. Am. Chem. Soc. 78, 2657–2857. [Google Scholar]
- 26.Woodward, R. B., Bader, F. E., Bickel, H., Frey, A. J. & Kierstead, R. W. (1958) Tetrahedron 2, 1–57. [Google Scholar]
- 27.Schmid, G., Fukuyama, T., Akasaka, K. & Kishi, Y. (1979) J. Am. Chem. Soc. 101, 259–260. [Google Scholar]
- 28.Fukuyama, T., Wang, C.-L. J. & Kishi, Y. (1979) J. Am. Chem. Soc. 101, 260–262. [Google Scholar]
- 29.Fukuyama, T., Akasaka, K., Karanewsky, D. S., Wang, C.-L. J., Schmid, G. & Kishi, Y. (1979) J. Am. Chem. Soc. 101, 262–264. [Google Scholar]
- 30.Chavez, D. E. & Jacobsen, E. N. (2003) Org. Lett. 5, 2563–2565. [DOI] [PubMed] [Google Scholar]
- 31.Akutagawa, S. (1999) in Comprehensive Asymmetric Catalysis, eds. Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (Springer, New York), Vol. 3, Chap. 41.4.
- 32.Wehn, P. M. & Du Bois, J. (2002) J. Am. Chem. Soc. 124, 12950–12951. [DOI] [PubMed] [Google Scholar]
- 33.Raheem, I. T., Goodman, S. N. & Jacobsen, E. N. (2004) J. Am. Chem. Soc. 126, 706–707. [DOI] [PubMed] [Google Scholar]
- 34.Fujii, K., Maki, K., Kanai, M. & Shibasaki, M. (2003) Org. Lett. 5, 733–736. [DOI] [PubMed] [Google Scholar]
- 35.Liu, P. & Jacobsen, E. N. (2001) J. Am. Chem. Soc. 123, 10772–10773. [DOI] [PubMed] [Google Scholar]
- 36.Sculimbrene, B. R. & Miller, S. J. (2001) J. Am. Chem. Soc. 123, 10125–10126. [DOI] [PubMed] [Google Scholar]
- 37.Lebsack, A. D., Link, J. T., Overman, L. E. & Stearns, B. A. (2002) J. Am. Chem. Soc. 124, 9008–9009. [DOI] [PubMed] [Google Scholar]
- 38.Xioing, Z. & Corey, E. J. (2000) J. Am. Chem. Soc. 122, 9328–9329. [Google Scholar]
- 39.Arnold, L. A., Naasz, R., Minnaard, A. J. & Feringa, B. L. (2001) J. Am. Chem. Soc. 123, 5841–5842. [DOI] [PubMed] [Google Scholar]
- 40.Shibasaki, M., Sasai, H. & Arai, T. (1997) Angew. Chem. Int. Ed. Engl. 36, 1236–1256. [Google Scholar]
- 41.Jacobsen, E. N. (2000) Acc. Chem. Res. 33, 421–431. [DOI] [PubMed] [Google Scholar]