Abstract
Ethyl 4-amino-2-fluorophenylpiperazin-1-carboxylates containing a 1,3-oxazol(idin)e, 5-thioxo-1,2,4-triazole, 1,3,4-thiadiazole, 5-thioxo-1,3,4-oxadiazole, or 1,3-thiazole nucleus were obtained starting from ethyl piperazine-1-carboxylate (1) by several steps. The treatment of amine, 3 or hydrazide, 9 with several aromatic aldehydes generated the corresponding arylmethyleneamino (3a–f) or arylidenehydrazino (12a–c) compounds. The Mannich reaction between the 1,2,4-triazole or 1,3,4-oxadiazole compounds and 7-aca produced cephalosporanic acid derivatives. Penicillanic acid derivatives were obtained when 6-apa was used in the Mannich reactions. The synthesized compounds were screened for their antimicrobial, antilipase, and antiurease activities. Some of them were found to possess good-moderate antimicrobial activity against the test microorganisms. Two compounds exhibited antiurease activity, and four of them displayed antilipase activity.
Keywords: Piperazine; 1,3-Oxa(thia)zole; 5-Oxo-1,3-oxazolidine; 1,2,4-Triazole; 7-Aminocephalosporanic acid; 6-Aminopenicillanic acid; Biological activity
Introduction
The limitations of the existing antibacterial drugs caused by various reasons including drug resistance, the serious side effects, and/or lack of efficacy made infectious diseases a vicious cycle. In addition, the treatment of resistant strains requires a prolonged therapy containing the use of more toxic drugs and increases the financial burden. The rising prevalence of multi-drug resistant bacteria continues to serve medicinal chemists to search and discove novel antimicrobial agents effective against pathogenic microorganisms resistant to current treatment.
Among the strategies addressed to the synthesis of compounds possessing antimicrobial activity, the syntheses of hybrid molecules incorporating different heterocyclic moieties have been attracting widespread attention (Mallikarjuna et al., 2009).
A number of N-containing heterocyclic compounds constitute important building blocks in organic and medicinal chemistry. For example, triazoles have been shown to possess a number of desirable activities in the context of medicinal chemistry. Ribavirin (antiviral), rizatriptan (antimigraine), alprazolam (psychotropic), fluconazole, and itraconazole (antifungal) are the best examples for potent drugs possessing triazole nucleus (Holla et al., 2006; Walczak et al., 2004; Jones et al., 1965; Ashok et al., 2007). Tazobactam, a β-lactamase inhibitor is the other best known example of triazole containing structures with the broad spectrum antibiotic piperacillin (Kategaonkar et al., 2010).
Substituted piperazines constitute another class of important pharmacophores, which are found in many marketed drugs, such as the HIV protease inhibitor, Crixivan (Chaudhary et al., 2006). Ciprofloxacin, norfloxacin, pefloxacine, ofloxacin, and enoxacin are fluoroquinolone class antibacterial drugs characterized by having a piperazine moiety at C-7 of quinolone skeleton, and they have been used for the treatment of bacterial infections (Foroumadi et al., 2005).
The compounds having a thiazolidinone nucleus are of interest due to their broad spectrum of biological activities such as bactericidal, fungicidal, antimicrobial, antiproliferative, antiviral, anticonvulsant, anticancer, and anti-inflammatory activities (Vicini et al., 2008; Wang et al., 2011; Lv et al., 2010; Metwally et al., 2010; Balzarini et al., 2009; Havrylyuk et al., 2009; Subtelna et al., 2010; Mushtaque et al., 2012).
Mannich bases, which are known to be physiologically reactive since their basic function rendering the molecule soluble in aqueous solvents when it is transformed into aminium salt, have been reported as potential biological agents (Karthikeyan et al., 2006). N-Mannich bases have been used successfully to obtain prodrugs of amine as well as amide-containing drugs (Zhao et al., 2009). Some Mannich bases derived from 1,2,4-triazole nucleus have been reported to possess protozocidal and antibacterial activity (Ashok et al., 2007; Almajan et al., 2009; Bayrak et al., 2009, 2010; Demirbas et al., 2009; Bektas et al., 2010; Patole et al., 2006).
Schiff bases have gained importance in medicinal and pharmaceutical fields due to their most versatile properties as organic synthetic intermediates and also possessing a broad range of biological activities, such as antituberculosis, anticancer, analgesic and anti-inflammatory, anticonvulsant, antibacterial, and antifungal activities (Patole et al., 2006, Hearn and Cynamon, 2004; Ren et al., 2002; Demirbas et al., 2002; Lohray et al., 2006).
We envisage that hybrid compound incorporating a 4-(2-fluorophenylene)-piperazine core with several heterocyclic moieties responsible for biological activity in a single molecular frame could lead to the novel potent antimicrobial and antiurease agents. Highly substituted piperazines can be expected to increase antimicrobial activity probably by enhancing lipophilicity of molecule.
In continuation of our research program on the synthesis of hybrid molecules containing various heterocyclic moieties, we planned the synthesis of 4-(2-fluorophenyl)piperazine derivatives along with their antimicrobial and antiurease activities.
Results and discussion
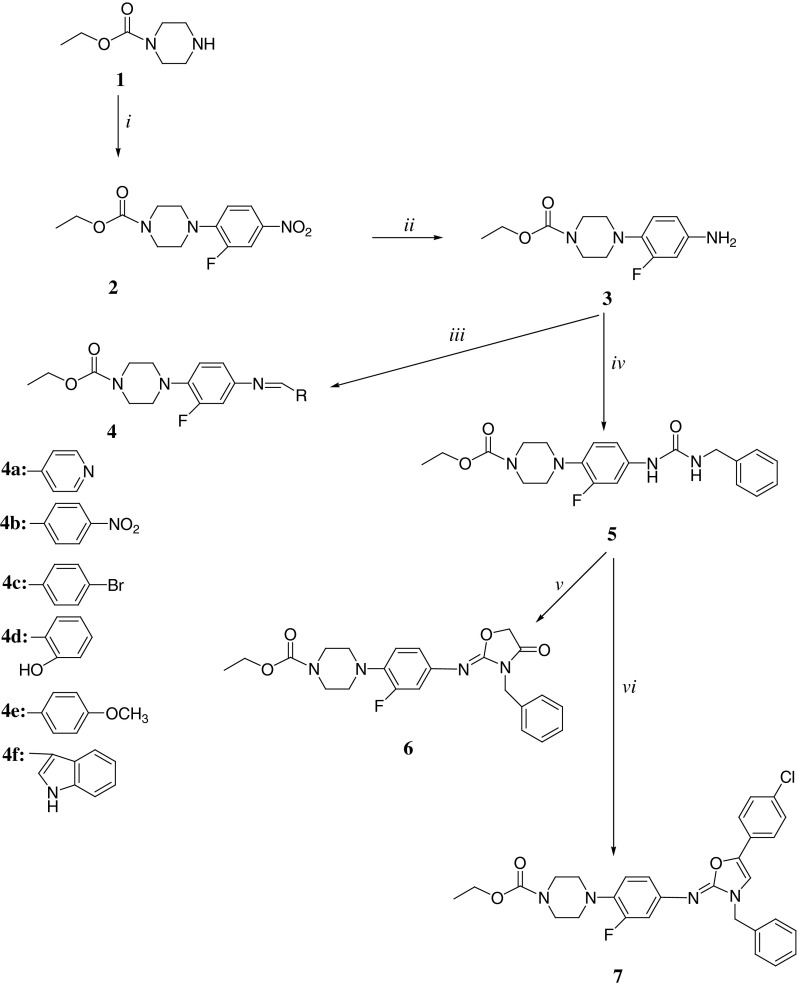
The main aim of the present study is the synthesis and antimicrobial activity evaluation of new piperazine derivatives incorporating several heterocyclic moieties including 1,3-oxadiazole, 1,2,4-triazole, 1,3-oxa(thia)zole, penicillanic acid, and/or cephalosporanic acid. Synthesis of the intermediate and target compounds was performed according to the reactions outlined in Schemes 1, 2, and 3. The starting compound ethyl 1-piperazinecarboxylate (1) was provided commercially.
Scheme 1.
i 3,4-Difluoronitrobenzene in ethanol, reflux for 6 h. ii Pd–C, hydrazine hydrate in n-butanol, reflux for 7 h. iii Indole-3-carboxaldehyde in absolute ethanol, irradiation by MW at 150 W, 110 °C for 30 min. iv Benzylisothiocyanate in absolute ethanol, reflux for 10 h. v Ethyl bromoacetate in absolute ethanol, dried sodium acetate, reflux for 13 h. vi 4-Chlorophenacylbromide in absolute ethanol, dried sodium acetate, reflux for 11 h
Scheme 2.
i Ethyl bromoacetate, Et3N, THF, rt for 14 h. ii Hydrazine hydrate in ethanol, reflux for 14 h. iii 4-Fluorophenylisothiocyanate or phenylisothiocyanate in absolute ethanol, reflux for 10 h. iv H2SO4, rt for 2 h. v NaOH in water, reflux for 3 h. vi 7-Aca, HCHO, Et3N in THF, rt, for 4 h. vii 6-Apa, HCHO, Et3N in THF, rt, for 4 h. vii 4-Chlorophenacylbromide in absolute ethanol, dried sodium acetate, reflux for 12 h
Scheme 3.
i 3-Hydroxy-4-phenoxybenzaldehyde, pyridine-4-carbaldehyde, 2-hydroxybenzaldehyde in absolute ethanol, irradiation by MW at 200 W, 140 °C for 30 min. ii CS2 and KOH in ethanol, reflux for 13 h. iii 7-Aca, HCHO, Et3N in THF, rt, for 4 h. iv 6-Apa, HCHO, Et3N in THF, rt, for 4 h
Ethyl 4-(4-amino-2-fluorophenyl)piperazine-1-carboxylate (3), that was obtained starting from compound 1 by two steps, was converted to the corresponding arylmethylenamino derivatives (4a–f) by the treatment with several aromatic aldehydes. In the FT-IR and 1H NMR spectra of these compounds, no signal pointing the –NH2 group was seen. Instead, additional signals derived from aldehyde moiety were recorded at the related chemical shift values in the 1H NMR spectra.
The cyclocondensation of compound 5, that was obtained from the reaction of 4 with benzylisocyanate, with ethyl bromoacetate or 4-chlorophenacyl bromide produced the corresponding hybrid molecules incorporating a 4-oxo-1,3-oxazolidine (6) or 4-chlorophenyl)-1,3-oxazole (7) nucleus in the 2-fluorophenylpiperazine-1-carboxylate skeleton. The 1H and 13C NMR spectra of compound 7 exhibited additional signals at aromatic region originated from 4-chlorophenyl nucleus as a result of condensation. Moreover, the elemental analyses and mass spectral data of derivatives 6 and 7 were compatible with the suggested structures.
The treatment of compound 3 with ethyl bromoacetate at room temperature in the presence of triethylamine resulted in the formation of compound 8. When compound 8 was converted to the corresponding hydrazide (9) by refluxing with hydrazine hydrate, the signals originated from ester function was disappeared in the 1H and 13C NMR spectra. Instead, new signals due to –NHNH2 protons were seen at 5.93 and 9.09 ppm. Meanwhile, the stretching frequency band of this group was recorded at 3,313 cm−1 as a wide signal characteristic for the hydrazide structure. Compounds 6 and 7 gave mass fragmentation confirming the proposed structures.
The synthesis of compounds 10 and 11 was carried out by the treatment of compound 7 with the corresponding isothiocanates. These compounds displayed spectroscopic data and elemental analysis results consistent with the assigned structures.
The intramolecular cyclization of compound 10 generated the corresponding 1,3,4-thiazole compound (12) in acidic media. On the other hand, the basic treatment of compounds 10 and 11 caused to the cyclization of the (arylamino)carbonothioylhydrazino side change leading to the formation of 5-thioxo-4,5-dihydro-1H-1,2,4-triazol derivatives (13 and 14). With the conversion of compounds 10 and 11 to compounds 12–14, two of NH signals were disappeared in the 1H NMR spectra. It is well-known that type of compounds can stay in thioxo or mercapto tautomeric form. In the present study, compounds 13 and 14 are present predominately in the thioxo form as it was shown by the C=S band at 1,244–1,250 cm−1 in the FT-IR spectra of these compounds. Furthermore, the 1H NMR spectra of compounds 13 and 14 revealed clearly the absence of the signal originated from SH proton, instead of that, two signals due to NH proton on 1,2,4-triazol ring was recorded at 10.45 (for 13) or 11.27 (for 14), that is characteristic for 4,5-dihydro-1H-1,2,4-triazoles.
The synthesis of Mannich bases (15–17) was performed by the reaction of compounds 13 and 14 with 6-aminopenicillanic acid, 6-apa (for 17) or 7-aminocephalosporanic acid, 7-aca (for 15 and 16) in tetrahydrofuran at room temperature in the presence of triethylamine and formaldehyde. The occurrence of the alkylaminomethylation was provided by the disappearance of signal for the proton at the N-1 nitrogen of the 1,2,4-triazole ring. Moreover, in 1H and 13C NMR spectra, additional signal corresponding to the 6-apa or 7-aca-ammonium salt was recorded at the related chemical shift value.
The conversion of arylcarbonothioylhydrazino side change to 4-chlorophenyl-3-phenyl-1,3-thiazole ring (18) was accomplished with the treatment of 4-chlorophenacyl bromide. This compound was characterized by spectroscopic techniques including 1H NMR, 13C NMR, FT-IR, EI-MS, and elemental analysis.
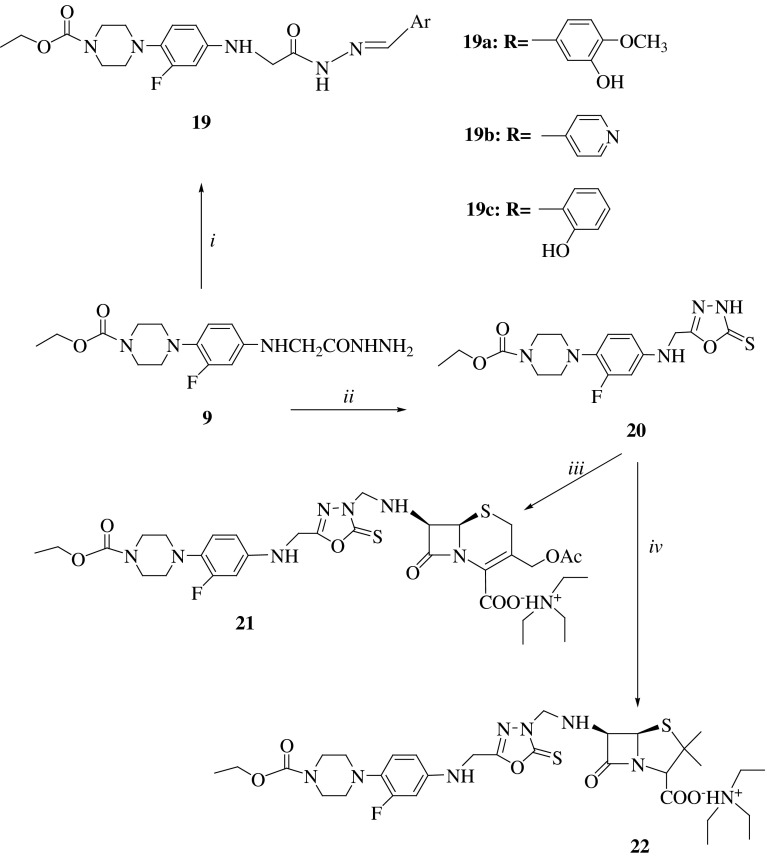
The synthesis of ethyl arylidenehydrazino-piperazine-1-carboxylate derivatives (19a–c) was performed by microwave irradiation of compound 9 with several aromatic aldehydes namely 3-hydroxy-4-methoxybenzaldehyde, pyridine-4-carbaldehyde, and 2-hydroxybenzaldehyde. In the FT-IR spectra of these arylidenehydrazino compounds, absorption bands characteristic for NH groups were visible in the ranges of 3,357–3,181 cm−1. Another piece of evidence for condensation was the appearance of a signal as singlet integrating for one proton in the 1H NMR spectra, which corresponds to the N=CH proton of azomethyne group. Moreover, these compounds gave mass fragmentation and elemental analysis confirming the proposed structures.
Ethyl 4-(2-fluoro-4-{[(5-thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl]amino} phenyl)piperazine-1-carboxylate (20) was prepared from the reaction of compound 9 with CS2 in the basic media. The attempts for aminoalkylations of compound (20) by Mannich reaction allowed the isolation of the corresponding products (21 and 22) after 4 (for 21) or 6 h (for 22) at room temperature. This idea originated from the intent to introduce the penicillanic acid or cephalosporanic acid nucleus to (piperazin-1-yl)-2-thioxo-1,3,4-oxadiazole skeleton. As different from 20, the NMR spectra of the obtained Mannich bases (21 and 22) displayed additional signals derived from penicillanic- or cephalosporanic-acid moiety and –CH2—linkage at the related shift and integral values as D2O nonexchangeable signals.
Among the synthesized compounds ethyl 4-(2-fluoro-4-nitrophenyl)piperazine-1-carboxylate (2) exhibited activity on Bacillus cereus (Bc), that is Gram positive spore bacillus. With the reduction of nitro group of 2 to amine (compound 3), additional activities towards Staphylococcus aureus (Sa), that is Gram positive coccus, Candida albicans (Ca), and Saccharomyces cerevisiae (Sc), which are yeast like fungi. For the imine compounds (4a–f), the highest activity was observed against Mycobacterium smegmatis (Ms) that is an atypical tuberculosis factor leading mortality, with the inhibition zone varying between 10 and 25 mm. The compounds containing 1,2,4-triazole and cephalosporanic- or penicillanic-acid moiety (compounds 15–17) displayed good-moderate activity on some of the test microorganisms. The highest activity was observed for compound 17 on Bc with the inhibition zone of 16 mm. This result is better than standard drug ampicillin. Other compounds containing penicillanic acid or cephalosporanic acid core (21 and 22) displayed good-moderate activity against the test microorganisms.
The synthesized compounds were assayed for their in vitro urease inhibitory activity against Jack bean urease. Two of those compounds showed perfect urease inhibition. No inhibitory effect was detected for other compounds. Thiourea with IC50 value 54.56 ± 4.17 μg mL−1 was used as standard inhibitor. Among tested compounds, compound 15 was found to be the best inhibitory effect against urease with an IC50 value of 4.67 ± 0.53 μg mL−1. At the various final concentrations the compound 15 showed more inhibitory effect than standard urease inhibitor thiourea. Also, compound 17 has the highest inhibitory activity than thiourea. These compounds might be considered as potential antibiotics to treat infections.
All compounds were evaluated with regard to pancreatic lipase activity and compounds 12, 13, 14, and 15, which are 1,3,4-thiadizole or 1,2,4-triazole derivatives including also 4-fluorophenylpiperazine nucleus, showed moderate anti-lipase activities at final concentration of 6.25 μg mL−1. No inhibitory effect was detected for other compounds. Orlistat, known pancreatic lipase inhibitor used as anti-obesity drug, showed inhibitory effect by 99 % at the same concentration.
Conclusion
This study reports microwave-assisted synthesis of some new hybrid molecules containing penicillanic acid or cephalosporanic acid moieties with some other pharmacophore heterocycles in a single structure. Hence herein we combined all these potential chemotherapeutic units, namely 1,2,4-triazole, 1,3-thiazole, 1,3-oxazole, 1,3,4-oxadiazole, piperazine, penicillanic acid, cephalosporanic acid moieties. The antimicrobial, antiurease, and antilipase screening studies were also performed in the study.
Among the synthesized compounds, the compounds containing 1,2,4-triazole and cephalosporanic- or penicillanic-acid moiety (15–17) displayed good-moderate activity on some of the test microorganisms. The highest activity was observed for compound 17 on Bc with the inhibition zone of 16 mm. This result is better than standard drug ampicillin. Moreover, compounds 15 and 17 exhibited an inhibitory effect against urease. Other compounds containing penicillanic acid or cephalosporanic acid core (21 and 22) displayed good-moderate activity against the test microorganisms. Furthermore, compounds 12, 13, 14, and 15, which are 1,3,4-thiadizole or 1,2,4-triazole derivatives including also 4-fluorophenylpiperazine nucleus, showed moderate anti-lipase activities at final concentration of 6.25 μg mL−1.
Experimental
Chemistry
General information for chemicals
All the chemicals were purchased from Fluka Chemie AG Buchs (Switzerland) and used without further purification. Melting points of the synthesized compounds were determined in open capillaries on a Büchi B-540 melting point apparatus and are uncorrected. Reactions were monitored by thin-layer chromatography (TLC) on silica gel 60 F254 aluminum sheets. The mobile phase was ethyl acetate:diethyl ether, 1:1, and detection was made using UV light. FT-IR spectra were recorded as potassium bromide pellets using a Perkin Elmer 1600 series FT-IR spectrometer. 1H NMR and 13C NMR spectra were registered in DMSO-d 6 on a BRUKER AVANCE II 400 MHz NMR Spectrometer (400.13 MHz for 1H and 100.62 MHz for 13C). The chemical shifts are given in ppm relative to Me4Si as an internal reference, J values are given in Hz. The elemental analysis was performed on a Costech Elemental Combustion System CHNS–O elemental analyzer. All the compounds gave C, H, and N analysis within ±0.4 % of the theoretical values. The mass spectra were obtained on a Quattro LC–MS (70 eV) instrument.
Ethyl 4-(2-fluoro-4-nitrophenyl)piperazine-1-carboxylate (2)
The solution of 3,4-difluoronitrobenzene (10 mmol) in excess amount of ethyl 1-piperazinecarboxylate (40 mmol) was allowed to reflux for 6 h (the progress of the reaction was monitored by TLC). Then, the mixture was poured into ice-water. The precipitated product was filtered off and recrystallized from ethanol. Yield 97 %, m.p: 90–93 °C. FT-IR (KBr, ν, cm−1): 3099 (ar–CH), 1509, and 1354 (NO2). Elemental analysis for C13H16FN3O4 calculated (%): C, 52.52; H, 5.42; N, 14.13. Found (%): C, 52.64; H, 5.70; N, 14.00. 1H NMR (DMSO-d 6, δ ppm): 1.19 (t, 3H, CH3, J = 7.0 Hz), 3.26 (s, 4H, 2CH2), 3.51 (s, 4H, 2CH2), 4.06 (q, 2H, CH2, J = 6.6 Hz), 7.16 (t, 1H, arH, J = 7.8 Hz), 8.00 (d, 2H, arH, J = 7.8 Hz). 13C NMR (DMSO-d 6, δ ppm): 11.47 (CH3), 40.46 (2CH2), 45.81 (2CH2), 57.92 (CH2), arC: [105.00 (CH), 109.09 (d, CH, J C–F = 26.0 Hz), 116.54 (d, CH, J C–F = 154.0 Hz), 136.43 (C), 142.01 (C), 146.05 (C)], 151.46 (C=O). MS m/z (%): 301.29 (32), 167.01 (18), 159.03 (19), 148.96 (100), 113.05 (34).
Ethyl 4-(4-amino-2-fluorophenyl)piperazine-1-carboxylate (3)
Pd–C (5 mmol) catalyst was added to the solution of compound (2) (10 mmol) in n-butanol, and the mixture was refluxed in the presence of hydrazine hydrate (50 mmol) for 7 h. The progress of the reaction was monitored by TLC. Then, the catalyst was separated by filtration and the solvent was evaporated under reduced pressure. The solid obtained was recrystallized from ethanol. Yield 65 %. M.p: 116–119 °C. FT-IR (KBr, ν, cm−1): 3,423 and 3,341 (NH2), 1682 (C=O). Elemental analysis for C13H18FN3O2 calculated (%): C, 58.41; H, 6.79; N, 15.72. Found (%): C, 58.31; H, 6.87; N, 15.78. 1H NMR (DMSO-d 6, δ ppm): 1.18 (t, 3H, CH3, J = 7.0 Hz), 2.76 (s, 4H, 2CH2), 3.45 (s, 4H, 2CH2), 4.04 (q, 2H, CH2, J = 7.4 Hz), 5.03 (s, 2H, NH2), 6.33 (d, 2H, arH, J = 12.4 Hz), 6.76 (t, 1H, arH, J = 9.0 Hz). 13C NMR (DMSO-d 6, δ ppm): 14.53 (CH3), 43.56 (2CH2), 51.07 (2CH2), 60.75 (CH2), arC: [101.66 (d, CH, J C–F = 23.0 Hz), 109.39 (CH), 120.92 (d, CH, J C–F = 4.05 Hz), 128.70 (d, C, J C–F = 9.5 Hz), 145.72 (d, C, J = 10.6 Hz), 154.18 (d, C, J C–F = 34.5 Hz)], 158.65 (C=O). MS m/z (%): 268.10 ([M+1]+,100).
Ethyl 4-(2-fluoro-4-{[pyridin-4-ylmethylene]amino}phenyl)piperazine-1-carboxylate (4a)
Indole-3-carboxaldehyde (10 mmol) was added to the solution of compound 3 (10 mmol) in absolute ethanol and the reaction mixture was irradiated by microwave at 150 W and 110 °C for 30 min. After removing in the solvent under reduced pressure, an oily product obtained. This was recrystallized from butyl acetate and diethyl ether (1:2). Yield: 81 %, M.p: 162–163 °C. FT-IR (KBr, ν, cm−1): 1686 (C=O), 1508 (C=N), 1224 (C–O). Elemental analysis for C19H21FN4O2 calculated (%): C, 64.03; H, 5.94; N, 15.72. Found (%): C, 64.18; H, 6.14; N, 15.78. 1H NMR (DMSO-d 6, δ ppm): 1.19 (t, 3H, CH3, J = 6.6 Hz), 3.00 (s, 4H, 2CH2), 3.51 (s, 4H, 2CH2 + H2O), 4.04–4.11 (m, 2H, CH2), 7.04–7.34 (m, 3H, arH), 7.80 (d, 2H, arH, J = 4.2 Hz), 8.71 (s, 3H, arH + N=CH). 13C NMR (DMSO-d 6, δ ppm): 15.26 (CH3), 44.01 (CH2), 50.69 (CH2), 51.83 (2CH2), 61.57 (CH2), arC: [102.18 (CH), 109.63 (d, CH, J C–F = 21.0 Hz), 120.05 (d, CH, J C–F = 31.5 Hz), 121.37 (C), 122.77 (2CH), 139.48 (d, C, J C–F = 9.0 Hz), 144.37 (d, C, J C–F = 120.0 Hz), 151.14 (2CH), 154.23 (d, C, J C–F = 103.2 Hz)], 158.09 (N=CH), 158.90 (C=O). MS m/z (%): 357.11 ([M+1]+, 64), 302.10 (100), 342.24 (80).
Ethyl 4-(2-fluoro-4-{[(4-nitrophenyl)methylene]amino}phenyl)piperazine-1-carboxylate (4b)
The mixture of compound 3 (10 mmol) and 4-nitrobenzaldehyde (10 mmol) in absolute ethanol was irradiated by microwave at 150 W and 110 °C for 10 min. The solid obtained was recrystallized from ethyl acetate:petroleum ether (1:2). Yield: 58 %, M.p: 164–166 °C. FT-IR (KBr, ν, cm−1): 3074 (ar–CH), 1696 (C=O), 1510, and 1341 (NO2), 1433 (C=N), 1215 (C–O). Elemental analysis for C20H21FN4O4 calculated (%): C, 59.99; H, 5.29; N, 13.99. Found (%): C, 60.12; H, 5.45; N, 14.19. 1H NMR (DMSO-d 6, δ ppm): 1.19 (brs, 3H, CH3), 3.10 (s, 4H, 2CH2), 3.51 (s, 4H, 2CH2), 4.04–4.17 (m, 2H, CH2), 7.09–7.37 (m, 3H, arH), 8.14 (d, 2H, arH, J = 7.8 Hz), 8.35 (d, 2H, arH, J = 7.8 Hz), 8.84 (s, 1H, N=CH). 13C NMR (DMSO-d 6, δ ppm): 15.16 (CH3), 43.39 (CH2), 50.64 (CH2), 55.44 (2CH2), 61.57 (CH2), arC: [110.57 (d, CH, J C–F = 32.7 Hz), 117.30 (CH), 120.24 (d, CH, J C–F = 41.0 Hz), 123.62 (d, C, J C–F = 42.5 Hz), 124.76 (2CH), 130.18 (2CH), 139.53 (C), 142.26 (C), 146.09 (d, C, J C–F = 51.0 Hz), 150.35 (d, C, J C–F = 96.0 Hz)], 153.46 (C=O), 160.32 (N=CH).
Ethyl 4-(4-{[(4-bromophenyl)methylene]amino}-2-fluorophenyl)piperazine-1-carboxylate (4c)
The mixture of compound 3 (10 mmol) and 4-bromobenzaldehyde (10 mmol) in absolute ethanol was irradiated at 150 W and 150 °C for 30 min. The yellow solid obtained was recrystallized ethanol. Yield: 84 %, M.p: 124–126 °C. FT-IR (KBr, ν, cm−1): 3053 (ar–CH), 1671 (C=O), 1434 (C=N), 1210 (C–O). Elemental analysis for C20H21BrFN3O2 calculated (%): C, 55.31; H, 4.87; N, 9.68. Found (%): C, 55.71; H, 4.90; N, 9.79. 1H NMR (DMSO-d 6, δ ppm): 1.19 (t, 3H, CH3, J = 7.0 Hz), 2.98 (s, 4H, 2CH2), 3.51 (s, 4H, 2CH2), 4.05 (q, 2H, CH2, J = 7.0 Hz), 6.93–7.27 (m, 3H, arH), 7.71 (d, 2H, arH, J = 7.8 Hz), 7.84 (d, 2H, arH, J = 8.2 Hz), 8.65 (s, 1H, N=CH). 13C NMR (DMSO-d 6, δ ppm): 15.26 (CH3), 41.40 (CH2), 44.04 (CH2), 50.78 (2CH2), 61.56 (CH2), arC: [105.00 (CH), 109.44 (d, CH, J C–F = 22.5 Hz), 119.80 (d, CH, J C–F = 58.2 Hz), 125.61 (C), 131.05 (2CH), 132.57 (2CH), 135.83 (C), 138.83 (d, C, J C–F = 8.75 Hz), 146.26 (d, C, J C–F = 8.5 Hz), 153.39 (C)], 155.27 (C=O), 159.44 (N=CH).
Ethyl 4-{2-fluoro-4-[(2-hydroxybenzylidene)amino]phenyl}piperazine-1-carboxylate (4d)
The solution of compound 3 (10 mmol) in absolute ethanol was refluxed with 2-hydroxybenzaldehyde (10 mmol) for 7 h. On cooling the reaction content to room temperature, a solid appeared. This crude product was filtered off and recrystallized from acetone. Yield: 83 %. M.p: 136–137 °C. FT-IR (KBr, ν, cm−1):1697 (C=O), 1510 (C=N), 1225 (C–O). Elemental analysis for C20H22FN3O3 calculated (%): C, 64.68; H, 5.97; N, 11.31. Found (%): C: 64.31; H: 5.78; N: 11.48. 1H NMR (DMSO-d 6, δ ppm): 1.21 (brs, 3H, CH3), 3.00 (s, 4H, 2CH2), 3.52 (s, 4H, 2CH2), 4.06 (brs, 2H, CH2), 6.97–7.59 (m, 7H, arH), 8.95 (s, 1H, N=CH), 13.02 (s, 1H, OH). 13C NMR (DMSO-d 6, δ ppm): 15.26 (CH3), 44.40 (2CH2), 50.66 (2CH2), 61.59 (CH2), arC: [109.50 (d, CH, J C–F = 22.0 Hz), 117.24 (2CH), 119.33 (CH), 119.87 (C), 120.22 (d, CH, J C–F = 28.5 Hz), 133.18 (CH), 133.86 (CH), 139.28 (d, C, J C–F = 9.0 Hz), 143.26 (d, C, J C–F = 8.5 Hz), 153.32 (C), 156.74 (d, C, J C–F = 145.5 Hz)], 160.82 (C=O), 163.17 (N=CH).
Ethyl 4-(2-fluoro-4-{[(4-methoxyphenyl)methylene]amino}phenyl)piperazine-1-carboxylate (4e)
The solution of compound 3 (10 mmol) in absolute ethanol was refluxed with 4-methoxybenzaldehyde (10 mmol) for 7 h. On cooling the reaction content to room temperature, a solid appeared. This crude product was filtered off and recrystallized from ethanol. Yield: 42 %. M.p: 122–124 °C. FT-IR (KBr, ν, cm−1): 1688 (C=O), 1509 (C=N), 1225 (C–O). Elemental analysis for C21H24FN3O3 calculated (%): C, 65.44; H, 6.28; N, 10.90. Found (%): C, 65.56; H, 6.52; N, 11.12. 1H NMR (DMSO-d 6, δ ppm): 1.19 (t, 3H, CH3, J = 6.6 Hz), 2.96 (s, 4H, 2CH2), 3.49 (s, 4H, 2CH2), 3.82 (s, 3H, O–CH3), 4.06 (q, 2H, CH2, J = 6.8 Hz), 6.71–6.78 (m, 1H, arH), 7.04–7.22 (m, 5H, arH), 7.86 (d, 1H, arH, J = 8.2 Hz), 8.58 (s, 1H, N=CH). 13C NMR (DMSO-d 6, δ ppm): 15.27 (CH3), 44.13 (CH2), 50.85 (CH2), 51.35 (2CH2), 56.10 (O–CH3) 61.53 (CH2), arC: [109.73 (d, CH, J C–F = 38.9 Hz), 114.98 (2CH), 118.72 (CH), 121.90 (d, CH, J C–F = 66.3 Hz), 129.12 (C), 131.24 (2CH), 132.53 (C), 138.35 (d, C, J C–F = 21.0 Hz), 147.24 (C), 154.40 (d, C, J C–F = 94.5 Hz), 160.10 (N=CH), 162.24 (C=O).
Ethyl 4-(2-fluoro-4-{[1H-indol-3-ylmethylene]amino}phenyl)piperazine-1-carboxylate (4f)
The solution of compound 3 (10 mmol) in absolute ethanol was refluxed with indol-3-carbaldehyde (10 mmol) for 6 h. On cooling the reaction content to room temperature, a solid appeared. This crude product was filtered off and recrystallized from acetone. Yield: 82 %. M.p: 184–186 °C. FT-IR (KBr, ν, cm−1): 3484 (NH), 1678 (C=O), 1439 (C=N), 1220 (C–O). Elemental analysis for C22H23FN4O2 calculated (%): C, 66.99; H, 5.88; N, 14.20. Found (%): C, 66.76; H, 6.02; N, 14.01. 1H NMR (DMSO-d 6, δ ppm): 1.20 (brs, 3H, CH3), 3.01 (s, 4H, 2CH2), 3.53 (s, 4H, 2CH2), 4.06 (brs, 2H, CH2), 7.29 (brs, 5H, arH), 8.08 (s, 1H, arH), 8.38 (s, 2H, arH), 9.06 (s, 1H, N=CH), 9.29 (s, 1H, NH). 13C NMR (DMSO-d 6, δ ppm): 15.21 (CH3), 44.18 (CH2), 50.76 (CH2), 51.51 (2CH2), 62.46 (CH2), arC: [108.93 (d, CH, J C–F = 23.4 Hz), 113.47 (d, CH, J C–F = 34.4 Hz), 117.88 (CH), 118.82 (C), 120.71 (CH), 121.51 (CH), 121.84 (CH), 122.84 (CH), 123.76 (d, CH, J C–F = 41.0 Hz), 124.87 (C), 137.91 (d, C, J = 19.8 Hz), 139.24 (2C) 155.26 (d, C, J C–F = 4.0 Hz)], 153.18 (N=CH), 185.74 (C=O).
Ethyl 4-(4-{[(benzylamino)carbonyl]amino}-2-fluorophenyl)piperazine-1-carboxylate (5)
The mixture of compound 3 (10 mmol) and benzylisothiocyanate (10 mmol) in absolute ethanol was refluxed for 10 h. On cooling the reaction mixture to room temperature, a solid formed. This crude product was collected by filtration and recrystallized from ethanol. Yield: 93 %. M.p: 153–155 °C. FT-IR (KBr, ν, cm−1): 3346, 3284 (2NH), 3063 (ar–CH), 1694, 1638 (2C=O), 1236 (C–O). Elemental analysis for C21H25FN4O3 calculated (%): C, 62.99, H, 6.29; N, 13.99. Found (%): C, 62.78; H, 6.07; N, 14.04. 1H NMR (DMSO-d 6, δ ppm): 1.17 (t, 3H, CH3, J = 7.6 Hz), 2.85 (s, 4H, 2CH2), 3.40 (s, 4H, 2CH2 + H2O), 4.02 (q, 2H, CH2, J = 7.0 Hz), 4.26 (d, 2H, CH2, J = 6.0 Hz), 6.61 (brs, 1H, NH), 6.95 (s, 2H, arH), 7.21–7.31 (m, 6H, arH), 8.62 (s, 1H, NH). 13C NMR (DMSO-d 6, δ ppm): 15.27 (CH3), 41.39 (CH2), 43.39 (CH2), 44.15 (CH2), 51.23 (CH2), 60.45 (CH2), 61.52 (CH2), arC: [106.69 (d, CH, J C–F = 25.6 Hz), 114.19 (CH), 120.59 (CH), 127.42 (CH), 127.79 (2CH), 128.99 (2CH), 133.98 (d, C, J C–F = 9.55 Hz), 137.02 (d, C, J C–F = 9.85 Hz), 140.98 (C), 156.65 (d, C, J C–F = 137.5 Hz)], 155.83 (2C=O).
Ethyl 4-(4-{[3-benzyl-4-oxo-1,3-oxazolidin-2-ylidene]amino}-2-fluorophenyl)piperazine-1-carboxylate (6)
The mixture of compound 5 (10 mmol) and ethyl bromoacetate in absolute ethanol was refluxed in the presence of dried sodium acetate (50 mmol) for 13 h. After removing the solvent under reduced pressure, a solid appeared. This crude product was washed water and the precipitated solid was recrystallized from ethanol:water (1:2). Yield: 64 %. M.p: 158–159 °C. FT-IR (KBr, ν, cm−1): 1696, 1638 (2C=O), 1429 (C=N), 1210 (C–O). Elemental analysis for C23H25FN4O4 calculated (%): C, 62.72; H, 5.72; N, 12.72. Found (%): C, 62.87; H, 5.98; N, 12.88. 1H NMR (DMSO-d 6, δ ppm): 1.35 (t, 3H, CH3, J = 8.0 Hz), 3.02 (brs, 4H, 2CH2), 3.53 (s, 4H, 2CH2 + H2O), 3.65 (brs, 2H, CH2), 4.22 (q, 2H, CH2, J = 7.0 Hz), 4.44 (d, 2H, CH2, J = 5.8 Hz), 7.08–7.12 (m, 3H, arH), 7.43–7.49 (m, 5H, arH). 13C NMR (DMSO-d 6, δ ppm): 15.26 (CH3), 43.37 (CH2), 44.16 (CH2), 51.24 (2CH2), 54.37 (CH2), 61.54 (CH2), 62.49 (CH2), arC: [105.9 (d, CH, J C–F = 95.7 Hz), 114.21 (CH), 119.98 (d, CH, J C–F = 61.1 Hz), 127.38 (CH), 127.78 (2CH), 128.97 (2CH), 133.72 (d, C, J C–F = 30.1 Hz), 136.95 (d, C, J C–F = 36.5 Hz), 142.15 (C), 143.15 (d, C, J C–F = 211.6 Hz)], 155.30 (C=O), 155.92 (C=N), 161.28 (C=O). MS m/z (%): 479.16 ([M+K]+, 100).
4-(4-{[3-Benzyl-5-(4-chlorophenyl)-1,3-oxazol-2(3H)-ylidene]amino}-2-fluorophenyl) piperazine-1-carboxylate (7)
The mixture of compound 5 (10 mmol) and 4-chlorophenacylbromide (10 mmol) in absolute ethanol was refluxed in the presence of dried sodium acetate (50 mmol) for 11 h. Then, the reaction mixture was cooled to room temperature and the precipitated salt was removed by filtration. After evaporating the solvent under reduced pressure, a solid appeared. This crude product recrystallized with ethyl acetate: petroleum ether (1:2). Yield: 40 %, M.p: 162–163 °C. FT-IR (KBr, ν, cm−1): 1697 (C=O), 1429 (C=N), 1209 (C–O). Elemental analysis for C23H28ClFN4O3 calculated (%): C, 65.10, H, 5.28; N, 10.47. Found (%): C, 65.14; H, 5.39; N, 10.49. 1H NMR (DMSO-d 6, δ ppm): 1.17 (t, 3H, CH3, J = 7.6 Hz), 2.85 (s, 4H, 2CH2), 3.47 (s, 4H, 2CH2), 4.04 (q, 2H, CH2, J = 6.2 Hz), 4.26 (brs, 2H, CH2), 6.85–6.94 (m, 4H, arH + CH), 7.28 (brs, 8H, arH), 7.45 (s, 1H, arH). 13C NMR (DMSO-d 6, δ ppm): 15.27 (CH3), 43.36 (2CH2), 44.14 (2CH2), 51.21 (CH2), 61.52 (CH2), 96.76 (CH), arC: [106.66 (d, CH, J C–F = 25.6 Hz), 114.13 (CH), 120.50 (CH), 124.20 (2CH), 124.97 (2CH), 127.38 (CH), 127.78 (2CH), 128.97 (2CH), 133.90 (d, C, J C–F = 21.9 Hz), 137.14 (d, C, J C–F = 11.0 Hz), 141.05 (2C), 155.28 (C), 155.63 (d, C, J C–F = 240.5 Hz)], 155.91 (C + C=O), 162.27 (C=N). MS m/z (%): 535.12 ([M]+, 14), 479.16 (100), 423.16 (97), 138.12 (50).
Ethyl 4-{4-[(2-ethoxy-2-oxoethyl)amino]-2-fluorophenyl}piperazine-1-carboxylate (8)
To the mixture of compound 3 (10 mmol) and triethylamine (10 mmol) in dry tetrahydrofurane, ethylbromoacetate (10 mmol) was added drop by drop at 0–5 °C. Then, the reaction mixture was allowed to reach room temperature and stirred for 14 h (the progress of the reaction was monitored by TLC). The precipitated triethylammonium salt was removed by filtration and the resulting solution was evaporated under reduced pressure to dryness. The obtained yellow solid was recrystallized from ethanol:water (1:2). Yield: 50.2 %. M.p: 71–73 °C. FT-IR (KBr, ν, cm−1): 3383 (NH), 1719 (C=O), 1697 (C=O), 1220 (C–O). Elemental analysis for C17H24FN3O4 calculated (%): C, 57.78; H, 6.85; N, 11.89. Found (%): C, 57.74; H, 6.77; N, 11.97. 1H NMR (DMSO-d 6, δ ppm): 1.35 (t, 6H, 2CH3, J = 7.0 Hz), 2.95 (s, 4H, 2CH2), 3.60 (s, 6H, 3CH2), 4.24 (q, 4H, 2CH2, J = 7.0 Hz), 5.24 (s, 1H, NH), 6.44–6.59 (m, 2H, arH), 6.94–7.05 (m, 1H, arH). 13C NMR (DMSO-d 6, δ ppm): 14.80 (CH3), 15.24 (CH3), 44.23 (CH2), 45.49 (2CH2), 51.33 (CH2), 51.75 (CH2), 61.01 (CH2), 61.52 (CH2), arC: [101.06 (d, CH, J C–F = 24.1 Hz), 121.47 (d, CH, J C–F = 4.0 Hz), 121.67 (d, CH, J C–F = 4.0 Hz), 129.97 (d, C, J C–F = 9.9 Hz), 145.96 (d, C, J C–F = 10.6 Hz), 157.02 (d, C, J C–F = 240.9 Hz)], 155.29 (C=O), 171.90 (C=O). MS m/z(%): 376.34 ([M+Na]+, 75), 354.38 ([M+1]+,100), 222.17 (22), 149.03 (49).
Ethyl 4-{2-fluoro-4-[(2-hydrazinyl-2-oxoethyl)amino]phenyl}piperazine-1-carboxylate (9)
Hydrazine hydrate (25 mmol) was added to the solution of compound 8 (10 mmol) in ethanol and the mixture was heated under reflux for 14 h. On cooling the mixture in cold overnight, a white solid appeared. The crude product was filtered off and recrystallized from ethyl acetate. Yield: 54 %. M.p: 153–155 °C. FT-IR (KBr, ν, cm−1): 3313 (2NH + NH2), 1675 (C=O), 1653 (C=O). Elemental analysis for C15H22FN5O3 calculated (%): C, 53.09; H, 6.53; N, 20.64. Found (%): C, 53.18; H, 6.79; N, 20.44. 1H NMR (DMSO-d 6, δ ppm): 1.18 (t, 3H, CH3, J = 6.2 Hz), 2.77 (s, 4H, 2CH2), 3.37 (s, 4H, 2CH2), 4.05 (d, 2H, CH2, J = 7.0 Hz), 4.24 (s, 2H, CH2), 5.93 (brs, 2H, NH2), 6.25–6.39 (m, 2H, arH), 6.83 (t, 1H, arH, J = 9.8 Hz), 9.09 (s, 2H, 2NH). 13C NMR (DMSO-d 6, δ ppm): 15.27 (CH3), 43.09 (CH2), 44.30 (CH2), 46.04 (CH2), 51.78 (2CH2), 61.48(CH2), arC: [101.10 (d, CH, J = 24.1 Hz), 108.53 (CH), 121.70 (CH), 130.00 (d, C, J C–F = 9.5 Hz), 146.18 (d, C, J C–F = 10.0 Hz), 157.03 (d, C, J C–F = 240.9 Hz)], 155.26 (C=O), 169.97 (C=O). MS m/z (%): 380.47 ([M+2+K]+,100), 379.41 ([M+1 + K]+, 30), 267.22 ([M–CH2CONHNH2]+, 33), 234.18 (28).
Ethyl 4-(2-fluoro-4-{[2-(2-{[(4-fluorophenyl)amino]carbonothioyl}hydrazino)-2-oxoethyl]amino}phenyl)piperazine-1-carboxylate (10)
The solution of compound 9 (10 mmol) in absolute ethanol was refluxed with 4-fluorophenylisothiocyanate (10 mmol) for 10 h. On cooling the reaction mixture to room temperature, an oily product appeared. This was recrystallized from butyl acetate: ethyl ether (1:2). Yield: 50 %. M.p: 78–80 °C. FT-IR (KBr, ν, cm−1): 3225 (2NH + NH2), 1671 (2C=O), 1210 (C–O). Elemental analysis for C22H26F2N6O3S calculated (%): C, 53.66; H, 5.32; N, 17.06. Found (%): C, 53.78; H, 5.47; N, 17.14. 1H NMR (DMSO-d 6, δ ppm): 1.19 (brs, 3H, CH3), 2.78 (s, 4H, 2CH2), 3.35 (s, 4H, 2CH2), 3.77 (brs, 2H, CH2), 4.06 (brs, 2H, CH2), 5.91 (brs, 2H, 2NH), 6.35 (brs, 2H, arH), 6.83 (brs, 1H, arH), 7.17 (brs, 2H, arH), 7.39 (brs, 2H, arH), 9.56 (brs, 1H, NH), 9.69 (brs, 1H, NH), 10.08 (brs, 1H, NH). 13C NMR (DMSO-d 6, δ ppm): 17.45 (CH3), 43.56 (CH2), 46.49 (CH2), 53.96 (2CH2), 63.67 (CH2), 67.10 (CH2), arC: [105.40 (d, CH, J C–F = 40.1 Hz), 114.19 (CH), 118.62 (d, CH, J C–F = 36.6 Hz), 121.70 (2CH), 124.54 (2CH), 128.55 (d, C, J C–F = 36.4 Hz), 140.19 (d, CH, J C–F = 37.0 Hz), 150.36 (d, C, J C–F = 184.7 Hz), 157.43 (2C)], 168.24 (C=O), 172.66 (C=O), 190.04 (C=S).
Ethyl 4-[4-({2-[2-(anilinocarbonothioyl)hydrazino]-2-oxoethyl}amino)-2-fluorophenyl] piperazine-1-carboxylate (11)
The mixture of compound 9 (10 mmol) and phenylisothiocyanate (10 mmol) in absolute ethanol was heated under reflux for 10 h. On cooling the reaction mixture to room temperature, a white solid appeared. This crude product was filtered off and recrystallized from ethanol. Yield: 85 %, M.p: 160–163 °C. FT-IR (KBr, ν, cm−1): 3340, 3256, 3193 (4NH), 1697 (C=O), 1633 (C=O), 1286 (C=S). Elemental analysis for C22H27FN6O3S calculated (%): C, 55.68; H, 5.73, N, 17.71. Found (%): C, 55.98; H, 5.78; N, 17.87. 1H NMR (DMSO-d 6, δ ppm): 1.19 (t, 3H, CH3, J = 7.0 Hz), 2.78 (s, 4H, 2CH2), 3.47 (s, 4H, 2CH2), 3.77 (s, 2H, CH2), 4.04 (q, 2H, CH2, J = 7.2 Hz), 6.34–6.51 (m, 2H, arH), 6.80–6.85 (m, 1H, arH), 7.17 (s, 1H, arH), 7.34–7.38 (d, 4H, arH, J = 8.2 Hz), 9.56 (s, 1H, NH), 9.69 (s, 1H, NH), 10.12 (s, 2H, 2NH). 13C NMR (DMSO-d 6, δ ppm): 15.29 (CH3), 44.25 (CH2), 45.92 (CH2), 51.83 (2CH2), 61.51 (2CH2), arC: [101.29 (d, CH, J C–F = 24.1 Hz), 108.72 (CH), 121.68 (CH), 125.92 (2CH), 126.48 (CH), 128.82 (2CH), 139.70 (C), 146.20 (d, C, J C–F = 10.0 Hz), 154.00 (d, C, J C–F = 63.3 Hz), 157.35 (d, C, J C–F = 209.8 Hz)], 168.64 (C=O), 170.64 (C=O), 181.58 (C=S). MS m/z (%): 475.41 ([M+1]+, 32), 414.53 (26), 413.53 (100), 149.03 (32).
Ethyl 4-(4-{[(5-anilino-1,3,4-thiadiazol-2-yl)methyl]amino}-2-fluorophenyl)piperazine-1-carboxylate (12)
Concentrated sulfuric acid (64 mmol) was added to compound 11 (10 mmol) dropwise while stirring, and the reaction mixture was stirred in an ice bath for 15 min. Then, the mixture was allowed to reach room temperature and stirred for additional 2 h. The resulting solution was poured into ice cold water and made alkaline (pH 8) with ammonia. The precipitated product was filtered, washed with water, and recrystallized from dimethysulfoxide:water (1:3). Yield 74 %. M.p: 93–95 °C. FT-IR (KBr, ν, cm−1): 3257 (2NH), 1677 (C=O), 1433 (C=N). Elemental analysis for C22H25F2N6O2S calculated (%): C, 57.88; H, 5.52; N, 18.41. Found (%): C, 58.08; H, 5.75; N, 18.78. 1H NMR (DMSO-d 6, δ ppm): 1.17 (t, 3H, CH3, J = 7.0 Hz), 2.78 (s, 4H, 2CH2), 3.46 (s, 6H, 3CH2 + H2O), 4.03 (q, 2H, CH2, J = 7.2 Hz), 4.48 (s, 1H, NH), 6.37–6.51 (m, 2H, arH), 6.80–6.99 (m, 2H, arH), 7.17 (brs, 1H, NH), 7.27–7.33 (m, 3H, arH), 7.56 (d, 1H, arH, J = 7.8 Hz). 13C NMR (DMSO-d 6, δ ppm): 14.47 (CH3), 42.36 (CH2), 43.37 (CH2), 45.14 (CH2), 50.03 (CH2), 50.92 (CH2), 60.72 (CH2), arC: [108.11 (d, CH, J C–F = 12.4 Hz), 116.95 (d, CH, J C–F = 19.4 Hz), 121.30 (d, CH, J C–F = 33.3 Hz), 128.03 (CH), 128.75 (2CH), 128.96 (2CH), 129.53 (d, C, J C–F = 9.5 Hz), 140.52 (C), 144.63 (d, C, J C–F = 10.6 Hz), 156.51 (d, C, J C–F = 204.2 Hz)], 160.77 (C), 164.32 (C), 169.87 (C=O). MS m/z (%): 458.16 ([M+2]+, 27), 457.16 ([M+1]+, 100).
Ethyl 4-[2-fluoro-4-({[4-(4-fluorophenyl)-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl]methyl}amino)phenyl]piperazine-1-carboxylate (13)
A solution of compound 10 (10 mmol) in water was refluxed in the presence of 2 N NaOH for 3 h. Then, the resulting solution was cooled to room temperature and acidified to pH 4 with 37 % HCl. The precipitate formed was filtered off, washed with water, and recrystallized from ethanol. Yield: 61 %. M.p: 100–101 °C. FT-IR (KBr, ν, cm−1): 1675 (C=O), 1244 (C=S). 1H NMR (DMSO-d 6, δ ppm): elemental analysis for C22H24F2N6O2S calculated (%): C, 55.68; H, 5.10; N, 17.71. Found (%): C, 55.54; H, 5.28; N, 17.89. 1H NMR (DMSO-d 6, δ ppm): 1.18 (brs, 3H, CH3) 2.78 (brs, 4H, 2CH2), 3.41 (brs, 4H, 2CH2), 4.08 (brs, 4H, 2CH2), 5.87 (brs, 2H, 2NH), 6.27 (brs, 2H, arH), 6.79 (brs, 1H, arH), 7.45 (brs, 4H, arH). 13C NMR (DMSO-d 6, δ ppm): 15.26 (CH3), 44.25 (2CH2), 51.67 (2CH2), 61.50 (2CH2), arC: [101.18 (d, CH, J C–F = 9.5 Hz), 108.66 (CH), 116.98 (d, CH, J C–F = 23.0 Hz), 121.53 (C), 130.31 (d, C, J C–F = 10.2 Hz), 131.07 (2CH), 131.25 (2CH), 145.33 (d, C, J C–F = 10.6 Hz), 153.16 (d, C, J C–F = 213.8 Hz), 159.87 (d, C, J C–F = 57.2 Hz)], 151.02 (C), 165.34 (C=O), 168.98 (C=S).
Ethyl 4-(2-fluoro-4-{[(4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-3-yl)methyl] amino}phenyl)piperazine-1-carboxylate (14)
A solution of compound 11 (10 mmol) in ethanol water (1:1) was refluxed in the presence of 2 N NaOH for 3 h. Then, the resulting solution was cooled to room temperature and acidified to pH 7 with 37 % HCl. The precipitate formed was filtered off, washed with water, and recrystallized from ethyl acetate. Yield 70 %. M.p: 206–208 °C. FT-IR (KBr, ν, cm−1): 3248, 3117 (2NH), 3049 (ar CH), 1660 (C=O), 1250 (C=S). Elemental analysis for C22H25FN6O2S calculated (%): C, 57.88; H, 5.52; N, 18.41. Found (%): C, 57.51; H, 5.45; N, 18.49. 1H NMR (DMSO-d 6, δ ppm): 1.13 (t, 3H, CH3, J = 7.4 Hz), 2.73 (s, 4H, 2CH2), 3.42 (s, 4H, 2CH2), 3.99 (s, 4H, 2CH2), 6.25–6.32 (m, 2H, arH + NH), 6.76–6.80 (m, 1H, arH), 7.36 (s, 2H, ar–H), 7.49 (brs, 4H, ar–H), 10.45 (s, 1H, NH). 13C NMR (DMSO-d 6, δ ppm): 15.25 (CH3), 31.39 (CH2), 44.27 (2CH2), 51.68 (2CH2), 61.49 (CH2), arC: [101.27 (d, CH, J C–F = 24 Hz), 108.63 (CH), 121.59 (CH), 128.76 (CH), 130.05 (2CH), 130.15 (2CH), 134.09 (2C), 145.50 (C), 150.92 (C)], 155.25 (C), 168.75 (C=S + C=O). MS m/z (%): 480.48 ([M+1 + Na]+, 29), 479.54 ([M+Na]+, 100), 457.41 ([M+1]+, 85).
({[(6R,7R)-3-[(Acetyloxy)methyl]-7-({[3-[({4-[4-(ethoxycarbonyl)piperazin-1-yl]-3-fluorophenyl}amino)methyl]-4-(4-fluorophenyl)-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-1-yl]methyl}amino)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-2-yl]carbonyl}oxy)triethyl ammonium (15)
7-Aca (10 mmol) was added to the mixture of compound 13 (10 mmol), triethylamine (20 mmol), and formaldehyde (50 mmol) in tetrahydrofurane, and the mixture was stirred at room temperature 4 h. After removing the solvent under reduced pressure, an oily product appeared. This was recrystallized from ethanol:water (1:2). Yield: 43 %. M.p: 68–70 °C. FT-IR (KBr, ν, cm−1): 3359, 3263 (2NH), 3075 (ar–CH), 2988, 2973 (aliphatic CH), 1680, 1629 (4C=O), 1228 (C=S). Elemental analysis for C39H51F2N9O7S2 calculated (%): C, 54.47; H, 5.98; N, 14.66. Found (%): C, 54.70; H, 5.74; N, 14.55. 1H NMR (DMSO-d 6, δ ppm): 1.10 (brs, 12H, 4CH3) 1.74 (s, 3H, CH3), 2.86 (brs, 4H, 2CH2), 3.20 (s, 6H, 3CH2), 3.58 (brs, 6H, 3CH2), 4.04 (brs, 2H, CH2), 4.52 (brs, 2H, CH2), 4.67 (s, 4H, 2CH2), 4.89 (s, 2H, 2CH), 5.42 (s, 2H, 2NH), 6.51 (brs, 2H, arH), 6.89 (brs, 1H, arH), 7.35–7.44 (m, 4H, arH). 13C NMR (DMSO-d 6, δ ppm): 9.01 (3CH3), 15.04 (CH3), 23.44 (CH3), 25.69 (CH2), 44.05 (2CH2), 46.25 (CH2), 49.16 (3CH2), 51.29 (CH2), 51.56 (2CH2), 54.70 (2CH), 61.89 (CH2), 67.78 (CH2), arC: [103.99 (d, CH, J C–F = 12.45 Hz), 110.89 (CH), 117.08 (d, CH, J C–F = 23.45 Hz), 120.97 (2CH), 131.04 (2CH), 131.69 (C), 131.88 (C), 143.85 (d, C, J C–F = 9.85 Hz), 154.78 (d, C, J C–F = 92.61 Hz), 162.96 (d, C, J C–F = 246.0 Hz)], 130.41 (C), 130.49 (C), 150.18 (triazole-C), 165.79 (C=O), 168.64 (C=O), 168.86 (C=S), 171.93 (C=O), 175.76 (C=O).
[({(6R,7R)-3-[(Acetyloxy)methyl]-7-[({3-[({4-[4-(ethoxycarbonyl)piperazin-1-yl]-3-fluorophenyl}amino)methyl]-4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-1-yl}methyl)amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-2-yl}carbonyl)oxy](triethyl)ammonium (16)
To the mixture of compound 14 (10 mmol), triethylamine (20 mmol) and formaldehyde (50 mmol) in tetrahydrofurane, 7-aca (10 mmol) was added. The mixture was stirred at room temperature 4 h. After removing the solvent under reduced pressure, an oily product appeared. This product recrystallized ethyl acetate:hexane (1:2). Yield: 47 %. M.p: 64–66 °C. FT-IR (KBr, ν, cm−1): 3662 (OH), 3374 (NH), 2988, 2901 (aliphatic CH), 1762 (C=O), 1687 (2C=O), 1629 (C=O), 1227 (C=S). Elemental analysis for C39H52FN9O7S2 calculated (%): C, 55.63; H, 6.22; N, 14.97. Found (%): C, 55.87; H, 6.33; N, 15.05. 1H NMR (DMSO-d 6, δ ppm): 1.11 (t, 12H, 4CH3, J = 7.0 Hz), 1.99 (s, 3H, CH3), 2.99 (q, 8H, 4CH2, J = 8.0 Hz), 3.87 (brs, 10H, 5CH2), 4.55 (s, 2H, CH2), 4.68–4.80 (m, 4H, 2CH2), 5.40 (s, 2H, CH), 6.22 (brs, 2H, 2NH), 7.33 (brs, 3H, ar–H), 7.50–7.75 (m, 5H, ar–H).13C-NMR (DMSO-d 6, δ ppm): 9.31 (3CH3), 15.22 (CH3), 21.38 (CH3), 25.79 (CH2), 41.30 (2CH2), 44.17 (2CH2), 45.79 (3CH2), 51.40 (CH2), 51.64 (CH2), 61.49 (CH2), 66.68 (CH2), 67.69 (CH), 71.09 (CH), arC: [110.41 (d, CH, J C–F = 34.2 Hz), 118.31 (d, CH, J C–F = 18.7 Hz), 123.22 (d, C, J C–F = 22.1 Hz), 126.01 (CH), 128.74 (CH), 130.14 (2CH), 130.33 (2CH), 134.47 (d, C, J C–F = 7.3 Hz), 148.01 (d, C, J C–F = 172.2 Hz), 149.99 (C)], 138.25 (2C), 155.28 (C), 166.21 (C=S), 169.90 (C=O), 170.92 (C=O), 171.19 (C=O), 172.95 (C=O).
[({(5R,6R)-6-[({3-[({4-[4-(Ethoxycarbonyl)piperazin-1-yl]-3-fluorophenyl}amino)methyl]-4-phenyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-1-yl}methyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-yl}carbonyl)oxy](triethyl)ammonium (17)
To the mixture of compound 14 (10 mmol), triethylamine (20 mmol), and formaldehyde (50 mmol) in tetrahydrofurane, 6-apa (10 mmol) was added. The mixture was stirred at room temperature 4 h. After removing the solvent under reduced pressure, an oily product appeared. This product recrystallized ethyl acetate:hexane (1:2). Yield: 41 %, M.p: 64–66 °C. FT-IR (KBr, ν, cm−1): 3393 (NH), 3073 (ar–CH), 2980 (aliphatic CH), 1764 (C=O), 1692 (C=O), 1609 (C=O), 1230 (C–O). Elemental analysis for C37H52FN9O7S2 calculated (%): C, 56.54; H, 6.67; N, 16.04. Found (%): C, 56.65; H, 6.79; N, 16.87. 1H NMR (DMSO-d 6, δ ppm): 1.13 (t, 12H, 4CH3, J = 6.2 Hz), 1.39 (brs, 3H, CH3), 1.42 (brs, 3H, CH3), 3.02 (q, 8H, 4CH2, J = 7.0 Hz), 3.43 (s, 8H, 4CH2), 3.73 (brs, 2H, CH2), 4.56 (s, 2H, 2CH), 5.41 (s, 2H, CH2), 6.24 (s, 1H, CH), 6.77 (brs, 1H, NH), 7.36 (brs, 3H, ar–H), 7.50 (s, 5H, ar–H). 13C-NMR (DMSO-d 6, δ ppm): 8.99 (3CH3), 14.53 (CH3), 27.13 (2CH3), 43.49 (2CH2), 44.96 (2CH2), 50.58 (CH2), 50.70 (3CH2), 50.94 (2CH2), 60.75 (C-(CH3)2), 70.39 (CH), 73.89 (CH), 81.90 (CH), arC: [100.44 (d, CH, J C–F = 24.1 Hz), 108.87 (d, CH, J C–F = 213.1 Hz), 120.53 (d, CH, J C–F = 60.2 Hz), 128.18 (CH), 129.57 (2CH), 129.64 (2CH), 133.79 (d, C, J C–F = 14.9 Hz), 144.08 (d, C, J C–F = 99.5 Hz), 146.84 (d, C, J C–F = 442.1 Hz)] 149.26 (C), 154.53 (C), 156.88 (C=S), 167.90 (C=O), 168.09 (C=O), 170.16 (C=O).
Ethyl 4-[4-(3-{2-[5-(4-chlorophenyl)-3-phenyl-1,3-thiazol-2(3H)-ylidene]hydrazino}-3-oxoethyl)-2-fluorophenylamino]piperazine-1-carboxylate (18)
The mixture of compound 11 (10 mmol) and 4-chlorophenacylbromide (10 mmol) in absolute ethanol was refluxed in the presence of dried sodium acetate (50 mmol) for 12 h. After removing the solvent under reduced pressure, an orange solid appeared. This product washed water and recrystallized ethanol. Yield: 45 %. M.p: 60–62 °C. FT-IR (KBr, ν, cm−1): 3345, 3259 (2NH), 3054 (ar–CH), 1677 (C=O), 1628 (C=O). Elemental analysis for C30H30ClFN6O3S calculated (%): C, 59.15; H, 4.96; N, 13.80. Found (%): C, 59.05; H, 5.06; N, 13.87. 1H NMR (DMSO-d 6, δ ppm): 1.15 (brs, 3H, CH3), 2.76 (s, 4H, 2CH2), 3.61 (s, 6H, 3CH2 + H2O), 4.03 (brs, 2H, CH2), 5.40 (s, 1H, NH), 6.44–6.54 (m, 1H, arH), 6.84–6.96 (m, 2H, arH + CH), 7.29–7.52 (m, 9H, arH), 7.95 (s, 1H, arH), 10.45 (s, 1H, NH). 13C NMR (DMSO-d 6, δ ppm): 15.24 (CH3), 41.37 (CH2), 44.26 (CH2), 51.68 (CH2), 52.46 (2CH2), 61.48 (CH2), arC: [101.24 (d, CH, J = 24.5 Hz), 108.66 (CH), 117.54 (2CH), 120.12 (C), 121.75 (2CH), 122.41 (2CH), 128.76 (CH), 129.71 (2CH), 130.37 (2CH), 130.76 (2C), 131.82 (C), 139.37 (2C), 146.87 (d, C, J C–F = 133.95 Hz)], 155.26 (C=N), 158.92 (C=O), 160.62 (C=O). MS m/z (%): 631.64 ([M−1 + Na]+, 25), 464.59 (26), 463.58 (83), 441.62 (26), 360.57 (61), 267.31 (29), 195.00 (40), 149.00 (100), 135.03 (50), 121.06 (65).
Ethyl 4-[2-fluoro-4-({2-[2-(3-hydroxy-4-methoxybenzylidene)hydrazino]-2-oxoethyl} amino)phenyl]piperazine-1-carboxylate (19a)
The mixture of solution of compound 9 (10 mmol) and 3-hydroxy-4-methoxybenzaldehyde (10 mmol) in absolute ethanol was irradiated by microwave at 200 W and 140 °C for 30 min. On cooling the reaction mixture to room temperature a solid was appeared. This crude product was recrystallized from ethanol. Yield: 72 %. M.p: 183–185 °C. FT-IR (KBr, ν, cm−1): 3342, 3181 (2NH), 3096 (ar–CH), 1678 (2C=O), 1437 (C=N), 1211 (C–O). Elemental analysis for C23H28FN5O5 calculated (%): C, 58.34; H, 5.96; N, 14.79. Found (%): C, 58.65; H, 6.06; N, 14.98. 1H NMR (DMSO-d 6, δ ppm): 1.17 (t, 3H, CH3, J = 6.8 Hz), 2.77 (s, 4H, 2CH2), 3.36 (s, 6H, 3CH2), 3.78 (s, 3H, O–CH3), 3.99 (q, 2H, CH2, J = 6.6 Hz), 5.80 (brs, 1H, NH), 6.04 (brs, 1H, NH), 6.32–6.37 (m, 3H, arH), 6.84–6.98 (m, 3H, arH), 9.27 (s, 1H, N=CH), 11.35 (s, 1H, OH). 13C NMR (DMSO-d 6, δ ppm): 15.26 (CH3), 44.29 (CH2), 44.62 (2CH2), 51.78 (2CH2), 56.22 (OCH3), 61.48 (CH2), arC: [101.23 (d, CH, J C–F = 22.0 Hz), 108.47 (CH), 112.58 (d, CH, J C–F = 15.0 Hz), 120.73 (CH), 120.96 (CH), 121.72 (CH), 127.64 (C), 129.83 (d, C, J C–F = 9.1 Hz), 146.25 (C), 146.46 (C), 150.34 (d, C, J C–F = 6.5 Hz), 151.36 (d, C, J C–F = 388.7 Hz)], 144.44 (N=CH), 167.17 (C=O), 171.66 (C=O). MS m/z (%): 497.56 ([M+1 + Na]+, 31) 496.56 ([M+Na]+,100), 370.41 (19), 360.65 (22).
Ethyl 4-[2-fluoro-4-({2-oxo-2-[2-(pyridin-4-ylmethylene)hydrazino]ethyl}amino)phenyl] piperazine-1-carboxylate (19b)
The mixture of compound 9 (10 mmol) and pyridine-4-carbaldehyde (10 mmol) in absolute ethanol was irradiated by microwave at 200 W and 140 °C for 30 min. On cooling the reaction mixture to room temperature a solid was appeared. This crude product was recrystallized from ethanol. Yield: 85 %. M.p: 184–185 °C. FT-IR (KBr, ν, cm−1): 3356, 3269 (2NH), 3057 (ar–CH), 1707, 1679 (2C=O), 1428 (C=N), 1230 (C–O). Elemental analysis for C21H25FN6O3 calculated (%): C, 58.87; H, 5.88; N, 19.61. Found (%): C, 58.97; H, 6.00; N, 19.97. 1H NMR (DMSO-d 6, δ ppm): 1.16 (brs, 3H, CH3), 2.76 (s, 4H, 2CH2), 3.41 (s, 4H, 2CH2), 4.02–4.03 (m, 2H, CH2), 4.21 (s, 2H, CH2), 6.35–6.51 (m, 2H, arH), 6.83 (brs, 1H, arH), 7.69 (brs, 2H, arH), 8.63 (s, 3H, 2arH + CH), 11.80 (s, 2H, 2NH). 13C NMR (DMSO-d 6, δ ppm): 15.26 (CH3), 47.25 (CH2), 51.79 (2CH2), 52.85 (2CH2), 61.37 (CH2), arC: [107.70 (d, CH, J C–F = 45.1 Hz), 114.07 (C), 118.26 (d, CH, J C–F = 29.3 Hz), 120.15 (CH), 124.56 (2CH), 137.02 (C), 141.37 (d, C, J C–F = 50.6 Hz), 146.20 (2CH), 152.26 (d, C, J C–F = 161.2 Hz)], 150.31 (N=CH), 160.00 (C=O), 166.71 (C=O). MS m/z (%): 467.51 ([M+K]+, 19) 451.55 ([M+Na]+,65), 429.53 ([M+1]+, 76), 267.35 (45), 201.02 (23), 162.98 (25), 160.98 (30), 149.03 (100), 135.01 (72), 118.99 (71).
Ethyl 4-[2-fluoro-4-({2-[2-(2-hydroxybenzylidene)hydrazino]-2-oxoethyl}amino)phenyl] piperazine-1-carboxylate (19c)
The mixture of compound 9 (10 mmol) and 2-hydroxybenzaldehyde (10 mmol) in absolute ethanol was irradiated by microwave at 200 W and 140 °C for 30 min. On cooling the reaction mixture to room temperature a solid was appeared. This crude product was recrystallized from ethanol. Yield: 50 %. M.p: 155–157 °C. FT-IR (KBr, ν, cm−1): 3675 (OH), 3357, 3270 (2NH), 3059 (ar–CH), 1707, 1676 (2C=O), 1428 (C=N), 1230 (C–O). Elemental analysis for C22H26FN5O4 calculated (%): C, 59.58; H, 5.91; N, 15.79. Found (%): C, 59.72; H, 6.16; N, 15.77. 1H NMR (DMSO-d 6, δ ppm): 1.17 (brs, 3H, CH3), 2.78 (s, 4H, 2CH2), 3.45 (s, 6H, 3CH2), 4.02–4.03 (m, 2H, CH2), 6.39 (brs, 2H, 2NH), 6.85 (brs, 4H, arH), 7.41 (brs, 3H, arH), 8.70 (s, 1H, N=CH), 10.56 (brs, 1H, OH). 13C NMR (DMSO-d 6, δ ppm): 15.25 (CH3), 41.29 (CH2), 44.18 (2CH2), 51.51 (2CH2), 61.52 (CH2), arC: [108.24 (CH), 116.79 (d, CH, J C–F = 36.2 Hz), 119.18 (C), 120.18 (CH), 122.19 (d, CH, J C–F = 53.4 Hz), 126.61 (CH), 131.22 (CH), 132.68 (CH), 137.00 (C), 141.26 (d, C, J C–F = 10.6 Hz), 152.71 (d, C, J C–F = 252.9 Hz), 157.86 (C)], 146.15 (N=CH), 159.33 (C=O), 163.12 (C=O). MS m/z (%): 466.51 ([M+1+Na]+, 16), 444.55 ([M+1]+, 25), 249.20 (19), 241.19 (18), 149.03 (100), 135.07 (33), 121.06 (45), 103.04 (40).
Ethyl 4-(2-fluoro-4-{[(5-thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl]amino}phenyl) piperazine-1-carboxylate (20)
The mixture of compound 9 (10 mmol) and carbon disulfide (20 mmol) in absolute ethanol was refluxed in the presence of dried potassium hydroxide (10 mmol) for 13 h. Then, the resulting solution was cooled to room temperature and acidified with acetic acid. The precipitate formed was filtered off, washed with water, and recrystallized from ethyl acetate:petroleum ether (1:3) Yield 68 %. M.p: 210–212 °C. FT-IR (KBr, ν, cm−1): 3300 (2NH), 1675 (C=O), 1428 (C=N), 1249 (C=S). Elemental analysis for C16H20FN5O3S calculated (%): C, 50.38; H, 5.29; N, 18.36. Found (%): C, 50.51; H, 5.66; N, 18.74. 1H NMR (DMSO-d 6, δ ppm): 1.17 (t, 3H, CH3, J = 6.6 Hz), 2.77 (s, 4H, 2CH2), 3.47 (s, 2H, CH2), 4.03 (q, 2H, CH2, J = 7.0 Hz), 4.34 (d, 2H, CH2, J = 5.0 Hz), 6.33–6.52 (m, 4H, ar-2H + 2NH), 6.85 (t, 1H, arH, J = 8.6 Hz). 13C NMR (DMSO-d 6, δ ppm): 15.25 (CH3), 41.37 (2CH2), 44.25 (2CH2), 51.64 (CH2), 61.50 (CH2), arC: [101.41 (d, CH, J C–F = 24.1 Hz), 108.78 (CH), 121.78 (CH), 130.67 (d, C, J C–F = 9.9 Hz), 144.97 (d, C, J C–F = 10.6 Hz), 156.95 (d, C, J C–F = 241.9 Hz)], 155.28 (C=O), 163.00 (C), 185 (C=S).
({[(6R,7R)-3-[(Acetyloxy)methyl]-7-({[5-[({4-[4-(ethoxycarbonyl)piperazin-1-yl]-3-fluorophenyl}amino)methyl]-2-thioxo-1,3,4-oxadiazol-3(2H)-yl]methyl}amino)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-2-yl]carbonyl}oxy)(triethyl)ammonium (21)
To the mixture of compound 20 (10 mmol), triethylamine (20 mmol) and formaldehyde (50 mmol) in tetrahydrofurane, 7-aca (10 mmol) was added. The mixture was stirred at room temperature 4 h. After removing the solvent under reduced pressure, a liquid product appeared. This was recrystallized by column chromatography (n-hexane:ethyl acetate, 4:1). Yield 58 %. FT-IR (KBr, ν, cm−1): 3373 (OH + NH), 2980, 2974 (aliphatic CH), 1676 (4C=O), 1432 (C=N), 1232 (C=S). Elemental analysis for C33H47FN8O8S2 calculated (%) C: 51.68; H: 6.18; N: 14.61. Found (%): C: 51.47; H: 6.00; N: 14.67. 1H-NMR (DMSO-d 6) δ ppm: 1.12 (t, 12H, 4CH3, J = 7.0 Hz), 1.99 (s, 3H, CH3), 2.98–3.18 (m, 12H, 6CH2), 3.82 (brs, 8H, 4CH2), 4.00 (s, 2H, CH2), 4.56 (s, 2H, CH2), 4.65 (s, 1H, CH), 5.19 (s, 1H, CH), 6.40 (brs, 2H, 2NH), 6.90 (brs, 1H, ar–H), 6.94 (brs, 2H, ar–H). 13C-NMR (DMSO-d 6) δ ppm: 9.33 (3CH3), 15.15 (CH3), 21.39 (CH3), 25.75 (CH2), 40.94 (CH2), 43.66 (CH2), 44.04 (CH2), 46.26 (2CH2), 48.64 (CH2), 50.95 (3CH2), 61.71 (CH2), 67.38 (CH2), 67.73 (CH), 70.89 (CH), arC: [107.63 (d, CH, J C–F = 11.8 Hz), 113.45 (CH), 115.47 (CH), 120.42 (d, C, J C–F = 34.7 Hz), 122.05 (C), 150.83 (d, C, J C–F = 273.3 Hz)], 130.04 (C), 134.26 (C), 155.50 (C=O), 155.65 (C=O), 162.28 (C), 175.25 (2C=O), 189.74 (C=S).
({[(5R,6R)-6-({[5-[({4-[4-(Ethoxycarbonyl)piperazin-1-yl]-3-fluorophenyl}amino)methyl]-2-thioxo-1,3,4-oxadiazol-3(2H)-yl]methyl}amino)-3,3-dimethyl-7-oxo-4-thia-1-aza bicyclo[3.2.0]hept-2-yl]carbonyl}oxy)(triethyl)ammonium (22)
To the mixture of compound 20 (10 mmol), triethylamine (20 mmol), and formaldehyde (50 mmol) in tetrahydrofurane, 6-apa (10 mmol) was added. The mixture was stirred at room temperature 6 h. After removing the solvent under reduced pressure, a liquid product appeared. This was recrystallized by column chromatography (n-hexane:ethyl acetate, 4:1). Yield 66 %. FT-IR (KBr, ν, cm−1): 3676 (OH), 2901, 2987 (aliphatic CH), 1768 (C=O), 1683 (2 C=O), 1431 (C=N), 1231 (C=S). Elemental analysis for C31H47FN8O6S2 calculated (%): C, 52.38; H, 6.66; N, 15.76. Found (%): C, 52.18; H, 6.79; N, 15.55. 1H-NMR (DMSO-d 6, δ ppm): 0.99–1.21 (m, 18H, 6CH3), 2.90 (q, 8H, 4CH2, J = 7.0 Hz), 3.38 (q, 8H, 4CH2, J = 7.2 Hz), 3.98–4.08 (m, 4H, 2CH2), 4.55 (s, 1H, CH), 5.26 (s, 1H, CH), 5.30 (s, 1H, CH), 5.38, 5.45 (brs, 2H, 2NH), 6.80 (brs, 1H, ar–H), 6.94 (brs, 2H, ar–H). 13C-NMR (DMSO-d 6, δ ppm): 9.32 (3CH3), 15.25 (CH3), 27.77 (CH3), 32.62 (CH3), 44.13 (CH2), 45.67 (2CH2), 51.09 (CH2), 51.50 (CH2), 52.61 (CH2), 56.73 (C–(CH3)2), 61.52 (CH2), 62.23 (CH2), 62.99 (CH2), 63.59 (CH2), 65.39 (CH), 67.00 (CH), 73.68 (CH), arC: [107.41 (d, CH, J C–F = 9.8 Hz),113.72 (d, CH, J C–F = 33.0 Hz), 120.07 (CH), 134,64 (d, C, J C–F = 9.1 Hz), 143.12 (d, C, J C–F = 9.5 Hz), 154.47 (d, C, J C–F = 81.2 Hz)], 163.63 (C), 170.45 (C=O), 170.91 (C=O), 172.13 (C=O), 175.29 (C=S).
Antimicrobial activity assessment
All bacterial and yeast strains were obtained from the Hifzissihha Institute of Refik Saydam (Ankara, Turkey) and were as follows: Pseudomonas aeruginosa ATCC 27853, Enterococcus faecalis ATCC 29212, S. aureus ATCC 25923, B. cereus 709 ROMA, Ms: M. smegmatis ATCC607, C. albicans ATCC 60193, Sc: S. cerevisiae RSKK 251. All the newly synthesized compounds were dissolved in dimethyl sulfoxide (DMSO) and ethanol to prepare chemicals of stock solution of 10 mg mL−1.
Agar-well diffusion method
Simple susceptibility screening test using agar-well diffusion method as adapted earlier (Ahmad et al., 1998) was used. Each microorganism was suspended in Mueller–Hinton (MH) (Difco, Detroit, MI, USA) broth and diluted approximately to 106 colony forming unit (cfu) mL−1. They were “flood-inoculated” onto the surface of MH agar and Sabouraud dextrose agar (SDA) (Difco, Detriot, MI, USA) and then dried. For C. albicans and C. tropicalis, SDA were used. Five-millimeter diameter wells were cut from the agar using a sterile cork-borer, and 50 mL of the extract substances was delivered into the wells. The plates were incubated for 18 h at 35 °C. Antimicrobial activity was evaluated by measuring the zone of inhibition against the test organism. Ampicillin (10 mg) and Fluconazole (5 mg) were used as standard drugs. Dimethyl sulfoxide and ethanol were used as solvent controls. The antimicrobial activity results are summarized in Table 1.
Table 1.
Screening for antimicrobial activity of the compounds (50 μL)
| Comp. no | Microorganisms and inhibition zone (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ec | Yp | Pa | Sa | Ef | Bc | Ms | Ca | Sc | |
| 2 | – | – | – | – | – | 6 | – | – | – |
| 3 | – | – | – | 11 | – | 6 | – | 15 | 15 |
| 4a | 8 | 8 | – | – | – | 10 | 8 | 8 | |
| 4b | – | – | – | – | – | – | – | – | – |
| 4c | – | – | – | – | – | – | – | 8 | 8 |
| 4d | 6 | 6 | – | – | – | 8 | 20 | 15 | 15 |
| 4e | – | – | – | – | – | 20 | 10 | 10 | |
| 4f | 8 | 8 | 6 | 6 | – | 6 | 25 | 20 | 10 |
| 5 | – | – | – | – | – | – | – | 6 | 7 |
| 6 | – | – | – | – | – | – | – | – | – |
| 7 | – | – | – | – | – | – | – | – | – |
| 8 | – | – | – | – | – | 6 | – | – | – |
| 9 | – | – | – | – | – | 6 | – | 7 | – |
| 10 | – | – | – | – | – | 6 | – | – | – |
| 11 | – | – | – | 10 | – | 6 | – | – | – |
| 12 | – | – | – | – | – | – | – | 6 | 6 |
| 13 | – | – | 6 | – | – | – | – | 8 | 10 |
| 14 | – | – | – | 6 | 6 | – | – | 8 | – |
| 15 | – | 6 | 6 | 6 | – | – | – | 10 | – |
| 16 | 8 | – | – | 6 | 10 | – | – | 6 | 10 |
| 17 | 9 | 9 | 8 | 13 | – | 16 | 14 | 6 | 12 |
| 18 | – | – | 6 | 10 | – | 6 | – | 8 | 12 |
| 19a | – | – | 6 | – | 8 | – | – | 9 | 6 |
| 19b | – | – | – | – | – | – | – | 8 | – |
| 19c | – | – | 6 | – | 8 | – | – | 8 | 6 |
| 20 | – | – | – | 10 | 6 | 6 | 15 | 8 | 12 |
| 21 | 8 | 8 | – | 6 | 10 | 10 | 20 | 10 | 8 |
| 22 | 9 | 8 | 15 | 9 | 10 | 18 | 8 | 12 | |
| Amp. | 10 | 18 | 18 | 35 | 10 | 15 | |||
| Strep. | 35 | ||||||||
| Flu. | 25 | >25 | |||||||
(–), no activity
Ec, Escherichia coli ATCC 25922; Yp, Yersinia pseudotuberculosis ATCC 911; Pa, Pseudomonas aeruginosa ATCC 43288; Sa, Staphylococcus aureus ATCC 25923; Ef, Enterococcus faecalis ATCC 29212; Bc, Bacillus cereus 702 Roma; Ms, M. smegmatis ATCC607; Ca, Candida albicans ATCC 60193; Sc, Saccharomyces cerevisiae RSKK 251; Amp., Ampicillin; Strep., Streptomycin; Flu., Fluconazole
Urease inhibition assay
Reaction mixtures comprising 25 μL of Jack bean urease, 55 μL of buffer (100 mM urea, 0.01 M K2HPO4, 1 mM EDTA, and 0.01 M LiCl, pH 8.2), and 100 mM urea were incubated with 5 μL of the test compounds at room temperature for 15 min in microtiter plates. The production of ammonia was measured by indophenol method and used to determine the urease inhibitory activity. The phenol reagent (45 μL, 1 % w/v phenol, and 0.005 % w/v sodium nitroprusside) and alkali reagent (70 μL, 0.5 % w/v sodium hydroxide, and 0.1 % v/v NaOCl) were added to each well and the increasing absorbance at 625 nm was measured after 20 min, using a microplate reader (Molecular Device, USA). The percentage inhibition was calculated from the formula 100 − (OD test well/OD control) × 100. Thiourea was used as the standard inhibitor. In order to calculate IC50 values, different concentrations of synthesized compounds and standard were assayed at the same reaction conditions (Weatherburn, 1967). The obtained results are presented in Table 2.
Table 2.
Inhibitory activities of the synthesized compounds against Jack Bean urease
| Compound | % Inhibition ± S.D. | IC50 ± S.D. |
|---|---|---|
| Thiourea | 100 ± 0.1 | 54.56 ± 4.17 |
| 2 | -a | –b |
| 3 | 11 ± 3.3 | – |
| 4a | N.s. | – |
| 4b | N.s. | – |
| 4d | - | – |
| 4e | 1 ± 0.2 | – |
| 4f | - | – |
| 5 | - | – |
| 6 | 3 ± 3.0 | – |
| 7 | N.s. | – |
| 8 | 7 ± 3.1 | – |
| 9 | 7 ± 3.0 | – |
| 10 | 4 ± 1 | – |
| 12 | 56 ± 4 | – |
| 14 | - | – |
| 15 | 100 ± 1.5 | 4.67 ± 0.53 |
| 17 | 100 ± 2.1 | 45.37 ± 0.78 |
| 18 | - | – |
| 19a | - | – |
| 19b | 47 ± 0.1 | – |
| 19c | - | – |
| 20 | N.s. | – |
N.s. Not soluble
aNo inhibition
bNot determined
Anti-lipase activity assay
The inhibitory effects of those compounds were evaluated against porcine pancreatic lipase (PPL) (15 ng mL−1). Lipase activity assay was done according to Verger et al., (Woods et al., 2003). Microtiter plates were coated with purified tung oil TAGs. Compounds were mixed with PPL 1:2 (v/v) and incubated for 30 min. The microtiter plates containing purified tung oil, lipase solution, and assay buffer (10 mM Tris–HCl buffer, pH 8.0, containing 150 mM NaCl, 6 mM CaCl2, 1 mM EDTA, and 3 mg mL−1 β-cyclodextrin) were recorded continuously for 40 min against the buffer alone by using microplate reader (SpectraMax M5, Molecular Devices) at 272 nm. The inhibitory activity of those compounds and Orlistat, a positive control against pancreatic lipase, were measured at concentration of 6.25, 2.08, and 1.04 μg mL−1. Residual activities were calculated by comparing to control without inhibitor (T+). The assays were done in triplicate. The IC50 value was determined as the concentration of compound that give 50 % inhibition of maximal activity. The results are presented in Table 3.
Table 3.
Porcine pancreatic lipase inhibitory activity of synthesized compounds
| Compound no. | % Inhibition |
|---|---|
| 2 | – |
| 3 | – |
| 5 | – |
| 6 | 16 |
| 7 | 33 |
| 8 | 22 |
| 9 | 20 |
| 10 | – |
| 11 | – |
| 12 | 68 |
| 13 | 63 |
| 14 | 75 |
| 15 | 73 |
| 16 | 6 |
| 17 | – |
| 18 | 1 |
| 19a | – |
| 19b | – |
| 19c | – |
| 20 | 33 |
| Orlistat | 99 |
| DMSO control | – |
| Positive control | – |
All compounds were screened at concentration of 6.25 μg mL−1
Acknowledgments
This Project was supported by Scientific and Technological Research Council of Turkey (TUBITAK, Project No: 107T333) and Karadeniz Technical University, BAP, Turkey (Ref. No. 8623) and is gratefully acknowledged.
References
- Ahmad I, Mehmood Z, Mohammed F. Screening of some Indian plants for their antimicrobial properties. J Ethnopharmacol. 1998;62:183–193. doi: 10.1016/S0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- Almajan GL, Barbucenau SF, Almajan ER, Draghici C, Saramet G. Synthesis, characterization and antibacterial activity of some triazole Mannich bases carrying diphenylsulfone moieties. Eur J Med Chem. 2009;44:3083–3089. doi: 10.1016/j.ejmech.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Ashok M, Holla BS, Poojary B. Convenient one pot synthesis and antimicrobial evaluation of some new Mannich bases carrying 4-methylthiobenzyl moiety. Eur J Med Chem. 2007;42:1095–1101. doi: 10.1016/j.ejmech.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Orzeszko-Krzesinska B, Maurin JK, Orzeszko A. Synthesis and anti-HIV studies of 2- and 3-adamantyl-substituted thiazolidin-4-ones. Eur J Med Chem. 2009;44:303–311. doi: 10.1016/j.ejmech.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayrak H, Demirbas A, Demirbas N, Alpay Karaoglu S. Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Eur J Med Chem. 2009;44:4362–4366. doi: 10.1016/j.ejmech.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Bayrak H, Demirbas A, Demirbas N, Alpay-Karaoglu S. Cyclization of some carbothioamide derivatives containing antipyrine and triazole moieties and investigation of their antimicrobial activities. Eur J Med Chem. 2010;45:4726–4732. doi: 10.1016/j.ejmech.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Bektas H, Karaali N, Sahin D, Demirbas A, Alpay Karaoglu S, Demirbas N. Synthesis and antimicrobial activities of some new 1,2,4-triazole derivatives. Molecules. 2010;15:2427–2438. doi: 10.3390/molecules15042427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary P, Kumar R, Verma AK, Singh D, Yadav V, Hillar AK, Sharmab GL, Chandraa R. Synthesis and antimicrobial activity of N-alkyl and N-aryl piperazine derivatives. Bioorg Med Chem. 2006;14:1819–1826. doi: 10.1016/j.bmc.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Demirbas N, Ugurluoğlu R, Demirbas A. Synthesis of 3-alkyl(aryl)-4-alkylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-ones and 3-alkyl-4-alkylamino-4,5-dihydro-1H-1,2,4-triazol-5-ones as antitumor agents. Bioorg Med Chem. 2002;10:3717–3723. doi: 10.1016/S0968-0896(02)00420-0. [DOI] [PubMed] [Google Scholar]
- Demirbas A, Sahin D, Demirbas N, Alpay Karaoglu S. Synthesis of some new 1,3,4-thiadiazol-2-ylmethyl-1,2,4-triazole derivatives and investigation of their antimicrobial activities. Eur J Med Chem. 2009;44:2896–2903. doi: 10.1016/j.ejmech.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Foroumadi A, Emami S, Mehni M, Hassan M, Shafiee A. Synthesis and antibacterial activity of N-[2-(5-bromothiophen-2-yl)-2-oxoethyl] and N-[(2-5-bromothiophen-2-yl)-2-oximinoethyl] derivatives of piperazinyl quinolones. Bioorg Med Chem Lett. 2005;15:4536–4539. doi: 10.1016/j.bmcl.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Havrylyuk D, Zimenkovsky B, Vasylenko O, Zaprutko L, Gzella A, Lesyk R. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur J Med Chem. 2009;44:1396–1404. doi: 10.1016/j.ejmech.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Hearn MJ, Cynamon MH. Design and synthesis of antituberculars: preparation and evaluation against Mycobacterium tuberculosis of an isoniazid Schiff base. J Antimic. Chemother. 2004;53:185–191. doi: 10.1093/jac/dkh041. [DOI] [PubMed] [Google Scholar]
- Holla BS, Rao BS, Sarojini Holla BK, Akberali PM, Kumari NS. Synthesis and studies on some new fluorine containing triazolothiadiazines as possible antibacterial, antifungal and anticancer agents. Eur J Med Chem. 2006;41:657–663. doi: 10.1016/j.ejmech.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Jones DH, Slack R, Squires S, Wooldridge KRH. Antiviral chemotherapy. I. The activity of pyridine and quinoline derivatives against neurovaccinia in mice. J Med Chem. 1965;8:676–680. doi: 10.1021/jm00329a026. [DOI] [Google Scholar]
- Karthikeyan MS, Prasad DJ, Poojary B, Bhat KS, Holla BS, Kumari NS. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorg Med Chem. 2006;14:7482–7489. doi: 10.1016/j.bmc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Kategaonkar AH, Shinde PV, Kategaonkar AH, Pasale SK, Shingate BB, Shingare MS. Synthesis and biological evaluation of new 2-chloro-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)quinoline derivatives via click chemistry approach. Eur J Med Chem. 2010;45:3142–3146. doi: 10.1016/j.ejmech.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Lohray BB, Lohray VB, Srivastava BK, Gupta S, Solanki M, Pandya P, Kapadnis P. Novel 4-N-substituted aryl pent-2-ene-1,4-dione derivatives of piperazinyloxazolidinones as antibacterials. Bioorg Med Chem Lett. 2006;16:1557–1561. doi: 10.1016/j.bmcl.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Lv PC, Zhou CF, Chen J, Liu PG, Wang KR, Mao WJ, Li HQ, Yang Y, Xiong J, Zhu HL. Design, synthesis and biological evaluation of thiazolidinone derivatives as potential EGFR and HER-2 kinase inhibitors. Bioorg Med Chem. 2010;18(2010):314–319. doi: 10.1016/j.bmc.2009.10.051. [DOI] [PubMed] [Google Scholar]
- Mallikarjuna BP, Sastry BS, Kumar GVS, Rajendraprasad Y, Chandrashekar SM, Sathisha K. Synthesis of new 4-isopropylthiazole hydrazide analogs and some derived clubbed triazole, oxadiazole ring system—a novel class of potential antibacterial, antifungal and antitubercular agents. Eur J Med Chem. 2009;44:4739–4746. doi: 10.1016/j.ejmech.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Metwally NH, Abdalla MA, Mosselhi MAN, El-Desoky EA. Synthesis and antimicrobial activity of some new N-glycosides of 2-thioxo-4-thiazolidinone derivatives. Carbohydr. Res. 2010;345:1135–1141. doi: 10.1016/j.carres.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Mushtaque M, Avecilla F, Azam A. Synthesis, characterization and structure optimization of a series of thiazolidinone derivatives as Entamoeba histolytica inhibitors. Eur J Med Chem. 2012;55:439–448. doi: 10.1016/j.ejmech.2012.06.052. [DOI] [PubMed] [Google Scholar]
- Patole J, Shingnapurkar D, Padhye S, Ratledge C. Schiff base conjugates of p-aminosalicylic acid as antimycobacterial agents. Bioorg Med Chem Lett. 2006;16:1514–1517. doi: 10.1016/j.bmcl.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Ren S, Wang R, Komatsu K, Bonaz-Krause P, Zyrianov Y, McKenna CE, Csipke C, Tokes ZA, Lien EJ. Synthesis, biological evaluation, and quantitative structure-activity relationship analysis of new Schiff bases of hydroxysemicarbazide as potential antitumor agents. J Med Chem. 2002;45:410–419. doi: 10.1021/jm010252q. [DOI] [PubMed] [Google Scholar]
- Subtelna I, Atamanyuk D, Szymanska E, Konowicz KK, Zimenkovsky B, Vasylenko O, Gzella A, Lesyk R. Synthesis of 5-arylidene-2-amino-4-azolones and evaluation of their anticancer activity. Bioorg Med Chem. 2010;18:5090–5102. doi: 10.1016/j.bmc.2010.05.073. [DOI] [PubMed] [Google Scholar]
- Vicini P, Geronikaki A, Incerti M, Zani F, Dearden J, Hewitt M. Bioorg Med Chem. 2008;16:3714–3724. doi: 10.1016/j.bmc.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Walczak K, Gondela A, Suwinski J. Synthesis and anti-tuberculosis activity of N-aryl-C-nitroazoles. Eur J Med Chem. 2004;39:849–853. doi: 10.1016/j.ejmech.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhao Y, Zhang G, Lv Y, Zhang N, Gong P. Design, synthesis and biological evaluation of novel 4-thiazolidinones containing indolin-2-one moiety as potential antitumor agent. Eur J Med Chem. 2011;46:3509–3518. doi: 10.1016/j.ejmech.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Weatherburn AW. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39:971–974. doi: 10.1021/ac60252a045. [DOI] [Google Scholar]
- Woods GL, Brown-Elliott BA, Desmond EP, Hall GS, Heifets L, Pfyffer EG, Ridderhof JC, Wallace RJ, Warren NC, Witebsky FG (2003) Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes. App. Stand. NCCLS document M24-A: 18–23 [PubMed]
- Zhao YJ, Wei W, Su ZG, Ma GH. Poly (ethylene glycol) prodrug for anthracyclines via N-Mannich base linker: design, synthesis and biological evaluation. Int J Pharm. 2009;379:90–99. doi: 10.1016/j.ijpharm.2009.06.013. [DOI] [PubMed] [Google Scholar]