Abstract
Background
The accurate diagnosis of lymph node (LN) metastasis is important for making treatment decisions for gastric cancer patients. This multicenter study evaluated the clinical performance of the one-step nucleic acid amplification (OSNA) assay (Sysmex Corp.), an automated system that detects cytokeratin 19 (CK19) mRNA, in detecting LN metastases in gastric cancer patients.
Methods
LNs retrieved from patients who had undergone gastric cancer surgery at one of the four Japanese hospitals involved in this study were divided into blocks at 2-mm intervals. Alternate blocks were examined with the OSNA assay and the remaining blocks were assessed histologically.
Results
A total of 394 LNs from 61 patients were examined. The concordance rate between the OSNA assay and the histological examination was 0.942 (95 % CI, 0.914–0.963). Sensitivity and specificity of the OSNA assay compared to the histological examination were 0.833 (95 % CI, 0.707–0.921) and 0.959 (95 % CI, 0.932–0.977), respectively. Discordant results were observed in 23 LNs (5.8 %), and these were mainly the result of tissue allocation bias and/or low CK19 protein expression.
Conclusion
The OSNA assay can detect lymph node metastases in gastric cancer patients as accurately as the histological examination of blocks sectioned at 2-mm intervals. The OSNA assay is a useful tool for the intraoperative diagnosis of LN metastasis in gastric cancer patients.
Keywords: Stomach neoplasm, Lymphatic metastasis, Intraoperative period, Nucleic acid amplification techniques, Keratin-19
Introduction
The accurate diagnosis of lymph node (LN) metastasis in gastric cancer patients is important for identifying prognostic factors, accurate staging, and planning further additional treatment strategies and the provision of postoperative adjuvant chemotherapy. The detection of LN metastases is commonly conducted using hematoxylin and eosin (H&E) staining of one section containing the largest dimension of the LN. However, LN metastases may be overlooked because of the random distribution of tumor cells throughout the LN. More accurate information about LN metastases can be obtained by conducting a more detailed histological examination, which involves the preparation and examination of many slides. However, this is time consuming and increases the workload of the surgeons and pathologists involved. It would therefore be ideal to replace histological examinations with a quick and simple molecular approach that is also cost effective.
Molecular biological approaches, such as the reverse transcription polymerase chain reaction (RT-PCR), have been developed for the detection of localized metastatic deposits in the LNs of patients with gastric cancer [1–3]. These methods can examine the whole lymph node and are expected to become alternatives to conventional histological examination. However, these tests are not used in clinical practice, probably because they are complex and time consuming. For example, although RT-PCR shows a higher sensitivity for revealing minor tumor deposits in LNs compared to conventional techniques, it requires several hours to obtain a final result.
Recently, Sysmex Corp. (Kobe, Japan) developed a one-step nucleic acid amplification (OSNA) assay, an automated system that uses the reverse-transcription loop-mediated isothermal amplification (RT-LAMP) method for gene amplification. In this system, the mRNA of the molecular marker cytokeratin 19 (CK19) is directly and rapidly amplified from the supernatant of homogenized LNs. The mRNA purification process that is usually performed in RT-PCR methods is not required in this assay. Results are available within 30 min for one LN, and four LNs can be analyzed simultaneously. The OSNA assay is already in clinical use for the diagnosis of LN metastases in breast cancer and colorectal cancer in Japan [4–6]. Furthermore, Yaguchi et al. [7] have defined the CK19 mRNA cutoff value required when using the OSNA assay to detect LN metastases in gastric cancer patients.
Although most colorectal cancer consists of well-differentiated adenocarcinomas, approximately 60 % of gastric cancer is composed of poorly differentiated adenocarcinomas or signet ring cell carcinomas, which may affect the OSNA assay [8].
The current multicenter study was conducted to evaluate the clinical performance of the OSNA assay in detecting LN metastases in gastric cancer patients.
Materials and methods
Patients and lymph nodes
Perigastric and suprapancreatic LNs were obtained from patients who underwent surgery for gastric cancer between June 2010 and December 2010 at four Japanese hospitals: Cancer Institute Hospital, Osaka Medical Center for Cancer and Cardiovascular Diseases, National Cancer Center Hospital, and Keio University Hospital. Patients who received preoperative or intraoperative adjuvant therapy and patients with a previous history of gastric cancer or other cancers were excluded from this study. Only lymph nodes from patients who had given their consent were used. During the postoperative LN retrieval procedure, four to eight LNs with a minor axis diameter of 8 mm or less were randomly selected for this study.
Methods
The fresh LNs retrieved from the resected stomach were directly cut into blocks at 2-mm intervals with cutting devices developed by Tsujimoto et al. [9], and nonadjacent blocks were subjected to either the OSNA assay or histological examination (Fig. 1). Blocks “a” and “c” were subjected to the OSNA assay, and blocks “b” and “d” were cut into 5-μm-thick sections, stained with H&E, and analyzed by histological examination. The concordance rate between the two methods was evaluated to confirm the accuracy of the OSNA assay. The sensitivity and specificity of the OSNA assay compared to histological examination were also evaluated.
Fig. 1.
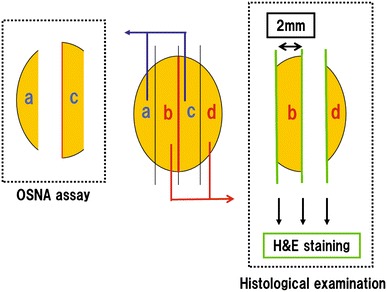
Lymph node processing. Lymph nodes with a short axis diameter of 8 mm or less were cut at 2-mm intervals, and nonadjacent blocks were subjected to either histological examination or the one-step nucleic acid amplification (OSNA assay). A subset of the sections prepared from the cut surfaces was stained with hematoxylin and eosin (H&E)
Patients with surgically retrieved LNs scored as negative by histological examination were classified as “node-negative patients”. “Node-positive patients” were defined as patients with LNs that had been histologically classified as positive. The specificity of the OSNA assay for detecting LN metastases in “node-negative patients” only was also evaluated.
For blinding purposes, identification codes were randomly assigned to blocks analyzed by either the OSNA assay or histological examination, to eliminate the possibility that the results from one method would bias those of the other method.
Histological examination
Histological examination was performed on three sections at 2-mm intervals on each LN, two sections on both sides of block “b” and one section of block “d” (Fig. 1). LNs with at least one observed tumor cell detected by H&E staining were classified as positive, and LNs lacking one observed tumor cell were classified as negative. To ensure objectivity, the final histological results were based on the conclusions of two independent pathologists.
OSNA assay
CK19 mRNA was used as a marker in the OSNA assay. Blocks “a” and “c” were homogenized using LYNORHAG lysis buffer (Sysmex, Kobe, Japan) and centrifuged at 10,000 g. LYNOAMP BC gene amplification reagent (Sysmex) was added to the supernatant, and CK19 mRNA in each lysate was amplified and detected with the RD-100i system (Sysmex), an automated molecular detection system that uses the RT-LAMP method. This method measures the time taken to exceed a predetermined threshold turbidity caused by magnesium pyrophosphate, a by-product of the amplification reaction. Amplification times were analyzed based on a previously generated standard curve, and the results of the assay were expressed as the number of CK19 mRNA copies per microliter (copies/μl). In this study, the cutoff value that distinguished between positive and negative LNs was set at 250 copies/μl, on the basis of on a previous study [7].
Further analysis of discordant cases
In this study, several LNs showed discordant results between the OSNA assay and the histological examination: these were subjected to further analysis to determine the causes of the discordance. To confirm the presence of tumor cells in the LNs, the remains of blocks “b” and “d”, which had been used for histological examination, were prepared as pairs of 5-μm-thick serial sections cut at 0.2-mm intervals. These were then stained with H&E and the anti-CK19 antibody (RCK108; DAKO, Glostrup, Denmark), respectively. These slides were evaluated for the presence or absence of tumor cells and their distribution. The remaining LN lysates obtained from blocks “a” and “c”, which had been used for the OSNA assay, were examined for CK19 protein expression by Western blot analysis.
Ethics
The entire study was conducted with the approval of the institutional review boards at each of the four institutes.
Statistical analysis
The target value for the concordance rate between the OSNA assay and the 2-mm-interval histological examination was set at 0.93. This value was obtained by defining the two methods as sufficiently equivalent providing the discordance rate was not more than 7 %. The 95 % confidence interval (95 % CI) for the concordance rate was calculated based on the F distribution. The study was judged to be effective if the lower limit did not fall below 0.83 (target value, 0.93–∆0.1).
Results
Three of the 64 participating patients were excluded by technical failure. A total of 431 samples were dissected from the 61 gastric cancer patients enrolled in this study (Table 1). Of these, 37 samples were not confirmed as LNs and were therefore excluded. The remaining 394 LNs were analyzed by the OSNA assay and histological examination. Histological examination confirmed that 32 of the 61 patients were “node-negative patients”. The specificity of the OSNA assay for detecting LN metastases in “node-negative patients” was determined using 211 LNs obtained from these 32 patients.
Table 1.
Patient demographics and baseline characteristics
| Categories | Number of patients (%) | Number of lymph nodes (%) |
|---|---|---|
| Total | 61 | 394 |
| Facilities | ||
| Cancer Institute Hospital | 21 (34 %) | 150 (38 %) |
| Osaka Medical Center for Cancer and Cardiovascular Diseases | 20 (33 %) | 137 (35 %) |
| National Cancer Center Hospital | 17 (28 %) | 95 (24 %) |
| Keio University Hospital | 3 (5 %) | 12 (3 %) |
| Age (years) | ||
| Median (range) | 63 (33–86) | |
| Lymph node dissection | ||
| D1 or D1+ | 23 (38 %) | |
| D2 | 38 (62 %) | |
| Histological type | ||
| Pap | 0 (0 %) | |
| tub1 | 4 (7 %) | |
| tub2 | 17 (28 %) | |
| por1 | 8 (13 %) | |
| por2 | 21 (33 %) | |
| Sig | 9 (15 %) | |
| Muc | 1 (2 %) | |
| Other | 1 (2 %) | |
| T factor in TNM (7th edition) | ||
| T1 | 24 (39 %) | |
| T2 | 8 (13 %) | |
| T3 | 11 (18 %) | |
| T4a | 17 (28 %) | |
| T4b | 1 (2 %) | |
| N factor in TNM (7th edition) | ||
| N0 | 34 (57 %) | |
| N1 | 10 (16 %) | |
| N2 | 10 (16 %) | |
| N3 | 7 (11 %) | |
| Lymphatic invasion | ||
| ly0 | 33 (53 %) | |
| ly1 | 18 (30 %) | |
| ly2 | 4 (7 %) | |
| ly3 | 5 (8 %) | |
| Not specified | 1 (2 %) | |
| Venous invasion | ||
| v0 | 35 (56 %) | |
| v1 | 20 (33 %) | |
| v2 | 4 (7 %) | |
| v3 | 1 (2 %) | |
| Not specified | 1 (2 %) | |
pap papillary adenocarcinoma, tub1 well-differentiated tubular adenocarcinoma, tub2 moderately differentiated tubular adenocarcinoma, por1 solid-type poorly differentiated adenocarcinoma, por2 non-solid type poorly differentiated adenocarcinoma, sig signet ring cell adenocarcinoma, muc mucinous adenocarcinoma
Concordance between the OSNA assay and histological examination
A total of 394 LNs obtained from 61 gastric cancer patients were used to determine concordance between the OSNA assay and histological examination (Table 2). The same diagnosis for 371 LNs (94.2 %) was made by both methods. Of the 371 LNs, 45 (11.4 %) were assessed as positive and 326 (82.7 %) as negative by both methods. Hence, the concordance rate between both methods was 0.942 [(45 + 326)/394 = 0.942; 95 % CI, 0.914–0.963], and the lower limit of the 95 % CI was higher than the target value of 0.83. Histological examination identified 54 positive LNs, 45 of which were also classified as positive by the OSNA assay. Thus, the sensitivity of the OSNA assay compared to histological examination was 0.833 (45/54 = 0.833; 95 % CI, 0.707–0.921). Histological examination identified 340 negative LNs, 326 of which were also classified as negative by the OSNA assay. Therefore, the specificity of the OSNA assay compared to histological examination was 0.959 (326/340 = 0.959; 95 % CI, 0.932–0.977). The 23 LNs (5.8 %) with discordant results underwent further analysis.
Table 2.
Results from the one-step nucleic acid amplification (OSNA) assay and the 2-mm-interval histological examination
| OSNA | Histological examination | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 45 | 14 | 59 |
| Negative | 9 | 326 | 335 |
| Total | 54 | 340 | 394 |
Specificity of the OSNA assay for detecting LN metastases in “node-negative patients”
Of the 61 gastric cancer patients enrolled in this study, 32 were classified as “node-negative patients” based on the results of histological examinations. The OSNA assay classified 209 of the 211 LNs obtained from these 32 patients as negative. Therefore, the specificity of the OSNA assay for detecting LN metastases in node-negative patients was 0.991 (95 % CI, 0.966–0.999).
Further analysis of discordant cases
Discordant results were recorded for 23 of 394 LNs (5.8 %): 14 LNs (3.6 %) from 11 patients were histologically negative but positive in the OSNA assay, and 9 LNs (2.3 %) from 5 patients were histologically positive but negative in the OSNA assay.
Of the 14 LNs that were histologically negative but positive in the OSNA assay, 12 (Nos. 1–12; Table 3) were derived from patients classified as “node-positive patients”. In addition, metastatic deposits were observed in the 0.2-mm-interval serial sections prepared from the remains of LN blocks “b” and “d” (Nos. 1 and 7; Table 3). CK19 protein expression was also observed by Western blot analysis in the remaining lysates obtained from five LNs prepared for the OSNA assay (Nos. 3, 7–10; Table 3). These results suggest that the OSNA assay is more sensitive than the histological examination of serial sections cut at 2-mm intervals.
Table 3.
Results from further analyses of the histologically negative, OSNA-positive lymph nodes
| Patient | LN no. | Histological type of primary tumor | Histological examination (0.2-mm interval) | Western blotting/CK19 protein | Metastasis in other LNs of the patient |
|---|---|---|---|---|---|
| A | 1 | por2 | Positive | − | − |
| 2 | Negative | − | |||
| B | 3 | por2 | Negative | + | + |
| 4 | Negative | − | |||
| C | 5 | por2 | Negative | − | + |
| 6 | Negative | − | |||
| D | 7 | tub2 | Positive | + | + |
| E | 8 | tub2 | Negative | + | + |
| F | 9 | por2 | Negative | + | + |
| G | 10 | tub2 | Negative | + | + |
| H | 11 | tub2 | Negative | − | + |
| I | 12 | por1 | Negative | − | + |
| J | 13 | por2 | Negative | − | − |
| K | 14 | por2 | Negative | − | − |
tub2 moderately differentiated tubular adenocarcinoma, por1 solid-type poorly differentiated adenocarcinoma, por2 non-solid type poorly differentiated adenocarcinoma
+ CK19 protein concentrations are >0.1 ng/μl
− CK19 protein concentrations are <0.1 ng/μl
In contrast, of the nine LNs that were histologically positive but negative in the OSNA assay, six (Nos. 15–20; Table 4) were obtained from two patients. Immunohistochemical staining of these six LNs with the anti-CK19 antibody revealed faint positive reactions in the metastatic deposits (Table 4). The primary tumors in these two patients also showed faint staining with the anti-CK19 antibody and were therefore believed to be gastric cancer with low CK19 expression levels. Metastatic deposits in the other three LNs (Nos. 21–23) showed positive immunohistochemical staining of CK19 protein; however, the Western blot results were negative for CK19 protein expression. The discordant results for these three LNs may be caused by the random distribution of cancer cells within each LN.
Table 4.
Results from further analyses of histologically positive, OSNA-negative lymph nodes
| Patient | LN no. | Histological type of primary tumor | CK19 expression of metastatic tumors in LNs by IHC | CK19 expression of primary tumors by IHC | Western blotting/CK19 protein | Metastasis in other LNs of the patient |
|---|---|---|---|---|---|---|
| L | 15 | por2 | Faint | Faint | − | − |
| 16 | Faint | − | ||||
| M | 17 | sig | Faint | Faint | − | + |
| 18 | Faint | − | ||||
| 19 | Faint | − | ||||
| 20 | Faint | − | ||||
| N | 21 | por2 | Positive | Not assessed | − | − |
| O | 22 | por2 | Positive | Not assessed | − | + |
| P | 23 | sig | Positive | Not assessed | − | + |
por2 non-solid type poorly differentiated adenocarcinoma, sig signet ring cell adenocarcinoma, IHC immunohistochemistry
+ CK19 protein is >0.1 ng/μl
− CK19 protein is <0.1 ng/μl
Histological type of tumor and OSNA assay
Patient-based analyses were additionally performed to evaluate the risk for discordance between differentiated- and undifferentiated-type adenocarcinoma (Table 5). In 21 patients with differentiated adenocarcinoma (Table 5a), the concordance rate between both methods was 0.952 [(6 + 14)/21 = 0.952; 95 % CI, 0.762–0.999], and with sensitivity and specificity of the OSNA assay were 1.000 (6/6 = 1.000; 95 % CI, 0.541–1.000) and 0.933 (14/15 = 0.933; 95 % CI, 0.681–0.998), respectively. In 40 patients with undifferentiated-type adenocarcinoma (Table 5b), the concordance rate between both methods was 0.775 [(10 + 21)/40 = 0.775; 95 % CI, 0.616–0.892], and with sensitivity and specificity of the OSNA assay were 0.769 (10/13 = 0.769; 95 % CI, 0.462–0.950) and 0.778 (21/27 = 0.778; 95 % CI, 0.577–0.914), respectively. Although the discordance was seen more often in undifferentiated-type tumors, there were no statistically significant differences between the two cohorts in accuracy (p = 0.143), sensitivity (p = 0.517), and specificity (p = 0.390).
Table 5.
Patient-based results from the OSNA assay and the 2-mm interval histological examination
| OSNA | Histological examination | Total | |
|---|---|---|---|
| Positive | Negative | ||
| a. Differentiated adenocarcinoma | |||
| Positive | 6 | 1 | 7 |
| Negative | 0 | 14 | 14 |
| Total | 6 | 15 | 21 |
| b. Undifferentiated-type carcinoma | |||
| Positive | 10 | 6 | 16 |
| Negative | 3 | 21 | 24 |
| Total | 13 | 27 | 40 |
Discussion
Yaguchi et al. [7] recently published the results of their preliminary assessment of the OSNA assay for the diagnosis of LN metastasis in gastric cancer patients. They bisected 162 LNs from 32 patients: the half of each section containing the largest dimension of the LN was examined with H&E staining, and the other half was analyzed by the OSNA assay and Western blot analysis. They reported that when 250 copies/μl of CK19 mRNA was set as the cutoff value to distinguish between the presence and absence of LN metastasis in gastric cancer patients, the concordance rate between the two methods was 94.4 %, and the sensitivity and specificity of the OSNA assay compared to histological examination were 88.9 and 96.6 %, respectively [7]. However, they were not able to validate the efficacy of the OSNA assay in gastric cancer patients, one reason being the small number of LNs examined. In addition, the histological diagnoses were not as extensive as those in the current study, as only one LN section with the largest dimension was examined.
The present multicenter study was a validation study undertaken to evaluate the clinical performance of the OSNA assay with a predefined CK19 mRNA in detecting LN metastases in gastric cancer patients. The efficacy and clinical utility of the OSNA assay has been proven in breast cancer and colon cancer. As shown in Table 1, gastric cancer is different from breast and colon cancer as it has a relatively high incidence of poorly differentiated histological tumor types. Therefore, the efficacy of the OSNA assay in an undifferentiated type of cancer needed to be demonstrated.
In this study, the OSNA assay was shown to provide a diagnostic ability equivalent to that of the postoperative 2-mm-interval histological examination, as the concordance rate between the two methods for 394 LNs from 61 patients was 0.942 (95 % CI, 0.914–0.963), in which the lower limit of the 95 % CI exceeded the predetermined target value of 0.83. In addition, this study also showed that the specificity of the OSNA assay for detecting LN metastases in 32 “node-negative patients” was 0.991 (95 % CI, 0.966–0.999).
There were 23 discordant results between the OSNA assay and histological examination: 14 LNs were histologically negative but positive in the OSNA assay, and 9 LNs were histologically positive but negative in the OSNA assay. As the LN blocks used for the OSNA assay and histological examination were obtained from different parts of the LN, discordant results between the methods cannot be completely avoided for reasons of tissue allocation bias: the metastasis could be localized in the blocks used for the OSNA assay or in the blocks for histology. In fact, McGrath et al. [10] reported that examination of three section levels from paraffin blocks containing LN tissue detected more metastatic deposits than examination of one section level only. Metastatic deposits were also detected in the additional sections of histologically negative LNs when investigating the discordant results in the present study. Fluctuation of the assay results should be also considered. However, the accuracy and repeatability of the OSNA assay systems are guaranteed by the manufacturer with validation studies, and the risk is thought to be quite low.
Analysis of the 0.2-mm-interval serial sections or Western blot analysis confirmed the existence of cancer cells in 6 of the 14 LNs (Nos. 1, 3, 7–10) that were histologically negative but OSNA positive. Another 6 LNs (No. 2, 4–6, 11, 12) were derived from “node-positive patients”. It is likely that the discordant results recorded for these 12 LNs (Nos. 1–12) were the result of the random distribution of metastatic deposits within the lymph nodes. We should also consider the possibility of false-positive results caused by contamination or pseudogenes. Lymph nodes with contaminating epithelial cells, benign intranodal inclusions, or iatrogenic dissemination of benign epithelial or tumor cells can cause false-positive diagnoses. In this study, we used disposable pipette chips and clean blender shafts for each assay step. Both positive and negative control samples were also measured simultaneously in each assay for quality control. Therefore, the risk of contaminations was considered to be quite low. There are drawbacks using CK19 mRNA because the concomitant amplification of pseudogenes in genomic DNA can lead to false-positive results. However, the RT-LAMP primers that we used in this study were designed not to amplify the known CK19 pseudogenes. In addition, the lymph node solubilization step in the OSNA assay was carried out at pH 3.5. At this pH, almost all genomic DNA precipitates out. Even when the sample still contained genomic DNA, DNA amplification is unlikely to occur in the OSNA assay because the RT-LAMP step is carried out at 65 °C, a temperature at which genomic DNA typically does not denature.
In contrast, nine LNs were histologically positive but classified as negative by the OSNA assay. CK19-positive tumors were observed histologically in three of these LNs (Nos. 21–23), but Western blot analysis showed no expression of CK19 protein in the lysates obtained from the corresponding OSNA assay LN blocks. These three LNs were obtained from different hospitals. We evaluated the RNA integrity number for all lymph nodes, and that of those three LNs were 5.9, 6.6, and 7.6, respectively [11], which means their qualities were good enough to be analyzed. These data suggest that the discordant results were caused by the random distribution of metastatic deposits within these three LNs. The other six LNs (Nos. 15–20) were obtained from two patients with low CK19 expression levels. Low expression levels of CK19 were detected in only two of the 61 (3.3 %) cases. Yaguchi et al. [7] also reported high frequency of CK19 expression in gastric cancer tissues (206/209 cases, 98.6 %). However, it should be noted that some undifferentiated-type tumors do show low CK19 expression levels, and it is recommended to verify CK19 expression with immunohistochemical staining from a preoperative biopsy specimen in this type of tumor.
From the point of view of histological type, sensitivity in the patients with histologically differentiated adenocarcinoma was 1.000 (95 % CI, 0.541–1.000) and that in the patients with undifferentiated-type carcinoma was 0.769 (95 % CI, 0.462–0.950; p = 0.517). One of three patients with results of positive histology and negative OSNA had micrometastasis, which was 0.25 mm in diameter in 1 of 53 LNs (patient N; Table 4, data not shown). The discordance was thought to be caused by the random distribution of metastatic deposits within the LN. The other two patients had tumors with low CK19 expression levels (patients L and M; Table 4).
There are several limitations to the diagnostic accuracy of lymphatic metastasis detection, especially in intraoperative histological analysis using frozen sections. The main reason for the relatively low diagnostic accuracy of frozen section analyses is the distribution of metastatic cancer cells within LNs. LN metastasis examination is commonly conducted using H&E staining on only one LN section containing the largest dimension of the LN. LN metastases may be overlooked because of the random localization of tumor cells throughout the LN. Although more detailed histological examination of LNs can provide more accurate information about LN metastases, it may be impossible to examine the entire LN intraoperatively using frozen sections, and this may lead to false-negative results. To improve the sensitivity of the detection of micrometastases, immunohistochemistry (IHC) for a particular marker, for example pan-cytokeratin in breast cancer patients, has been employed [12]. However, this thorough assessment of formalin-fixed tissue is not suitable for intraoperative examinations. Therefore, a rapid, highly sensitive, and specific method that can be used for the intraoperative assessment of the metastatic status of LNs must be established.
In the present study, the OSNA assay was shown to possess a diagnostic ability equivalent to that of the postoperative 2-mm-interval histological examination. In this study, 39 of 61 patients (63.9 %) had poorly differentiated adenocarcinoma. The diagnostic accuracy of the OSNA assay was confirmed regardless of the degree of histological differentiation. In addition, results were available within 30 min for one LN, and four LNs could be analyzed simultaneously. As only some parts of each lymph node were analyzed by the OSNA assay and others were examined histologically, the results were influenced by tissue allocation bias. If the entire lymph node was examined by the OSNA assay, the results would not be affected by the random distribution of cancer cells within each lymph node. The accuracy and promptness of the OSNA assay make it a useful tool for the intraoperative diagnosis of lymph node metastasis.
The OSNA assay is already in clinical use for analyzing sentinel lymph node biopsies in breast cancer [5, 9]. The utility and feasibility of whole LN assay by OSNA in breast cancer have been reported [13, 14]. In a future clinical practice, the whole sentinel lymph node would be examined by the OSNA assay in gastric cancer surgery in the same way.
The usefulness of sentinel lymph node biopsies in gastric cancer surgery has been reported by Kitagawa et al. [15–18]. The Japan Society of Sentinel Node Navigation Surgery has conducted a prospective multicenter trial of sentinel node (SN) mapping by a dual tracer method using radioactive colloid and blue dye [19]. SN mapping has been performed for 397 patients with clinically diagnosed early gastric cancer. The detection rate of radioactive or blue sentinel nodes using this procedure was 97.5 % (387/397). The sensitivity of this method for detecting metastases based on SN status was therefore 93 % (53/57). The accuracy of metastatic status based on SN was 99 % (383/387). The OSNA assay may be useful in sentinel node navigation surgery for gastric cancer as well as for breast cancer.
The OSNA assay can facilitate the highly accurate detection of LN metastases in gastric cancer patients quickly and easily. The accuracy of intraoperative detection of LN metastases from frozen sections or imprint cytology is lower than that from postoperative histological examination in patients with gastric cancer, breast cancer, and melanoma [20, 21]. As the OSNA assay demonstrated a diagnostic ability equivalent to that of the postoperative 2-mm-interval histological examination, this assay will be a useful tool for the intraoperative diagnosis of LN metastases in gastric cancer patients.
Acknowledgments
We thank Mr. K. Nakabayashi and Mr. D. Kobayashi from Sysmex Corp. Mr. Nakabayashi made an enormous contribution to the current study in RT-PCR. Mr. Kobayashi has been tolerant and supportive in monitoring four hospitals. The study was supported by Sysmex Corporation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must, therefore, be hereby marked advertisement in accordance with 18 USC Section 1734 solely to indicate this fact.
Conflict of interest
No potential conflicts of interest were disclosed.
References
- 1.Ajisaka H, Miwa K. Micrometastases in sentinel nodes of gastric cancer. Br J Cancer. 2003;89:676–680. doi: 10.1038/sj.bjc.6601183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arigami T, Natsugoe S, Uenosono Y, Mataki Y, Ehi K, Higashi H, et al. Evaluation of sentinel node concept in gastric cancer based on lymph node micrometastasis determined by reverse transcription-polymerase chain reaction. Ann Surg. 2006;243:341–347. doi: 10.1097/01.sla.0000201453.65534.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumoto M, Natsugoe S, Ishigami S, Nakashima S, Nakajo A, Miyazono F, et al. Lymph node micrometastasis and lymphatic mapping determined by reverse transcriptase-polymerase chain reaction in pN0 gastric carcinoma. Surgery (St. Louis) 2002;131:630–635. doi: 10.1067/msy.2002.124632. [DOI] [PubMed] [Google Scholar]
- 4.Godey F, Leveque J, Tas P, Gandon G, Poree P, Mesbah H, et al. Sentinel lymph node analysis in breast cancer: contribution of one-step nucleic acid amplification (OSNA) Breast Cancer Res Treat. 2012;131:509–516. doi: 10.1007/s10549-011-1808-4. [DOI] [PubMed] [Google Scholar]
- 5.Tamaki Y, Akiyama F, Iwase T, Kaneko T, Tsuda H, Sato K, et al. Molecular detection of lymph node metastases in breast cancer patients: results of a multicenter trial using the one-step nucleic acid amplification assay. Clin Cancer Res. 2009;15:2879–2884. doi: 10.1158/1078-0432.CCR-08-1881. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto H, Sekimoto M, Oya M, Yamamoto N, Konishi F, Sasaki J, et al. OSNA-based novel molecular testing for lymph node metastases in colorectal cancer patients: results from a multicenter clinical performance study in Japan. Ann Surg Oncol. 2011;18:1891–1898. doi: 10.1245/s10434-010-1539-5. [DOI] [PubMed] [Google Scholar]
- 7.Yaguchi Y, Sugasawa H, Tsujimoto H, Takata H, Nakabayashi K, Ichikura T, et al. One-step nucleic acid amplification (OSNA) for the application of sentinel node concept in gastric cancer. Ann Surg Oncol. 2011;18:2289–2296. doi: 10.1245/s10434-011-1591-9. [DOI] [PubMed] [Google Scholar]
- 8.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 9.Tsujimoto M, Nakabayashi K, Yoshidome K, Kaneko T, Iwase T, Akiyama F, et al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res. 2007;13:4807–4816. doi: 10.1158/1078-0432.CCR-06-2512. [DOI] [PubMed] [Google Scholar]
- 10.McGrath S, Cross S, Pritchard SA. Histopathological assessment of lymph nodes in upper gastrointestinal cancer: does triple levelling detect significantly more metastases? J Clin Pathol. 2007;60:1222–1225. doi: 10.1136/jcp.2006.045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torrenga H, Rahusen FD, Meijer S, Borgstein PJ, van Diest PJ. Sentinel node investigation in breast cancer: detailed analysis of the yield from step sectioning and immunohistochemistry. J Clin Pathol. 2001;54:550–552. doi: 10.1136/jcp.54.7.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellano I, Macri L, Deambrogio C, Balmativola D, Bussone R, Ala A, et al. Reliability of whole sentinel lymph node analysis by one-step nucleic acid amplification for intraoperative diagnosis of breast cancer metastases. Ann Surg. 2012;255:334–342. doi: 10.1097/SLA.0b013e31823000ed. [DOI] [PubMed] [Google Scholar]
- 14.Osako T, Iwase T, Kimura K, Yamashita K, Horii R, Yanagisawa A, et al. Intraoperative molecular assay for sentinel lymph node metastases in early stage breast cancer: a comparative analysis between one-step nucleic acid amplification whole node assay and routine frozen section histology. Cancer (Phila) 2011;117:4365–4374. doi: 10.1002/cncr.26060. [DOI] [PubMed] [Google Scholar]
- 15.Kitagawa Y, Fujii H, Mukai M, Kubota T, Ando N, Watanabe M, et al. The role of the sentinel lymph node in gastrointestinal cancer. Surg Clin N Am. 2000;80:1799–1809. doi: 10.1016/S0039-6109(05)70262-0. [DOI] [PubMed] [Google Scholar]
- 16.Kitagawa Y, Kitajima M. Diagnostic validity of radio-guided sentinel node mapping for gastric cancer: a review of current status and future direction. Surg Technol Int. 2006;15:32–36. [PubMed] [Google Scholar]
- 17.Kitagawa Y, Kitano S, Kubota T, Kumai K, Otani Y, Saikawa Y, et al. Minimally invasive surgery for gastric cancer—toward a confluence of two major streams: a review. Gastric Cancer. 2005;8:103–110. doi: 10.1007/s10120-005-0326-7. [DOI] [PubMed] [Google Scholar]
- 18.Kitagawa Y, Fujii H, Kumai K, Kubota T, Otani Y, Saikawa Y, et al. Recent advances in sentinel node navigation for gastric cancer: a paradigm shift of surgical management. J Surg Oncol. 2005;90:147–151. doi: 10.1002/jso.20220. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, et al. Prospective multicenter trial of sentinel node mapping for gastric cancer. J Clin Oncol 2009;27(suppl): abstract 4518. [DOI] [PubMed]
- 20.Tanis PJ, Boom RP, Koops HS, Faneyte IF, Peterse JL, Nieweg OE, et al. Frozen section investigation of the sentinel node in malignant melanoma and breast cancer. Ann Surg Oncol. 2001;8:222–226. doi: 10.1007/s10434-001-0222-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ, Moon HG, Park ST, Choi SG, Hong SC, Jung EJ, et al. The value of intraoperative imprint cytology in the assessment of lymph node status in gastric cancer surgery. Gastric Cancer. 2005;8:245–248. doi: 10.1007/s10120-005-0347-2. [DOI] [PubMed] [Google Scholar]


