Abstract
Optically active 1,2-bis(alkylmethylphosphino)ethanes and bis(alkylmethylphosphino)methanes are unique diphosphine ligands combining the simple molecular structure and P-stereogenic asymmetric environment. This work shows that these ligands exhibit excellent enantioselectivity in rhodium-catalyzed asymmetric hydrogenation of α,β-unsaturated phosphonic acid derivatives. The enantioselective hydrogenation mechanism elucidated by NMR study is also described.
The α-hydroxy- and α-aminophosphonic acids have an extremely rich and varied spectrum of biological activity (1, 2) that is stipulated by their ability to act as antagonists for amino acids inhibiting enzymes involved in the amino acids' metabolism. They can be used as herbicides (3, 4), antiviral drugs (5), antibiotics (6), and neurodrugs (7). Being able to affect the biological activity of cells (8), some of them can inhibit HIV protease (9) and alanine racemase (alafosfalin) (10). Peptide α-hydroxyphosphonates are known to be rennin inhibitors (11).
The biological activity of the phosphonic acids highly depends on the absolute configuration of its α-chiral center. An excellent example is alafosfalin, [N-(l-alanyl)-l-1-aminoethyl]phosphonic acid (S,R)-diastereomer. Only this diastereomer unlike (R,R)-, (S,S)-, and (R,S)-diastereomers shows high antibacterial activity (12, 13).
Development of the synthesis of optically active α-hydroxy- and α-aminophosphonic acids has begun only recently (2, 15).∥ Processes applying the catalytic amounts of the source of chirality are especially attractive. Thus, hydrophosphonylation of imines catalyzed by heterobimetallic complexes Ln-K-BINOL (16) and hydrophosphonylation of aldehydes catalyzed by analogous complexes Al-Li-BINOL (17) give the α-amino- and α-hydroxyphosphonic acids, respectively, with high enantiomeric excesses (ee's). Furthermore, the catalytic asymmetric Michael addition to α,β-unsaturated phosphonates (18, 19) and the allylation reaction of α-acetylamino-β-ketophosphonates (20) have been successfully used for the synthesis of phosphonic acid derivatives with the stereogenic centers in the α- or β-position.
Another convenient approach to the synthesis of the acids is the asymmetric hydrogenation of the corresponding unsaturated precursors. By this means, the phosphorus analogs of α-amino- or α-hydroxycarboxylic acids were obtained with up to 96% ee by using the Rh complexes of chiral phosphine ligands such as (–)-BPPM (2S,4S) (21) and DuPHOS (22). Noyori and coworkers reported that α-substituted β-ketophosphonates were subjected to enantio- and diastereoselective hydrogenation by the use of the BINAP–Ru(II) complex to give the corresponding β-hydroxyphosphonates with exceedingly high ee's (94–98%) (23, 24). Also worth mentioning is that optically active α-arylsubstituted ethylphosphonates, phosphorus analogs of 2-arylpropionic acids, were produced by Ir- and Ru-catalyzed asymmetric hydrogenation of ethenylphosphonates (25, 26).
On the other hand, we previously designed and synthesized new bidentate phosphine ligands, (S,S)-1,2-bis(alkylmethylphosphino)ethanes (alkyl = tert-butyl, 1,1-diethyl-propyl, 1-adamantyl, 1-methylcyclohexyl, cyclohexyl, cyclopentyl, and isopropyl) (BisP*) (27) and (R,R)-bis(alkylmethylphosphino)methanes (alkyl = tert-butyl, cyclohexyl, isopropyl, and phenyl) (MiniPHOS) (28). An important feature of these ligands is that a bulky alkyl group and the smallest alkyl group (methyl group) are bound to each phosphorus atom. These ligands form five- or four-membered C2-symmetric chelates, and it is expected that the imposed asymmetric environment is responsible for high enantioselectivity in catalytic asymmetric reactions. The synthetic utility of these ligands, especially of t-Bu-BisP* and t-Bu-MiniPHOS, has been proven in a few representative asymmetric catalyses (27–33).
Based on these results we examined the applicability of these ligands in rhodium-catalyzed asymmetric reduction of several ethenephosphonate derivatives. In addition we studied the mechanism of this hydrogenation, in conjunction with our continuing mechanistic study on rhodium-catalyzed asymmetric hydrogenations of prochiral alkenes (31–38).
Scheme 1.
Methods
The reaction conditions were optimized with dimethyl α-benzoyloxyethenephosphonate or diethyl α-benzoyloxyethenephosphonate as the representative substrates (Table 1). The highest enantioselectivity was achieved in methanol. The use of t-Bu-MiniPHOS as the chiral ligand effected higher enantioselectivity than t-Bu-BisP* (entries 1 and 2). The alkyl group in the phosphonate component slightly affected the enantioselectivity; the methyl ester provided higher selectivity than the corresponding ethyl ester (entries 2 and 6). All reactions were complete within 18 h at room temperature at an initial H2 pressure of 4 atm. These relatively mild reaction conditions compare favorably with those applied in hydrogenations catalyzed by DuPHOS-Rh (22).
Table 1. Results of the asymmetric hydrogenation of α-benzoyloxyethenephosphonates under varied conditions (Eq. 1).
| Entry | R | Ligand | Solvent | ee, % |
|---|---|---|---|---|
| 1 | Me | (S,S)-t-Bu-BisP* | MeOH | 88 |
| 2 | Me | (R,R)-t-Bu-MiniPHOS | MeOH | 96 |
| 3 | Me | (R,R)-t-Bu-MiniPHOS | i-PrOH | 86 |
| 4 | Me | (R,R)-t-Bu-MiniPHOS | Toluene | 88 |
| 5 | Me | (R,R)-t-Bu-MiniPHOS | CH2Cl2 | 93 |
| 6 | Et | (R,R)-t-Bu-MiniPHOS | MeOH | 93 |
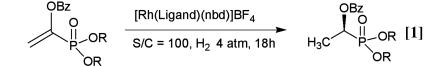 |
The results on the hydrogenation of a series of prochiral phosphonates are summarized in Table 2. All substrates except the β-phenyl derivative were converted to the hydrogenation products with high ee's up to 99%. The t-Bu-MiniPHOS-Rh-catalyzed reactions were uniformly superior to those using t-Bu-BisP*-Rh as a catalyst. The enantioselectivity was affected by the bulkiness of the substituent at the β-position; the more bulky group seems to increase the enantioselectivity. The selectivity observed in the reduction of cyclopentylmethyl derivative (99%, entry 9) is, to our knowledge, the highest among the asymmetric hydrogenations of enolbenzoate phosphonates.
Table 2. Results of the asymmetric hydrogenation of various ethenephosphonates under optimized conditions (Eq. 2).
| Entry | R | Ligand | ee, % |
|---|---|---|---|
| 1 | H | (S,S)-t-Bu-BisP* | 88 |
| 2 | H | (R,R)-t-Bu-MiniPHOS | 96 |
| 3 | CH3 | (S,S)-t-Bu-BisP* | 86 |
| 4 | CH3 | (R,R)-t-Bu-MiniPHOS | 89 |
| 5 | C2H5 | (S,S)-t-Bu-BisP* | 87 |
| 6 | C2H5 | (R,R)-t-Bu-MiniPHOS | 93 |
| 7 | (CH3)2CH | (S,S)-t-Bu-BisP* | 98 |
| 8 | (CH3)2CH | (R,R)-t-Bu-MiniPHOS | 98 |
| 9 | (cyclo-C5H9)CH2 | (S,S)-t-Bu-BisP* | 92 |
| 10 | (cyclo-C5H9)CH2 | (R,R)-t-Bu-MiniPHOS | 99 |
| 11 | Ph | (S,S)-t-Bu-BisP* | 50 |
| 12 | Ph | (R,R)-t-Bu-MiniPHOS | 70 |
| 13 | CH3O | (S,S)-t-Bu-BisP* | 87 |
| 14 | CH3O | (R,R)-t-Bu-MiniPHOS | 87 |
In all cases the same stereoselection leading to (R)-configuration of the products was observed. Note that (S,S)-t-Bu-BisP* and (R,R)-t-Bu-MiniPHOS constitute almost the same stereochemical environment around the stereogenic phosphorus centers, although the respective absolute configurations are different. The stereochemical sense of the hydrogenation is consistent with the results obtained for the hydrogenation of α-acylaminoacrylic acid derivatives and enamides (27–31). These facts suggest that the benzoate oxygen atom like amide oxygen interacts coordinatively with rhodium metal to control the stereoselection. The stereochemical outcome can be reasonably interpreted by assuming that the reaction proceeds through a dihydride mechanism (see below).
Two β,β-disubstituted phosphonates, dimethyl 1-benzoyloxy-2,2-dimethylethenephosphonate and dimethyl-1-benzoyloxy-2,2-pentamethylene-ethenephosphonate, were also tested for the hydrogenation. However, no reduction occurred even at 50 atm, probably because of both the steric and electronic effects.
The hydrogenation procedure was applied also to α-phenylethenephosphonic acid and its dimethyl ester. The reactions proceeded smoothly at a H2 pressure of 50 or 5 atm. However, no detectable enantiomeric excesses of the products were obtained. These results are ascribed to the absence of ester group at the α-position.
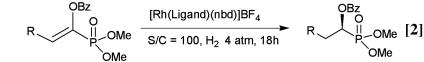 |
The asymmetric hydrogenation of dimethyl α-acetylaminoethenephosphonate with a rhodium complex of (R,R)-t-Bu-BisP* (39) proceeded in methanol at 4 atm of H2 pressure and was completed within 18 h to give in 90% ee the (R)-configuration product that is a precursor to alafosfalin (Eq. 1). This result demonstrates the potential usefulness of BisP*-Rh-catalyzed asymmetric hydrogenation for the preparation of optically active α-aminophosphonates.
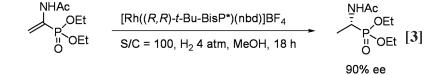 |
Results and Discussion
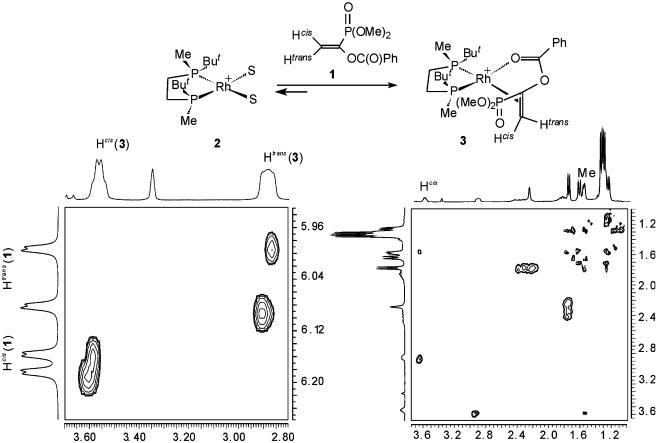
Formation of the Catalyst–Substrate Complex 3 and Determination of Its Structure in Solution. Addition of a 2-fold excess of substrate 1 to a deuteriomethanol solution of solvate complex 2 at –20°C yielded catalyst–substrate complex 3 (Fig. 1). The 1H, 13C, and 31P NMR spectra taken within the temperature interval from –100 to 60°C displayed only one set of signals corresponding to a single diastereomer of 3. We have attempted to detect the second possible diastereomer of 3 by carefully carrying out reactions 1 and 2 at –100°C and immediately placing of the sample thus obtained in the precooled to –100°C probe of the NMR spectrometer. However, this procedure gave NMR spectra identical with those obtained by cooling down a solution of preformed 3. The exchange cross-peaks observed in the phase-sensitive NOESY spectrum taken at 25°C (e.g., Fig. 1) confirmed the existence of the dynamic equilibrium between catalyst–substrate complex 3 and free substrate 1.
Fig. 1.
Section plots of the phase-sensitive 1H—1H NOESY spectrum of catalyst–substrate complex 3. (Left) Only negative (exchange) cross-peaks are shown. (Right) Only positive (NOE) cross-peaks are shown.
The assignment of signals in the NMR spectra of 3 was performed by using the routine 2D correlation NMR techniques. The signals of Hcis and Htrans in the 1H NMR spectrum could not be distinguished directly, because they displayed very similar sets of coupling constants (the largest couplings were 8 and 9 Hz, respectively). Nevertheless, the assignment was possible on the basis of the exchange spectroscopy data, because in 1 the assignment is straightforward, and the observation of the exchange cross-peaks between the corresponding signals (Fig. 1) was enough to make a confident assignment of the Hcis and Htrans protons in 3.
The chemical shift of the carbonyl carbon atom in 3 (δ = 178.9; compare with δ = 165.1 in uncoordinated substrate) and two vicinal C—P couplings observed for this signal (5 and 8 Hz) confirm the mode of the chelating coordination by the double bond and the benzoyloxy group of the substrate. By the use of phosphonate 1* labeled by 13C at α-carbon atom for the generation of catalyst–substrate complex 3*, the coupling 2J(α-C, Ptrans) was determined to be 53 Hz, which is more than two times higher than the previously determined values in the complexes of DIPHOS (40) and DIPAMP (41) with methyl (Z)-α-acetamidocinnamate. On the other hand, the coupling 2J(α-C, Ptrans) was <3 Hz in the loosely bound catalyst–substrate complexes of t-Bu-BisP* and alkyl-substituted enamides (32, 35). Hence, we conclude that the substrate is tightly bound in 3.
Furthermore, the nuclear Overhauser effect peak observed between the Hcis proton and one of the methyl groups (Fig. 1) allowed elucidation of the solution structure of 3, since in another diastereomer Hcis could display nuclear Overhauser effect only with a t-Bu group.
Thus, we conclude that the diastereomer observed in solution of catalyst–substrate complex 3 has the structure in which the substrate is si-coordinated. Therefore, similar to all catalyst–substrate complexes studied previously, the mode of the coordination of the substrate in 3 does not correspond to the stereochemistry of the hydrogenation product, and the stereoselective formation of 3 does not contribute to the enantioselection in asymmetric hydrogenation of 1.
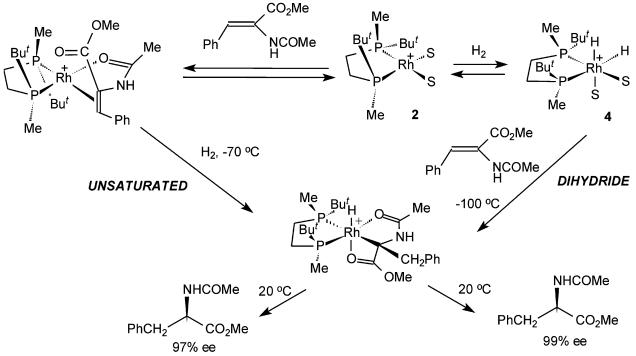
In our previous accounts we described the fast low-temperature reactions of solvate dihydride 4 produced by hydrogenation of the Rh-BisP* catalysts with methyl (Z)-α-acetamidocinnamate and other related substrates (31–37). These experiments simulated the dihydride mechanism of the asymmetric hydrogenation and demonstrated the possibility of an almost perfect enantioselection occurring after the hydrogenactivation step. In other experiments the catalyst–substrate complexes were hydrogenated to give the same monohydride intermediates as the reactions of dihydride 4 with substrates with comparable enantioselectivities (Fig. 2) (31, 32). The very similar results obtained in both methods did not allow comparison of the results of the stoichiometric experiments simulating dihydride or unsaturated mechanism with the results obtained under the catalytic conditions.
Fig. 2.
Operation of either unsaturated or dihydride pathway is possible on low-temperature hydrogenation of a catalyst–substrate complex.
The substrate is tightly bound in 3; hence, we presumed that, by comparing the results of the low-temperature reaction of 1 and 4 with the low-temperature hydrogenation of 3, more clear indications might be obtained on the nature of the pathway taking place under catalytic conditions in this case.
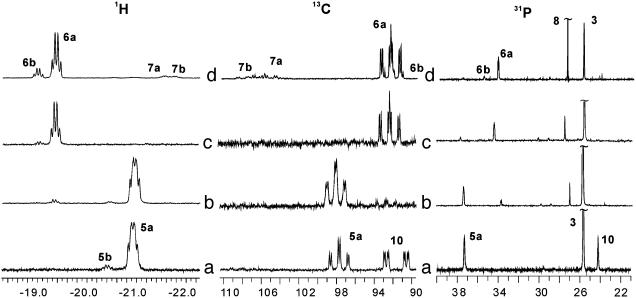
Low-Temperature Reaction of Solvate Dihydride 4 with Phosphonate 1. Two equivalents of phosphonate 1 were added to a deuteriomethanol solution of dihydride 4 (in equilibrium with solvate complex 2 and dihydrogen) at –100°C in an NMR tube, and the resulting sample was immediately placed into the probe of a NMR spectrometer precooled to –100°C. The immediately taken 1H NMR spectrum indicated the quantitative transformation of 4 into two monohydrides 5a and 5b formed in a 100:4 ratio (Table 3). In the 31P NMR spectrum each monohydride gave two doublets of the diphosphine ligands together with a singlet from the P(O)(OMe)2 group (Table 3 and Fig. 3a; assignments were proven with the 2D correlation and heteronucleus homodecoupling experiments). The chemical shifts of the hydride protons testify for the electron-acceptor substituent in trans-position to the hydride ligands in both 5a and 5b. Use of a 13C-labeled phosphonate 1a for the similar experiment allowed to detect the rhodium- and phosphorus-coupled signal of a quaternary carbon atom in 5a (Fig. 3 a and b), thus confirming that the first hydride is transferred to the β-position, similar to the hydrogenations of α-dehydroamino acids and phenyl-substituted enamides (31, 32).
Table 3.
Important data from 1H NMR (400 MHz, CD3OD, –50°C), 31P NMR (162 MHz, CD3OD, –50°C), and 13C NMR (100 MHz, CD3OD, –50°C) spectra and of the monohydride intermediates
| Compound | 5a | 5b | 6a | 6b |
|---|---|---|---|---|
| δH (1JRh—H, 2JP—H, 2JP—H) | -21.17 (32, 7, 14) | -20.67 (23, 23, 26) | -19.55 (25, 23, 23) | -19.17 (24, 22, 22) |
| δP1 (1JRh—P, 2JP—P) | 74.8 (84, 7) | 65.3 (85, <5) | 72.1 (96, 7) | 68.9 (96, 10) |
| δP2 (1JRh—P) | 83.9 (142) | 78.2 (140) | 81.4 (152) | 91.3 (149) |
| δP(OMe)2 | 37.28 | 37.08 | 32.9 | 34.3 |
| δCtert (1JP—C, 2JP—C, 1JRh—C) | 97.7 (94, 94, 19) | * | 92.8 (108, 92, 18) | 92.6 (110, 93, 19) |
Signal not found because of low concentration of 5b.
Fig. 3.
Section plots of 1H (400 MHz, CD3OD), 13C (100 MHz, CD3OD; spectra obtained by using α-13C-labeled 1), and 31P (162 MHz, CD3OD) NMR spectra of intermediates observed in low-temperature experiments. (a) The sample obtained by mixing the CD3OD solutions of 1 and 2 at –100°C, spectrum taken at –95°C. (b) The same sample as in a after raising the temperature to –70°C. (c) The same sample as in a after raising the temperature to –30°C and recooling to –60°C. (d) The sample obtained by hydrogenation of the CD3OD solution of 4 for 10 min at –30°C, spectrum taken at –60°C.
When the temperature of the sample was gradually raised, a rearrangement of the monohydride intermediates was observed by NMR (Fig. 3c). Thus, on raising temperature to –70°C, a new signal with δ = –19.55 (compound 6a) appeared in the hydride region of the 1H NMR spectrum. When temperature was raised to –30°C, the signals of 5 disappeared, and two species, 6a and 6b, were observed (Fig. 3c) in a ratio equal to that of 5a and 5b in the starting spectrum. The rearrangement of 5a,b to 6a,b is irreversible; recooling the sample to –60°C did not result in the reverse transformation.
Monohydrides 6a and 6b are relatively stable below –20°C; at higher temperatures they decompose, producing hydrogenation product 8 and solvate complex 2, which was immediately bound by the excess of the substrate yielding 3. The ee of 8 obtained after quenching of the NMR sample was 95–97%, which corresponds to the ratios 5a:5b and 6a:6b. We conclude, therefore, that major and minor diastereomers in both pairs of monohydride intermediates differ by configuration of the α-carbon atom.
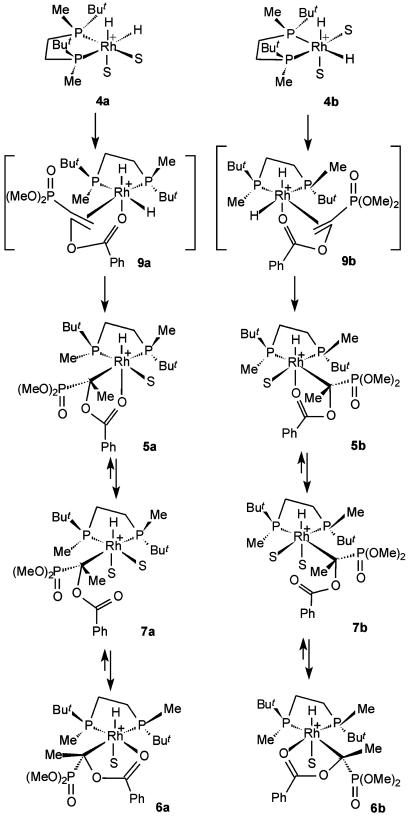
Fig. 4 displays the possible transformations occurring on reacting 4 and 1. Among eight possible dihydride intermediates, which can arise by displacement of two solvent molecules in either 4a or 4b, compounds 9a and 9b seem to be the most preferable candidates for the occurrence of the migratory insertion. Four isomers can be excluded, since they produce β-monohydrides, which are not observed experimentally, and another two have the coordinated double bond in the apical position, which seems to be less favorable due to the steric reasons.
Fig. 4.
Transformations taking place when 1 was reacted with 4 at –100°C.
The only substantial difference in structures 9a and 9b is the nature of the alkyl substituent being in relatively close contact with the chelate cycle of the substrate; it is methyl in 9a and t-Bu in 9b. Following the early suggestion made by Seebach et al. (42), we assume that this difference may be the main stereoregulating factor in the asymmetric hydrogenation, affecting the stability of both dihydride intermediates and transition states of the migratory insertion step, since it is generally accepted that the chelating coordination of a substrate must be conserved in the stereodetermining step.
Monohydrides 5a,b are directly formed after occurring of the migratory insertion step in 9a and 9b, respectively. The methyl group formed in the migratory insertion step remains at the same side of the chelate cycle as the vacant coordination site previously occupied by the hydride. Accordingly, the dimethoxyphosphinoyl group is located in the trans-position to the vacant site. We suggest that the driving force of the rearrangement of 5a,b into new species is the realization of terdentate coordination with the participation of the P(O)(OMe)2 group. Tridentate coordination may be achieved by reversible dissociation of the Rh—O bond yielding relatively unstable monohydrides 7a,b with monodentate coordination, rotation around the Rh—C bond, and reassociation of the carbonyl group (Fig. 4), affording compounds 6a,b in which the relative position of the P(O)(OMe)2 group and the vacant coordination site allows additional stabilization by the terdentate coordination. A direct evidence of the interconversion between the monohydride intermediates was obtained in an overnight 1H–1H exchange spectroscopy experiment at –60°C. Two cross-peaks were observed between 6a and 7a and between 6b and 7b. This observation corresponds to the scheme of interconversion of monohydrides 5–7 proposed above (Fig. 4). Apparently, cross-peaks between 5 and 7 escaped observation because of either their small concentration or relatively slow exchange rate.
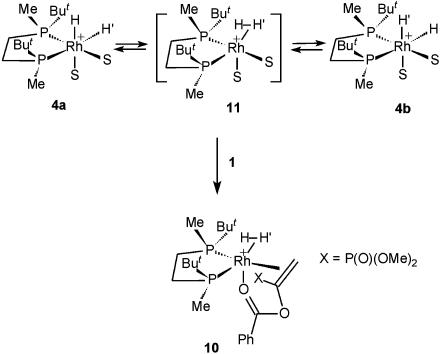
Another noteworthy observation was made in the experiments described above. An additional species stable only below –80°C was found in the 31P and 13C (of the labeled compound) NMR spectra of the sample obtained by mixing 1 and 4 at –100°C (Fig. 3a). In the 31P NMR spectrum two double doublets at δ = 62.2 (1JRh—P = 136 Hz, 2JP—P = 27 Hz) and δ = 73.8 (1JRh—P = 147 Hz, 2JP—P = 27 Hz) and singlet of the P(O)(OMe)2 group at δ = 23.0 were observed. In the 13C NMR spectrum this species displayed a multiplet of a quaternary carbon atom at δ = 91.7 (1JP—C = 213 Hz, 2JP—C = 39 Hz, 1JRh—C = 13 Hz). No hydride signals corresponding to this species were found in the 1H NMR spectrum. Several specially designed experiments showed that this species definitely does not form in the absence of hydrogen. Hence, we assume that intermediate 10 might be a complex of 3 with molecular hydrogen (Fig. 5). Recent computational studies showed that such complexes are real minima on the potential energy surface of the asymmetric catalytic hydrogenation (14, 43, 44) and, therefore, their experimental observation at low temperature is quite possible. Molecular hydrogen complex 10 can form by the reaction of substrate 1 with complex 11, which is equilibrating with 4a and 4b (Fig. 5) (31). It is not necessarily a precursor of 5, because the activation barrier of the oxidative addition in an observable isomer of the molecular hydrogen complex can be higher than dissociation of dihydrogen yielding 3 (14, 43, 44). Our attempts to prove the presence of bound hydrogen in 10 failed, probably because the signal is broad and the concentration of 10 is relatively small.
Fig. 5.
Possible side process in the low-temperature reaction of 1 with 4.
Hydrogenation of the Catalyst–Substrate Complex 3. Attempts to hydrogenate catalyst–substrate complex 3 at temperatures below –30°C were unsuccessful; no reaction occurred under these conditions. Hydrogenation under 2 atm of hydrogen for 10 min at –30°C gave an equilibrium mixture of monohydride intermediates 5–7 (Fig. 3d) together with some amount of the hydrogenation product 8. The main difference with the previous experiment is the increased concentration of 6b; the ratio 6a:6b was ≈5:1. It corresponded roughly to the 75% ee of 8 obtained after raising the temperature and quenching the sample.
The optical yield of 8 obtained in the catalytic conditions (88%) is intermediate between those obtained in the NMR experiments simulating dihydride (95%) and unsaturated (75%) mechanisms. Hence, the experimental data most probably testify to the possibility of the mixed mechanism in asymmetric hydrogenation of 1 catalyzed by 2. However, the dihydride mechanism itself seems to be more effective than the unsaturated pathway.
Experimental techniques and physicochemical data for all products of asymmetric hydrogenation are presented in Supporting Text, which is published as supporting information on the PNAS web site.
Supplementary Material
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BisP*, (S,S)-1,2-bis(alkylmethylphosphino)ethanes (alkyl = tert-butyl, 1,1-diethyl propyl, 1-adamantyl, 1-methylcyclohexyl, cyclohexyl, cyclopentyl, and isopropyl); MiniPHOS, (R,R)-bis(alkylmethylphosphino)methanes (alkyl = tert-butyl, cyclohexyl, isopropyl, and phenyl); ee, enantiomeric excess.
Footnotes
Mikolajezyk, M., Drawbowicz, J. & Kielbasinski, P. (1996) J. Stereoselective Synth. 9, 570A.
References
- 1.Kafarski, P. & Lejczak, B. (1991) Phosphorus Sulfur Silicon Relat. Elem. 63, 193–215. [Google Scholar]
- 2.Dhawan, B. & Redmore, D. (1987) Phosphorus Sulfur Relat. Elem. 32, 119–144. [Google Scholar]
- 3.Zeiss, H.-J. (1991) J. Org. Chem. 56, 1783–1788. [Google Scholar]
- 4.Zeiss, H.-J. (1994) Pestic. Sci. 41, 269–277. [Google Scholar]
- 5.Cull-Candy, S. G., Donnellan, J. F., James, R. W. & Lunt, G. G. (1976) Nature 262, 408–409. [DOI] [PubMed] [Google Scholar]
- 6.Hemmi, K., Takeno, H., Hashimoto, M. & Kamiya, T. (1982) Chem. Pharm. Bull. 30, 111–118. [DOI] [PubMed] [Google Scholar]
- 7.Jane, D. E., Jones, P. L. Pook, P. C., Tse, H.-W. & Watkins, J. C. (1994) Br. J. Pharmacol. 112, 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird, J., De Mello, R. C., Harper, G. P., Hunter, D. J., Karran, E. H., Markwell, R. E., Miles-Williams, A. J., Rahman, S. S. & Ward, R. W. (1994) J. Med. Chem. 37, 158–169. [DOI] [PubMed] [Google Scholar]
- 9.Stowasser, B., Budt, K.-H., Jian-Qi, L., Peyman, A. & Ruppert, D. (1992) Tetrahedron Lett. 33, 6625–6628. [Google Scholar]
- 10.Atherton, F. R., Hall, M. J., Hassel, C. H., Lambert, R. W., Lloyd, W. Y. & Ringrose, P. S. (1979) Antimicrob. Agents Chemother. 15, 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel, D. V., Rielly-Gauvin, K. & Ryono, D. E. (1990) Tetrahedron Lett. 31, 5587–5590. [Google Scholar]
- 12.Allen, J. G., Atherton, F. R., Hall, M. J., Hassall, C. H., Holmes, S. W., Lambert, R. W., Nisbet, L. J. & Ringrose, P. S. (1978) Nature 272, 56–58. [DOI] [PubMed] [Google Scholar]
- 13.Atherton, F. R., Hassall, C. H. & Lambert, R. W. (1986) J. Med. Chem. 29, 29–40. [DOI] [PubMed] [Google Scholar]
- 14.Landis, C. R. & Feldgus, S. (2001) Angew. Chem. Int. Ed. Engl. 39, 2863–2866. [DOI] [PubMed] [Google Scholar]
- 15.Kukhar, V. P., Soloshonok, V. A. & Solodenko, V. A. (1994) Phosphorus Sulfur Silicon Relat. Elem. 92, 239–264. [Google Scholar]
- 16.Sasai, H., Arai, S., Tahara, Y. & Shibasaki, M. (1995) J. Org. Chem. 60, 6656–6657. [Google Scholar]
- 17.Arai, T., Bongauchi, M., Sasai, H. & Shibasaki, M. (1996) J. Org. Chem. 61, 2926–2927. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi, T., Senda, T., Takaya, Y. & Ogasawara, M. (1999) J. Am. Chem. Soc. 121, 11591–11592. [Google Scholar]
- 19.Sawamura, M., Hamashima, H. & Ito, Y. (2000) Bull. Chem. Soc. Jpn. 73, 2559–2562. [Google Scholar]
- 20.Kuwano, R., Nishio, R. & Ito, Y. (1999) Org. Lett. 1, 837–839. [Google Scholar]
- 21.Schmidt, U., Oehme, G. & Krause, H. (1996) Synth. Commun. 26, 777–781. [Google Scholar]
- 22.Burk, M. J., Stammers, T. A. & Straub, J. A. (1994) Org. Lett. 1, 387–390. [Google Scholar]
- 23.Kitamura, M. Tokunaga, M. & Noyori, R. (1995) J. Am. Chem. Soc. 117, 2931–2932. [Google Scholar]
- 24.Kitamura, M., Tokunaga, M., Pham, T., Lubell, W. D. & Noyori, R. (1995) Tetrahedron Lett. 36, 5769–5772. [Google Scholar]
- 25.Goulioukina, N. S., Dolgina, T. M., Bondarenko, G. N., Beletskaya, I. P., Ilyin, M. M., Davankov, V. A. & Pfaltz, A. (2002) Tetrahedron: Asymmetry 14, 1397–1401. [Google Scholar]
- 26.Goulioukina, N. S., Dolgina, T. M., Beletskaya, I. P., Henry, J.-C., Lavergne, D., Ratovelomanana-Vidal, V. & Genet, J.-P. (2001) Tetrahedron: Asymmetry 12, 319–327. [Google Scholar]
- 27.Imamoto, T., Watanabe, J., Wada, Y., Masuda, H., Yamada, H., Tsuruta, H., Matsukawa, S. & Yamaguchi, K. (1998) J. Am. Chem. Soc. 120, 1635–1636. [Google Scholar]
- 28.Yamanoi, Y. & Imamoto T. (1999) J. Org. Chem. 64, 2988–2989. [DOI] [PubMed] [Google Scholar]
- 29.Gridnev, I. D., Yamanoi, Y., Higashi, N., Tsuruta, H., Yasutake, M. & Imamoto, T. (2001) Adv. Synth. Catal. 343, 118–136. [Google Scholar]
- 30.Imamoto, T. (2001) Pure Appl. Chem. 73, 373–376. [Google Scholar]
- 31.Gridnev, I. D., Higashi, N., Asakura, K. & Imamoto, T. (2000) J. Am. Chem. Soc. 122, 7183–7194. [Google Scholar]
- 32.Gridnev, I. D., Higashi, N. & Imamoto, T. (2000) J. Am. Chem. Soc. 122, 10486–10487. [Google Scholar]
- 33.Gridnev, I. D. & Imamoto, T. (2001) Organometallics 20, 545–549. [Google Scholar]
- 34.Gridnev, I. D., Higashi, N. & Imamoto, T. (2001) J. Am. Chem. Soc. 123, 4631–4632. [DOI] [PubMed] [Google Scholar]
- 35.Gridnev, I. D., Yasutake, M., Higashi, N. & Imamoto, T. (2001) J. Am. Chem. Soc. 123, 5268–5276. [DOI] [PubMed] [Google Scholar]
- 36.Yasutake, M., Gridnev, I. D., Higashi, N. & Imamoto, T. (2001) Org. Lett. 3, 1701–1704. [DOI] [PubMed] [Google Scholar]
- 37.Gridnev, I. D., Higashi, N. & Imamoto, T. (2001) Organometallics 20, 4542–4553. [Google Scholar]
- 38.Crepy, V.-L. & Imamoto, T. (2003) Adv. Synth. Catal. 345, 79–101. [Google Scholar]
- 39.Crépy, K. V. L. & Imamoto, T. (2002) Tetrahedron Lett. 43, 7735–7737. [Google Scholar]
- 40.Chan, A. S. S., Pluth, J. J. & Halpern, J. (1980) J. Am. Chem. Soc. 102, 5952–5954. [Google Scholar]
- 41.Brown, J. M., Chaloner, P. A. (1980) J. Am. Chem. Soc. 122, 3040–3048. [Google Scholar]
- 42.Seebach, D., Plattner, D. A., Beck, A., Wang, Y. M., Hunziker, D. & Petter, W. (1992) Helv. Chim. Acta 75, 2171–2209. [Google Scholar]
- 43.Landis, C. R., Hilfenhaus, P. & Feldgus, S. (1999) J. Am. Chem. Soc. 121, 8741–8754. [Google Scholar]
- 44.Feldgus, S. & Landis, C. R. (2000) J. Am. Chem. Soc. 122, 12714–12727. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.