Abstract
Enantiomerically enriched, deuterated branched carbonates (Z)-(S)-PhCH(OCO2Me)-CH = CHD (1-D), (Z)-(R)-PhCH(OCO2Me)CH = CHD (2-D), and linear carbonate (E)-(S)-PhCH = CHCHD(OCO2Me) (3-D) were used as probes in the Mo-catalyzed asymmetric allylic alkylation with sodium dimethyl malonate, catalyzed by ligand-complex 11 derived from the mixed benzamide/picolinamide of (S,S)-transdiaminocyclohexane and (norbornadiene)Mo(CO)4. The results of these studies, along with x-ray crystallography and solution NMR structural analysis of the π-allyl intermediate, conclusively established the reaction proceeded by a retention–retention pathway. This mechanism contrasts with that defined for Pd-catalyzed allylic alkylations, which proceed by an inversion–inversion pathway. A proposed rationale for the retention pathway for nucleophilic substitution involves CO-coordination to form a tri-CO intermediate, followed by complexation with the anion of dimethyl malonate to produce a seven-coordinate intermediate, which reductively eliminates to afford product with retention of configuration.
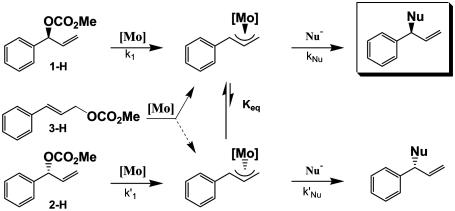
Metal-catalyzed asymmetric allylic alkylations are widely used in organic synthesis as efficient and practical methods to form carbon–carbon bonds with high enantio- and regioselectivity. Early work in this area focused on Pd complexes as catalysts, but their utility in organic synthesis was limited because, in general, unsymmetrical substrates afforded the achiral linear product (1–5). More recently, a limited number of ligands have been designed such that the branched product is now accessible with high enantiomeric excess (ee) by using Pd catalysis (6–9). Additionally, several other transition metals and ligands have been developed to regioselectively provide the branched product. These reactions can be grouped into two categories: (i) those that retain the stereochemistry of the branched substrate, and (ii) those in which the stereochemistry is lost. When starting with branched substrates, Ru (10–13), Rh (14, 15), Ir (16–19), Fe (20, 21), and W (22) complexes generally provide product wherein the stereochemistry of the substrate is retained, although recent exceptions have now been reported (23, 24). These reactions may proceed by means of intermediates in which isomerization (Keq in Scheme 1) is slow relative to nucleophilic reaction. In contrast, Mo-based catalysis (25–39) proceeds with loss of substrate stereochemistry and provides the opportunity in reactions with asymmetric ligands to obtain high product ee, starting with either the linear substrate or racemic branched product (Scheme 1). These reactions proceed by a dynamic kinetic asymmetric transformation (40) with rapid equilibration of the two diastereomeric π-allyl complexes before nucleophilic attack (Scheme 1).
Scheme 1.
The stereochemistry of the two steps shown in Scheme 1 is arbitrarily shown as retention for oxidative addition and retention for the nucleophilic displacement. Extensive work on the Pd-catalyzed allylic alkylation has shown that the overall stereochemical outcome is generally retention, with the two steps of the process both proceeding with inversion (41–46). More recently, the Ir-catalyzed alkylation has also been shown to proceed by inversion–inversion in constrained systems (19). For the Mo-catalyzed reaction, Trost and coworkers (47, 48) have determined that the reaction proceeds with overall retention, but the stereochemistry of each step in the reaction has not been elucidated.
A few studies have been carried out to determine the stereochemistry of the oxidative addition reaction in the Mocatalyzed reaction. Faller and Linebarrier (49), Rubio and Liebeskind (50), and Kuhl et al. (51) have shown that oxidative addition in stoichiometric reactions of Mo(CO)3(MeCN)3 with cyclic and acyclic allylic acetates proceeds with retention of configuration. On the other hand, Ward et al. (52) discovered the stereochemistry of the oxidative addition is influenced by steric factors and experimental conditions, and oxidative addition of a single substrate can follow either an inversion or retention pathway, depending on the solvent and concentration, suggesting both stereochemical avenues may be available to Mo-catalyzed reactions.
In 1995, Kočovský and coworkers (53) used sterically constrained substrates to further address the stereochemical preference in Mo-catalyzed allylic alkylations. In these constrained systems, a clear stereochemical differentiation was observed between the Pd- and Mo-catalyzed reactions, with the Mo-catalyzed reactions apparently reacting by a retention–retention pathway.
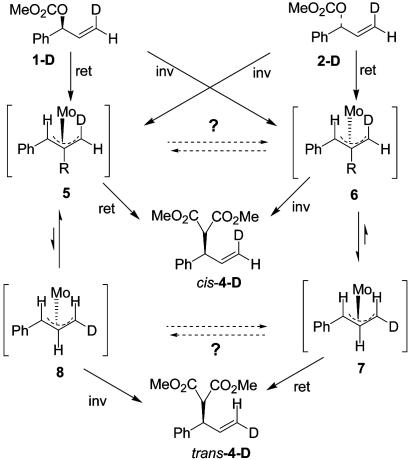
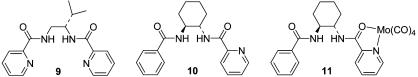
To further probe the mechanism of the Mo-catalyzed dynamic kinetic asymmetric transformation process, Malkov et al. (A. Malkov, I. Starý, L.G., G.C.L.-J., V. Langer, P. Spoor, V. Vinader, and P. Kočovský, unpublished results) used the labeled substrates 1-D and 2-D (Scheme 2) and valine-derived ligand 9. The cis-d-(S)-carbonate 1-D reacted with sodium dimethyl malonate to provide product cis-4-D with retention (the (R)-product based on convention) and with no transposition of the deuterium. With the cis-d-(R)-carbonate 2-D, the major product was again the (R)-product, this time derived from overall inversion, and the deuterium in product trans-4-D had been transposed to the trans position. These studies demonstrated that equilibration of the π-allyl complex necessary for the dynamic kinetic asymmetric transformation pathway to operate was occurring by a π–σ–π mechanism (Scheme 2). The overall stereochemistry was consistent with either an inversion–inversion or retention–retention pathway.
Scheme 2.
As outlined herein, the availability of the labeled substrates 1-D and 2-D, along with solution NMR and x-ray crystal structure studies of the π-allyl intermediate with ligand 10, have provided the means to conclusively establish the stereochemistry for both the oxidative addition and nucleophilic displacement steps for the Mo-catalyzed asymmetric allylic alkylation.
Experimental Procedures
Experimental procedures for stoichiometric and catalytic reactions of 1-D are outlined below. Experimental procedures for 2-D and 3 are similar and are provided as Supporting Text, which is published as supporting information on the PNAS web site. The procedures for the competition experiments are also included in Supporting Text. The preparation of ligand 10 has been described (54).
Stoichiometric Reaction of Branched Carbonate 1-D. Complex 11 was formed as follows. Ligand 10 (10.7 mg, 0.033 mmol) and 0.7 ml of tetrahydrofuran (THF)-d8 were placed in an NMR tube with a Teflon valve, and the solution was degassed by passing a stream of argon through the solution for 2 min. Molybdenum tetracarbonyl norbornadiene (10.0 mg, 0.033 mmol) was added, and the resulting red solution was held at ambient temperature for 1.5 h. 1H NMR analysis confirmed 90% conversion to complex 11. Branched carbonate 1-D (6.0 mg, 0.031 mmol, 91% ee) was added, and the solution was held at room temperature. 1H NMR analysis indicated 50% conversion to the π-allyl complex in 5 h and 90% conversion in 22 h. (The conversion is based on the ratio of complex 11 to π-allyl complex. Because the reaction stoichiometry (54) is 2 mol of complex 11, affording 1 mol each of π-allyl complex, free ligand, and molybdenum hexacarbonyl, the maximum yield of π-allyl complex is 50% based on complex 11.) Relative to the protio complex (12), the signal at 3.11 ppm was nearly absent (≈5%, corresponding to the level of the minor enantiomer in 1-D), confirming the location of the deuterium in the complex as that shown in complex 5. Solid sodium dimethyl malonate (10 mg, 0.065 mmol) was added and the solution was warmed in a 50°C oil bath. 1H NMR analysis after 5.5 h at 50°C indicated the reaction was >90% complete with formation of 95% cis-4-D and ≈5% trans-4-D, the ratio corresponding to the enantiomeric ratio (er) of the initial carbonate 1-D. Relative to the protio product, the resonance at 5.05 ppm due to Hc is absent in cis-4-D, and Hd at 4.99 ppm is collapsed to doublet of doublets with a coupling constant of 10.4 Hz consistent with a cisorientation relative to Hb. (A small (<1 Hz) coupling to Ha also occurs.) A 0.1-ml sample of the reaction mixture was added to 2 ml of 10% i-PrOH/90% hexanes and 2 ml of 0.5 M HCl. The organic layer was filtered, and a chiral HPLC analysis of this solution was carried out by using a Whelko column as described (30). The ee of combined products cis-4-D and trans-4-D was 99%.
Catalytic Reaction of Branched Carbonate 1-D. Complex 11 was formed as follows. Ligand (3.0 mg, 0.0092 mmol) and 0.8 ml of THF-d8 were placed in an NMR tube with a Teflon valve, and the solution was degassed by passing a stream of argon through the solution for 2 min. Molybdenum tetracarbonyl norbornadiene (2.6 mg, 0.0087 mmol) was added, and the resulting red solution was held at ambient temperature for 1.5 h. 1H NMR analysis indicated 70% conversion to complex 11, with 30% unreacted ligand and <5% unreacted molybdenum tetracarbonyl norbornadiene. Branched carbonate 1-D (12.0 mg, 0.063 mmol, 91% ee) and solid sodium dimethyl malonate (12 mg, 0.078 mmol) were added and the reaction was heated in an oil bath at 50°C. 1H NMR analysis showed 50% conversion in 25 min, 90% conversion in 2 h, and 94% conversion in 20 h. The primary product (94%) based on 1H NMR analysis was cis-4-D. HPLC analysis indicated >99% ee.
Results and Discussion
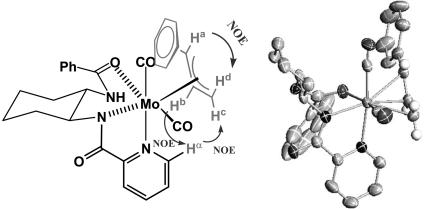
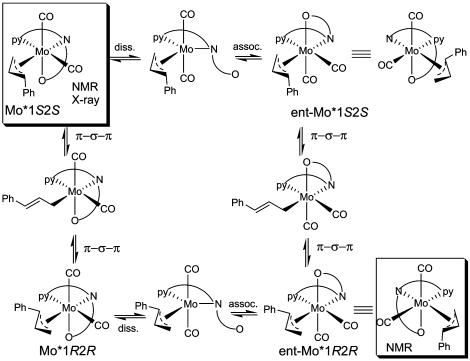
Formation and Characterization of the π-Allyl Complex 12. Mo–ligand complex 11 was prepared from reaction of ligand 10 with 1 eq of Mo(CO)4(norbornadiene) in THF solution. The reaction, as monitored by 1H NMR, was complete within 15 min at ambient temperature to provide a red solution of complex 11. Stoichiometric reaction of ligand complex 11 with either linear (3-H) or branched carbonates (1-H, 2-H) in THF in the absence of a nucleophile cleanly formed a single π-allyl intermediate. NMR studies of this complex in THF-d8 revealed a neutral Mo(II) complex, derived from deprotonated ligand 10, as the major species (Fig. 1). In the 15N NMR spectrum of this complex, the amide nitrogens appeared at δ 130 and 175 ppm and the pyridine nitrogen at δ 263 ppm in comparison with the free ligand, where the respective shifts were δ 118, 119, and 304 ppm. The 175-ppm resonance for one of the amide nitrogens, the absence of one NH proton in the 1H spectrum, and proton-coupling data clearly indicated one amide was deprotonated in the complex. The other amide remained protonated, but the downfield shift of the nitrogen in the 15N NMR and the NH proton in 1H NMR vs. the uncomplexed ligand suggested this amide was also involved in coordination to Mo in the complex. The complex displayed nuclear Overhauser effects (NOEs) between Hb and Hc on the allyl moiety, and the picoline hydrogen (Hα) at C-6. In addition, the other two protons (Ha and Hd) of the allyl moiety had NOE contacts and correlations to both COs. The NOEs and coupling constants establish the Ph group of the allyl moiety as syn relative to the central proton (Hb); thus, the stereochemistry of the two asymmetric carbons of the cinnamyl moiety in the complex is either 1R2R or 1S2S (not 1R2S or 1S2R, which would correspond to the anti-relationship), depending on which face is bound to the metal. A solution structure that fits the NMR data is shown in Fig. 1. However, the solution structure is actually consistent with either of two structures, denoted as Mo*1S2S or ent-Mo*1R2R (Scheme 3), where the S and R designations refer to the two stereogenic centers of the cinnamyl group. These structures are pseudoenantiomers of each other, with inverted stereochemistry at Mo (denoted Mo* and ent-Mo*) binding to different faces of the allyl fragment (1S2S or 1R2R), but with the same configuration at the chiral ligand. The two structures could potentially interconvert by dissociation/reassociation of the presumably weakly bound amide oxygen (shown in the horizontal equilibrations in Scheme 3) and π–σ–π isomerization (shown as the vertical equilibrations in Scheme 3).
Fig. 1.
(Left) Solution structure of 12. (Right) Crystal structure.
Scheme 3.
A suitable crystal of the π-allyl complex was grown, and the crystal structure was solved (as shown in Fig. 1). The crystal structure confirmed several features present in the solution structure, including (i) three-point binding of the ligand to Mo by the pyridine, the mono-deprotonated amide nitrogen, and the neutral amide oxygen; (ii) the presence of two Mo-bound COs; and (iii) the syn-relationship between the allyl moiety and the COs. The structure in the crystal corresponds to Mo*1S2S in Scheme 3.
The crystal structure of 12 revealed that one face of the π-allyl complex is clearly open for backside nucleophilic attack (inversion). However, reaction of a nucleophile in this way would produce the product with a stereochemistry opposite to that observed experimentally (4-H). This unexpected finding forced us to reconsider whether the solution and x-ray structures were in fact the same, or if the nucleophilic addition was perhaps not occurring by backside attack.
To form the product enantiomer observed experimentally, either (i) the ent-Mo*1R2R isomer is more stable in solution, but the Mo*1S2S isomer crystallizes preferentially and nucleophilic attack occurs with inversion on the isomer ent-Mo*1R2R; or(ii) the Mo*1S2S isomer is in equilibrium with an unobserved, highly reactive 1R2R isomer (Curtin–Hammett conditions) that reacts by inversion; or (iii) the Mo*1S2S isomer is the major isomer in solution and crystal, and the nucleophilic attack occurs with retention of configuration. To determine which of these mechanistic possibilities is valid, the enantiomerically enriched, deuterium-labeled substrates 1-D and 2-D were used.
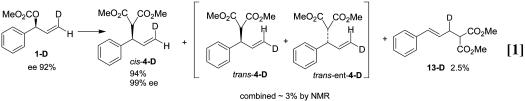
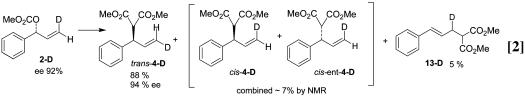
The overall catalytic reactions of substrates 1-D and 2-D using Mo–ligand complex 11 afforded the same products as reported with ligand 9. Substrate 1-D (96:4 er) provided cis-4-D (99.5:0.5 er) with no transposition of the deuterium along with ≈3% of trans-4-D in which the label had been transposed (see Eq. 1). The level of the transposed product (3%) corresponded well with the 4% enantiomer present initially in substrate 1-D, indicating that very little, if any, scrambling of the deuteron occurred in the reaction. The enantiomeric substrate 2-D (96:4 er) provided 88% of the deuterium-transposed product trans-4-D with 97:3 er along with ≈7% of the nontransposed labeled product cis-4-D (see Eq. 2). Again, the close correspondence of the original ee of the substrate (96:4 er) and the minimal formation of the nontransposed product (7%) indicated that no more than 3% of the reaction can be occurring with scrambling of the label.
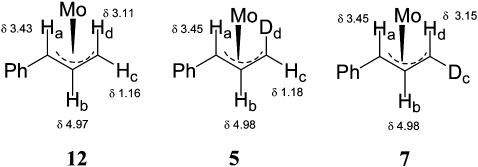
To characterize the π-allyl intermediate formed with the labeled substrates, stoichiometric reactions were carried out. The stoichiometric reaction of substrate 1-D (96:4 er) with ligand complex 11 in THF-d8 in the absence of a nucleophile cleanly afforded the π-allyl intermediate. 1H NMR examination of the major solution structure (95%), in comparison with the protio structure 12, indicated that the “d”-position (structure 5, Fig. 2) contained deuterium, whereas the minor structure (5%) had the label in the “c” position. For the major isomer in solution, no transposition of the deuterium label occurred on formation of the π-allyl complex, indicating no π–σ–π equilibration. The
 |
 |
minor isomer (≈5%) was formed at a level consistent with the amount of enantiomer present in the starting substrate (≈4%), indicating little if any leakage between pathways for formation of the π-allyl complex. The x-ray crystal structure of the protio complex is consistent with the stereochemistry shown in structure 5, indicating the major π-allyl complex is formed with retention of configuration. Addition of a stoichiometric quantity of sodium dimethyl malonate (in the presence of 1 eq of Mo(CO)6 (54) converted the π-allyl intermediate to product cis-4-D. Thus, conversion of the π-allyl intermediate 5 to product also occurred with retention and no transposition of the deuterium. This confirms that the reaction proceeded by a retention–retention pathway, 1-D to 5 to cis-4-D (Scheme 2).
Fig. 2.
′H NMR chemical shifts for deuterium-labeled complexes.
Reaction with the enantiomeric mismatched labeled substrate was examined next. Stoichiometric reaction of substrate 2-D (96:4 er) with ligand complex 11 in the absence of a nucleophile afforded the π-allyl intermediate with the deuterium at position “c” (Fig. 2, structure 7). In this case, the major π-allyl species (>95%) was that resulting from transposition of the label, with the minor isomer (no transposition of the label) present at a level similar to the amount of enantiomer present in the substrate. Comparison with the x-ray crystal structure of the protio complex established the stereochemistry of the π-allyl intermediate as that which corresponded to structure 7. In this case, formation of the π-allyl complex occurred with retention to produce the unobserved intermediate 6, which was rapidly converted to the observed 7 by π–σ–π equilibration (Scheme 2). Addition of a stoichiometric quantity of sodium dimethyl malonate [in the presence of Mo(CO)6] converted the π-allyl intermediate 7 to product trans-4-D, so conversion of the π-allyl intermediate observed in solution to the product occurred with retention and with no transposition of the label. Therefore, with substrate 2-D, oxidative addition and nucleophilic addition also both occurred with retention.
To complete the study, catalytic and stoichiometric reactions were carried out with the enantiomerically enriched deuterated linear carbonate 3-D. In the absence of a nucleophile, the deuterated linear carbonate (96:4 er) was reacted with a stoichiometric quantity of the Mo–ligand complex 11 in THF-d8. The major π-allyl complex observed by 1H NMR had the deuteron in the “c” position. Based on the x-ray crystal structure of the protio substrate, which corresponds to the stereochemistry shown in structure 7, the molybdenum was added from the same side as the leaving group. About 5% of the transposed, labeled complex 6 was observed by NMR, which is close to what is expected from the 96:4 er of the substrate. This indicated <2% of the oxidative addition was occurring with inverted stereochemistry. Stoichiometric addition of sodium dimethyl malonate
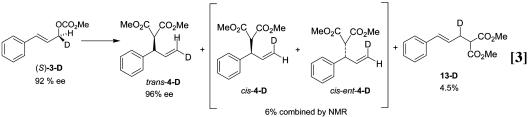 |
[in the presence of Mo(CO)6] converted the π-allyl complex to a 95:5 mixture of products, wherein retention of stereochemistry has occurred with no transposition of the deuterium. The 95:5 product mixture also closely mirrors the 96:4 er of the starting material and the π-allyl intermediate, suggesting that >98% of the nucleophilic substitution has occurred with retention of stereochemistry. The catalytic reaction (see Eq. 3) proceeded with similar results. Thus, the linear substrate also reacted by way of a retention–retention pathway.
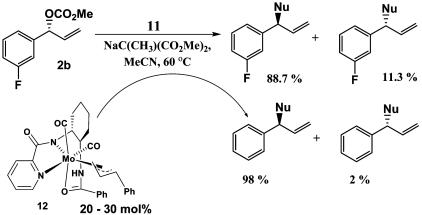
An assumption in the preceding analysis is that the major π-allyl complex observed in solution is that which crystallizes. Conceivably, the x-ray structure of the protio complex could be that of the minor isomer, because minor and major species are in equilibrium in solution. To address this possibility, competition experiments were carried out with the 3-F substrate 2b and π-allyl complex 12 (Scheme 4). Reaction of 2b with catalyst complex 11 represents the mismatched case wherein the minor diastereomer of the π-allyl complex (compare, 6) is kinetically generated and must equilibrate to the major complex (compare, 7) by π–σ–π isomerization before nucleophilic attack. The reactions were carried out with sodium dimethyl methylmalonate in MeCN, conditions that produce a substantial memory effect (30), such that the ee for the reaction with both 2-H and 2b is 74%. For these substrates, then, the equilibration (Keq) in Scheme 1 is competitive with nucleophilic attack (kNu), which results in a lower ee for the mismatched substrate. The experiments were conducted as follows. The catalytic reaction of the 3-F substrate 2b was initiated, and then complex 12 (20–30 mol%) derived from the unsubstituted analog was added after the reaction was smoothly turning over (15–45% conversion). If the isolated complex 12 was in fact the minor isomer (6) and required equilibration to the major isomer (7) before reaction with the nucleophile, then both the 3-F and unsubstituted products should exhibit a memory effect and have similar, low ee's. If the isolated complex 12 is the major complex (7) and does not require equilibration, then a high ee should result, similar to that observed with 1-H. The results of two experiments confirmed the latter case; the ee for the 3-F product was 77.5 ± 1.5%, whereas that derived from complex 12 was 96%. This confirms that the major isomer observed in solution is also that which crystallizes.
Scheme 4.
As another consideration, π-allyl complexes 5 and 6 (and 7 and 8) could potentially be interconverting by Mo–Mo exchange (equilibration by the dotted arrows in Scheme 2) and be consistent with a reaction pathway proceeding by inversion mechanisms. Exchange of this nature has been observed in Pd complexes (46). However, this mechanism can be excluded as outlined below. If 5/6 and 7/8 were equilibrating, then crossover between the 1-D and 2-D manifolds would occur (Scheme 2). The outcome would depend on the relative rates of equilibration relative to nucleophilic substitution. For example, if the rate of equilibration of 5/6 and 7/8 is slow relative to equilibration of 5/8 and 6/7, but similar in rate of nucleophilic addition, then 1-D could react by inversion to form 6, which would equilibrate rapidly to 7, which would partially equilibrate to 8, resulting in formation of trans-4-D with low ee. On the other hand, if the rates of all equilibrations and nucleophilic substitution were comparable, then all 4 π-allyl complexes (5–8) could potentially be generated, resulting in formation of both cis-4-D and trans-4-D, each with low ee. Finally, if the rates of all equilibrations are rapid relative to nucleophilic substitution, then both 1-D and 2-D would funnel into the same intermediate (5, 6, 7, or 8, which ever is the thermodynamic sink) and both form the same product. None of these scenarios applies. Substrate 1-D exclusively forms cis-4-D in high ee, whereas 2-D affords only trans-4-D in high ee. The highly stereospecific nature of the reaction demonstrates the two manifolds (1-D and 2-D) are not connected, excluding the possibility of 5/6 and 7/8 equilibration by Mo–Mo exchange.
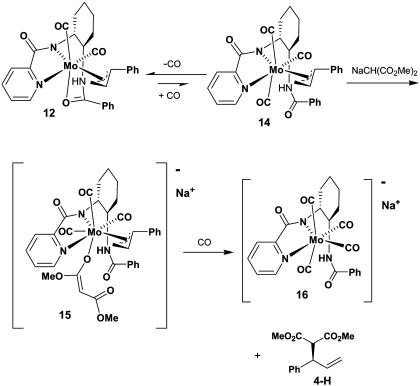
In conclusion, use of the enantiomerically enriched, labeled substrates, along with the x-ray crystal and solution structure of the π-allyl complex 12 and competition experiments, conclusively demonstrate the reaction of branched and linear substrates 1, 2, and 3 proceed by a retention–retention mechanism. As mentioned in the introduction, retentive oxidative addition catalyzed by Mo is precedented and has been attributed to precomplexation of the leaving group oxygen with Mo before coordination to the double bond (49–52). A retentive pathway for nucleophilic substitution is more surprising but may also be due to precomplexation of the nucleophile with Mo. However, since a CO source is imperative for the reaction to take place (54), and since the molybdenum-containing product of the reaction (16) contains four carbonyls, we believe a tricarbonyl species is a likely intermediate. A pathway that is consistent with the available data is outlined in Scheme 5. In the first step, the weakly bound neutral amide in the π-allyl complex (12) is displaced by CO to form tricarbonyl intermediate 14. This step must be reversible and favor the bis-CO adduct, since no tricarbonyl complex is observed under an atmosphere of CO. However, under an atmosphere of 13CO, rapid exchange occurs into the π-allyl complex, indicating that CO exchange, presumably by a tricarbonyl species, is viable. Next, coordination of malonate occurs to afford a seven-coordinate species 15, which should be an accessible intermediate because ample precedence exists for seven-coordinate Mo species (55–57). We depict this coordination occurring monodentate by an oxygen of the malonate rather than by the central carbon because of less steric crowding in the former. Two Mo–malonate complexes have been reported by Ito and coworkers (58), one with the malonate coordinated in a bidentate fashion through both oxygens, but the other is presumed to be monodentate by one oxygen, based on IR data (59). Several Pd complexes of malonate are known and have ligation either monodentate through the central carbon (60–63) or bidentate with both oxygens (60, 64, 65). A Rh–malonate complex has also been recently reported with ligation by the central carbon (66). Since no observable complexation of malonate occurs when sodium dimethyl malonate is added to the π-allyl complex in the absence of a CO source, we believe addition of CO to form the tricarbonyl complex 14 must occur before formation of the malonate complex 15. Finally, reductive elimination occurs to afford the enantio-enriched product and the anionic Mo(CO)4L complex (16), which is one of the observed resting states of the catalyst along with the π-allyl complex (54).
Scheme 5.
As an alternative to this mechanism, Kočovský et al. (27) have suggested the nucleophilic substitution step in Mo-catalyzed allylic alkylations may be occurring by an η1 complex with the nucleophile attacking in an SN2′ mode. Since the π–σ–π equilibration occurs by the η1 complex, it is clearly an accessible intermediate, but since the two substrate enantiomers would converge at this intermediate, it is not consistent with the memory effects observed in these reactions. An alternative is an enyl σ–π intermediate, such as that proposed by Evans and Nelson (15) to explain retention in the Rh-catalyzed allylic alkylations, yet steric effects in this complex may preclude addition from the same side as Mo to produce the product from retention. Kinetic and theoretical studies are needed to further probe the mechanism of this reaction.
Supplementary Material
Acknowledgments
G.C.L.-J. received support from the Engineering and Physical Sciences Research Council (GR/NO5208), the Royal Society of Chemistry, and AstraZeneca.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ee, enantiomeric excess; er, enantiomeric ratio; THF, tetrahydrofuran; NOE, nuclear Overhauser effect.
References
- 1.Pfaltz, A. & Lautens, M. (1999) Comprehensive Asymmetric Catalysis, eds. Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (Springer, Berlin), Vol. 2, pp. 833–884. [Google Scholar]
- 2.Trost, B. M. (1996) Acc. Chem. Res. 29, 355–364. [Google Scholar]
- 3.Frost, C. G., Howarth, J. & Williams, J. M. J. (1992) Tetrahedron: Asymmetry 3, 1089–1122. [Google Scholar]
- 4.Helmchen, G., Kudis, S., Sennhenn, P. & Steinhagen, H. (1997) Pure Appl. Chem. 69, 513–518. [Google Scholar]
- 5.Helmchen, G. & Pfaltz, A. (2000) Acc. Chem. Res. 33, 336–345. [DOI] [PubMed] [Google Scholar]
- 6.Trost, B. M. & Toste, F. D. J. (1998) J. Am. Chem. Soc. 110, 9074–9075. [Google Scholar]
- 7.Pretot, R. & Pfaltz, A. (1998) Angew. Chem. Int. Ed. Engl. 37, 323–325. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi, T., Ohno, A., Lu, S.-J., Matsumoto, Y., Fukuyo, E. & Yanagi, K. (1994) J. Am. Chem. Soc. 116, 4221–4226. [Google Scholar]
- 9.You, S.-L., Zhu, X.-Z, Luo, Y.-M., Shou, X.-L. & Dai, L.-X. (2001) J. Am. Chem. Soc. 123, 7471–7472. [DOI] [PubMed] [Google Scholar]
- 10.Trost, B. M., Fraisse, P. & Ball, Z. T. (2002) Angew. Chem. Int. Ed. Engl. 41, 1059–1061. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, S. W., Mitsudo, T., Kondo, T. & Watanabe, Y. (1993) J. Organomet. Chem. 450, 197–207. [Google Scholar]
- 12.Morisaki, Y., Kondo, T. & Mitsudo, T. (1999) Organometallics 18, 4742–4746. [Google Scholar]
- 13.Matsushita, Y., Onitsuka, K., Kondo, T., Mitsudo, T. & Takahashi, S. (2001) J. Am. Chem. Soc. 123, 10405–10406. [DOI] [PubMed] [Google Scholar]
- 14.Evans, P. A. & Kennedy, L. J. (2001) J. Am. Chem. Soc. 123, 1234–1235. [DOI] [PubMed] [Google Scholar]
- 15.Evans, P. A. & Nelson, J. D. (1998) J. Am. Chem. Soc. 120, 5581–5582. [Google Scholar]
- 16.Takeuchi, R., Ve, N., Tanabe, K., Yamashita, K. & Shiga, N. (2001) J. Am. Chem. Soc. 123, 9525–9534. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi, R. & Kashiro, M. (1998) J. Am. Chem. Soc. 120, 8647–8655. [Google Scholar]
- 18.Bartels, B. & Helmchen, G. (1999) Chem. Commun. 741–742.
- 19.Bartels, B., Garcia-Yebra, C., Rominger, F. & Helmchen, G. (2002) Eur. J. Inorg. Chem. 2569–2586.
- 20.Xu, Y. & Zhou, B. (1987) J. Org. Chem. 52, 974–977. [Google Scholar]
- 21.Eberhardt, U. & Mattern, G. (1988) Chem. Ber. 121, 1531–1534. [Google Scholar]
- 22.Lehmann, J. & Lloyd-Jones, G. C. (1995) Tetrahedron 51, 8863–8874. [Google Scholar]
- 23.Bartels, B., Garcia-Yebra, C. & Helmchen, G. (2003) Eur. J. Org. Chem. 1097–1103.
- 24.Hayashi, T., Okada, A., Suzuka, T. & Kawatsura, M. (2003) Org. Lett. 5, 1713–1715. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd-Jones, G. C. & Pfaltz, A. (1995) Angew. Chem. Int. Ed. Engl. 34, 462–464. [Google Scholar]
- 26.Malkov, A. V., Spoor, P., Vinader, V. & Kočovský, P. (2001) Tetrahedron Lett. 42, 509–512. [Google Scholar]
- 27.Kočovský, P., Malkov, A. V., Vyskočil, S. & Lloyd-Jones, G. C. (1999) Pure Appl. Chem. 71, 1425–1433. [Google Scholar]
- 28.Glorius, F. & Pfaltz, A. (1999) Org. Lett. 1, 141–144. [Google Scholar]
- 29.Trost, B. M., Dogra, K., Hachiya, I., Emura, T., Hughes, D. L., Krska, S., Reamer, R. A., Palucki, M. P., Yasuda, N. & Reider, P. J. (2002) Angew. Chem. Int. Ed. Engl. 41, 1929–1932. [PubMed] [Google Scholar]
- 30.Hughes, D. L., Palucki, M., Yasuda, N., Reamer, R. A. & Reider, P. J. (2002) J. Org. Chem. 67, 2762–2768. [DOI] [PubMed] [Google Scholar]
- 31.Trost, B. M. & Hachiya, I. (1998) J. Am. Chem. Soc. 120, 1104–1105. [Google Scholar]
- 32.Trost, B. M., Hilbrand, S. & Dogra, K. (1999) J. Am. Chem. Soc. 121, 10416–10417. [Google Scholar]
- 33.Trost, B. M. & Dogra, K. (2002) J. Am. Chem. Soc. 124, 7256–7257. [DOI] [PubMed] [Google Scholar]
- 34.Trost, B. M. & Andersen, N. G. (2002) J. Am. Chem. Soc. 124, 14320–14321. [DOI] [PubMed] [Google Scholar]
- 35.Kaiser, N.-F. K., Bremberg, U., Larhed, M., Moberg, C. & Hallberg, A. (2000) Angew. Chem Int. Ed. Engl. 39, 3595–3598. [DOI] [PubMed] [Google Scholar]
- 36.Belda, O., Kaiser, N. F. K., Bremberg, U., Larhed, M., Hallberg, A. & Moberg, C. (2000) J. Org. Chem. 65, 5868–5870. [DOI] [PubMed] [Google Scholar]
- 37.Glorious, F., Neuberger, M. & Pfaltz, A. (2001) Helv. Chim. Acta 84, 3178–3196. [Google Scholar]
- 38.Janssen, U. P. & Helmchen, G. (1997) Tetrahedron Lett. 38, 8025–8026. [Google Scholar]
- 39.Belda, O., Lundgren, S. & Moberg, C. (2003) Org. Lett. 5, 2275–2278. [DOI] [PubMed] [Google Scholar]
- 40.Faber, K. (2001) Chem. Eur. J. 7, 5004–5010. [DOI] [PubMed] [Google Scholar]
- 41.Trost, B. M. & Strege, P. E. (1975) J. Am. Chem. Soc. 97, 2534–2535. [Google Scholar]
- 42.Trost, B. M. & Verhoeven, T. R. (1976) J. Org. Chem. 41, 3215–3216. [Google Scholar]
- 43.Hayashi, T., Hagihara, T., Knonishi, M. & Kumada, M. (1983) J. Am. Chem. Soc. 105, 7767–7768. [Google Scholar]
- 44.Hayashi, T., Konishi, M. & Kumada, M. (1984) J. Chem. Soc. Chem. Commun. 107–108.
- 45.Hayashi, T., Yamamoto, A. & Hagihara, T. (1986) J. Org. Chem. 51, 723–727. [Google Scholar]
- 46.Mackenzie, P. B., Whelan, J. & Bosnich, B. (1985) J. Am. Chem. Soc. 107, 2046–2054. [Google Scholar]
- 47.Trost, B. M. & Lautens, M. (1987) J. Am. Chem. Soc. 109, 1469–1478. [Google Scholar]
- 48.Trost, B. M & Merlic, C. A. (1990) J. Am. Chem. Soc. 112, 9590–9600. [Google Scholar]
- 49.Faller, J. W. & Linebarrier, D. (1988) Organometallics 7, 1670–1672. [Google Scholar]
- 50.Rubio, A. & Liebeskind, L. S. (1993) J. Am. Chem. Soc. 115, 891–901. [Google Scholar]
- 51.Kuhl, A., Christopher, J. A., Farrugia, L. J. & Kocienski, P. J. (2000) Synlett 1765–1768.
- 52.Ward, Y. D., Villanueva, L. A., Allred, G. D. & Liebeskind, L. S. (1996) J. Am. Chem. Soc. 118, 897–898. [Google Scholar]
- 53.Dvorak, D., Starý, I. & Kočovský, P. (1995) J. Am. Chem. Soc. 117, 6130–6131. [Google Scholar]
- 54.Krska, S. W., Hughes, D. L., Reamer, R. A., Mathre, D. J., Sun, Y. & Trost, B. M. (2002) J. Am. Chem. Soc. 124, 12656–12657. [DOI] [PubMed] [Google Scholar]
- 55.Szalda, D. J., Dewan, J. C. & Lippard, S. J. (1981) Inorg. Chem. 20, 3851–3857. [Google Scholar]
- 56.Dewan, J. C. & Lippard, S. J. (1982) Inorg. Chem. 21, 1682–1684. [Google Scholar]
- 57.McAlpine, A. S., McEwan, A. G., Shaw, A. L. & Bailey, S. (1997) J. Biol. Inorg. Chem. 2, 690–701. [Google Scholar]
- 58.Minato, M., Kusishima, S., Nagai, K., Yamasaki, M. & Ito, T. (1994) Chem. Lett. 2339–2342.
- 59.Ito, T., Shimada, K., Ono, T., Minato, M. & Yamaguchi, Y. (2000) J. Organomet. Chem. 611, 308–313. [Google Scholar]
- 60.Wolkowski, J. P. & Hartwig, J. F. (2002) Angew. Chem. Int. Ed. Engl. 41, 4289–4291. [DOI] [PubMed] [Google Scholar]
- 61.Newkome, G. R., Gupta, V. K., Taylor, H. C. R. & Fronczek, F. R. (1984) Organometallics 3, 1549–1554. [Google Scholar]
- 62.Newkome, G. R., Kawato, T., Kohli, D. K., Puckett, W. E., Olivier, B. D., Chiari, G., Fronczek, F. R. & Deutsch, W. A. (1981) J. Am. Chem. Soc. 103, 3423–3429. [Google Scholar]
- 63.Kawato, T., Uechi, T., Koyama, H., Kanatomi, H. & Kawanami, Y. (1984) Inorg. Chem. 23, 764–769. [Google Scholar]
- 64.Ruiz, J., Rodriguez, V., Cutillas, N., Pardo, M., Perez, J., Lopez, G., Chaloner, P. & Hitchcock, P. B. (2001) Organometallics 20, 1973–1982. [Google Scholar]
- 65.Straub, B. F., Rominger, F. & Hofmann, P. (2000) Inorg. Chem. Commun. 3, 358–360. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe, M., Murata, K. & Ikariya, T. (2003) J. Am. Chem. Soc. 125, 7508–7509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.