Abstract
Monodentate phosphoramidite ligands have been developed based on enantiopure 6,6′-dimethylbiphenols with axial chirality. These chiral ligands are easy to prepare and flexible for modifications. The fine-tuning capability of these ligands plays a significant role in achieving high enantioselectivity in the asymmetric hydroformylation of allyl cyanide and the conjugate addition of diethylzinc to cycloalkenones.
Recently, chiral monodentate phosphorus ligands (phosphites, phosphoramidites, and phosphonites) have been attracting considerable interest for their use in catalytic asymmetric synthesis, because the chiral catalysts bearing these ligands have proven to be highly efficient in various asymmetric reactions. This is a previously univestigated wave in the design of chiral ligands, which makes a sharp contrast to almost three decades of predominance by C2 symmetrical bidentate phosphorus ligands for a variety of catalytic asymmetric transformations. This wave of designing simple and readily modifiable chiral structures, which are easy to synthesize, is fitting very well to the trendy and highly practical combinatorial approaches to the development of the most suitable chiral ligand for a particular catalytic asymmetric process of commercial value or academic interest. This approach is currently considered most practical rather than trying to develop a universal and almighty chiral ligand for different types of catalytic asymmetric transformations.
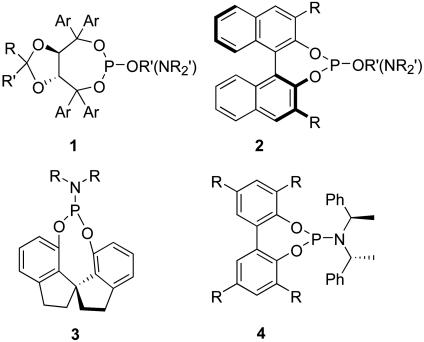
Before the launch of our research program on the development of chiral monodentate phosphorus ligands based on enantiopure 2,2′-dihydroxy-6,6′-dimethylbiphenyls, by far, the most studied monodentate ligands were phosphites and phosphoramidites based on TADDOL (1) (1) BINOL (2) (2–4), a spirobiindanediol (3) (5), or an achiral biphenol (4) (6, 7) bearing a chiral or achiral secondary alcohol or amine (Fig. 1). These ligands have found a wide range of applications in metal-catalyzed asymmetric transformations such as hydrogenation (5, 8–10), 1,4-additions of dialkylzinc (11, 12) and boronic acids (13) to enones, hydrovinylation (14), hydrosilylation (15), intramolecular Heck reaction (16), hydroformylation (17), allylic alkylation (18, 19), amination (20), and etherification (21).
Fig. 1.
Structures of known chiral phosphite and phosphoramidite ligands derived from diols.
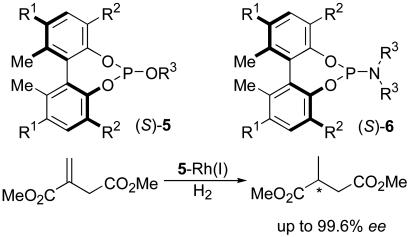
We have been developing a previously uninvestigated class of chiral monodentate phosphite and phosphoramidite ligands, 5 and 6, from readily accessible enantiopure, axially chiral biphenyls (Fig. 2) and have recently published the successful application of chiral monophosphite ligands 5 to the Rh(I)-catalyzed asymmetric hydrogenation of dimethyl itaconate (Fig. 2) (22).
Fig. 2.
Biphenol-based phosphites and phosphoramidites.
One of the salient and practical features of these chiral monophosphite ligands is the fine-tuning capability with modifiable substituents R1, R2, and R3 in the formula 5. This fine-tuning capability of ligands 5 (phosphites) and 6 (phosphoramidites) is expected to play a crucial role in the application of these ligands to a variety of catalytic asymmetric reactions. In fact, we have demonstrated that the substituents (R2) at the 3,3′ positions exert dramatic effects on the catalyst activity and enantioselectivity of the asymmetric hydrogenation of dimethyl itaconate (22).
We describe here the applications of a library of chiral monodentate phosphoramidite ligands 6 to the Rh-catalyzed hydroformylation of allyl cyanide and the Cu-catalyzed conjugate addition of diethylzinc to cycloalkenones, wherein remarkable effects of fine-tuning at the 3,3′ positions are observed on regioselectivity and enantioselectivity.
Materials and Methods
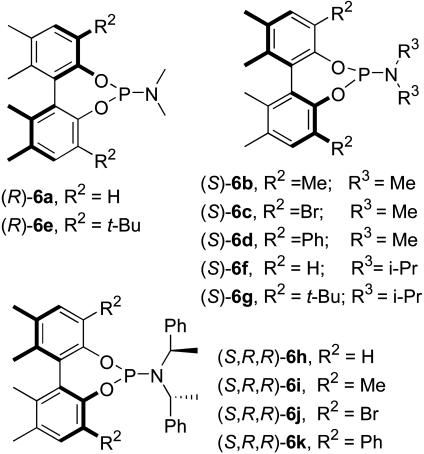
Monodentate phosphoramidites 6a–6e were synthesized by reacting the corresponding enantiopure biphenols (22) with hexamethylphosphorous triamide as described (23). Other new ligands 6f–6k were synthesized from the enantiopure biphenols, amines, and PCl3 as described (6). Fig. 3 summarizes a library of ligands 6 thus synthesized for this study.
Fig. 3.
Enantiopure biphenol-based chiral monodentate phosphoramidite ligands.
As Fig. 3 shows, H, Me, Br, Ph, and t-Bu are used as the 3,3′ substituents, and dimethylamine, diisopropylamine, and (R,R)-bis(phenylethyl)amine (6h–6k) are used as the amine moiety. The enantiopure biphenols were prepared by our method (22). The characterization data for the new phosphoramidites, 6a–6k, are presented in Supporting Text, which is published as supporting information on the PNAS web site. Allyl cyanide, cyclohexenone, cycloheptenone, cyclopentenone, and Cu(OTf)2 were purchased from Aldrich and used as received. Rh(acac)(CO)2 was obtained from Mitsubishi Chemical Corporation (Tokyo).
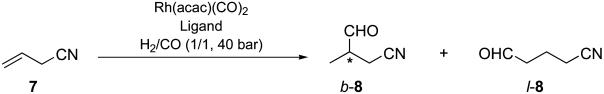
General Procedure for the Hydroformylation of Allyl Cyanide (7). In a 15-ml Pyrex reaction vessel with a magnetic stirring bar, Rh(acac)(CO)2 and a phosphoramidite ligand 6 were dissolved in a degassed solvent (5 ml), and the mixture was stirred at room temperature for 5 min under nitrogen. To this catalyst solution was added allyl cyanide (7, 80 μl, 1.0 mmol), and the reaction vessel was placed in a 300-ml stainless steel autoclave. The autoclave was purged with carbon monoxide and then charged with carbon monoxide and hydrogen [1:1, 40 bars (1 bar = 100 kPa)]. The reaction was carried out with stirring at a given temperature (25–60°C) and for a given time (5–74 h). The autoclave was allowed to cool to room temperature, and then the gases were carefully released. The solvent was removed and the crude reaction mixture was subjected to 1H NMR for determination of the conversion and the branched-to-linear ratio of the resulting aldehydes 8. To the aldehyde mixture, (S)-methylbenzylamine (130 μl) and anhydrous MgSO4 were added, and the mixture was stirred at room temperature for 1 h. The solid was filtered off, and the solution was concentrated in vacuo to give the corresponding diastereomeric mixture of aldimines. The enantioselectivity was determined by 1H NMR analysis of the diastereomer ratio of the aldimines. The absolute configuration of 3-formylbutanenitrile (b-8) was determined by converting the compound to 4-methyldihydrofuran-2-one (24), followed by the measurement of its specific optical rotation.
General Procedure for the Conjugate Addition of Diethylzinc to Cycloalkenones. All reactions at room temperature were performed by using a Miniblock XT parallel synthesis reactor. The reactions at low temperatures were carried out individually. In a 20-ml reaction tube, a mixture of Cu(OTf)2 (1.8 mg, 5 μmol) and a phosphoramidite ligand 6 (11 μmol) in a degassed solvent (5 ml) was stirred at room temperature for 20 min. To this mixture, Et2Zn (1.1 M in toluene, 1.4 ml) and a cycloalkenone (1.0 mmol) were added at a given temperature (–30 or 23°C). The mixture was stirred at the same temperature for 4 h. The reaction was quenched with 1 N HCl. The aqueous layer was extracted with Et2O. The combined extracts were washed with brine and dried over MgSO4. The crude product was filtered through a short silica gel column and subjected to 1H NMR and GC analysis. The conversion of the reaction was determined by 1H NMR and GC analysis. The enantioselectivity was determined by GC analysis by using a Supelco Beta Dex-225 column. The absolute configuration was determined by chiral GC analysis in comparison with authentic samples.
Results and Discussion
Rh-Catalyzed Asymmetric Hydroformylation of Allyl Cyanide. Asymmetric hydroformylation of allyl cyanide, giving (R)-3-formylbutanenitrile [(R)-b-8] offers an attractive entry to (R)-2-methyl-4-aminobutanol, which is a key building block for TAK-637, a drug candidate for treatment of urinary continence (17). The best result for this process so far obtained to date was reported by de Vries and coworkers (17), giving 8 in 72:28 branched-to-linear ratio and 66% enantiomeric excess (ee) by using (R,S)-BINAPHOS as the chiral ligand. Accordingly, this process is useful but quite demanding, and an obvious need exists for the improvement in the regioselectivity and enantioselectivity for this process to be practical. Thus, we selected allyl cyanide as the substrate to evaluate the efficacy of phosphoramidite ligands 6.
BINOL-based monophosphoramidite ligands 2 have been used in this process. However, the best result was in 76:24 branched-to-linear ratio and only 18% ee for b-8 (17). Also, the reaction rate was very low with only 31–45% conversion after 5 h by running the reactions at 60°C and 30 bars of CO/H2 (1:1) in toluene.
In sharp contrast to the BINOL-based ligands 2, the reactions by using chiral biphenol-based ligands 6a–6e and 6g at 60°C and 40 bars of CO/H2 (1:1) in benzene completed within 5 h, giving the aldehydes 8 as exclusive products, i.e., no hydrogenation product was detected. The results are summarized in Table 1.
Table 1.
| Entry | Ligand | Ligand/Rh | Solvent | Temperature, °C | Time, h | Conversion, %* | b/l† | % ee‡ |
|---|---|---|---|---|---|---|---|---|
| 1 | (R)-6a | 3 | C6H6 | 60 | 5 | 100 | 80:20 | 5 (S) |
| 2 | (S)-6b | 3 | C6H6 | 60 | 5 | 100 | 85:15 | 14 (R) |
| 3 | (S)-6c | 3 | C6H6 | 60 | 5 | 100 | 83:17 | 2 (R) |
| 4 | (S)-6d | 3 | C6H6 | 60 | 5 | 100 | 84:16 | 10 (R) |
| 5 | (R)-6e | 3 | C6H6 | 60 | 5 | 100 | 92: 8 | 69 (S) |
| 6 | (S)-6g | 3 | C6H6 | 60 | 5 | 100 | 81:19 | 0 |
| 7 | (R)-6e | 1 | C6H6 | 60 | 5 | 100 | 90:10 | 61 (S) |
| 8 | (R)-6e | 2 | C6H6 | 60 | 5 | 100 | 91: 9 | 65 (S) |
| 9 | (R)-6e | 3 | C6H6 | 60 | 5 | 100 | 92: 8 | 68 (S) |
| 10 | (R)-6e | 4 | C6H6 | 60 | 5 | 100 | 93: 7 | 65 (S) |
| 11 | (R)-6e | 3 | Toluene | 60 | 5 | 100 | 90:10 | 67 (S) |
| 12 | (R)-6e | 3 | THF | 60 | 5 | 100 | 86:14 | 58 (S) |
| 13 | (R)-6e | 3 | CH2Cl2 | 60 | 5 | 100 | 89:11 | 60 (S) |
| 14 | (R)-6e | 3 | CH3OH | 60 | 5 | 100 | 91: 9 | 49 (S) |
| 15 | (R)-6e | 3 | Toluene | 50 | 5 | 100 | 92: 8 | 76 (S) |
| 16 | (R)-6e | 3 | Toluene | 40 | 5 | 89 | 93: 7 | 78 (S) |
| 17 | (R)-6e | 3 | Toluene | 40 | 23 | 100 | 93: 7 | 78 (S) |
| 18 | (R)-6e | 3 | Toluene | 30 | 47 | 100 | 95: 5 | 79 (S) |
| 19 | (R)-6e | 3 | Toluene | 25 | 74 | 100 | 96: 4 | 80 (S) |
Reactions were performed on a 1.0-mmol scale with [Rh(acac)(CO)2] (0.5 mol%) and indicated amounts of ligand 6 in a degassed solvent (5 ml).
The conversion was determined by 1H NMR analysis, and no hydrogenation product was observed.
The branched-to-linear (b/l) ratio was determine by 1H NMR analysis.
Enantiopurity was determined by converting the aldehyde to the corresponding aldimine of (S)-methylbenzylamine and subjected to 1H NMR analysis. Absolute configuration was determined by converting b-8 to 4-methyldihydrofuran-2-one, followed by the measurement of its specific optical rotation.
As Table 1 shows, the best result under these conditions (i.e., 60°C and 40 bars, ligand/Rh = 3, benzene as solvent) was obtained with (R)-6e, bearing bulky t-Bu groups at the 3,3′ positions of the biphenol moiety, giving aldehyde 8 in 92:8 branched-to-linear ratio and 69% ee for (S)-b-8 (entry 5). The results also clearly indicate the dramatic effects of the 3,3′ substituents on the regioselectivity and enantioselectivity of the reaction. The ligands bearing smaller substituents, i.e., H, Me, Br, and Ph (6a–6d), at the 3,3′ positions gave 8 in lower branched-to-linear ratio (b/l = 80:20–84:16) and significantly lower enantiopurity (2–14% ee) for b-8 (entries 1–4). In addition, a larger group (i.e., isopropyl) at the amine moiety [ligand (S)-6g] is detrimental to the enantioselectivity (entry 6).
After initial screening to select the most efficient ligand (R)-6e from the library, we investigated the reaction variables with (R)-6e. First, the effect of the ligand/Rh ratio on the regioselectivity and enantioselectivity was examined. As Table 1 (entries 7–10) shows, the ligand/Rh ratio has only a minor influence on the reaction, i.e., a comparable good result was obtained even with the ligand/Rh ratio of 1:1, which is rather surprising (entry 7). The best ligand/Rh ratio appears to be 3:1 (entry 9). Thus, this ligand/Rh ratio was used for the further study. Next, effect of different solvents on the selectivities was examined. The reactions in toluene, tetrahydrofuran, CH2Cl2, and MeOH also gave 8 in complete conversion under the same reaction conditions and no hydrogenation product was observed (entries 11–14). However, the reactions in tetrahydrofuran and CH2Cl2 gave 8 with lower branched-to-linear ratio and enantiopurity (entries 12 and 13), and the reaction in MeOH gave lower enantioselectivity (entry 14) than those in benzene and toluene. Because the use of toluene provided almost the same results as benzene (entry 11 vs. entry 9), toluene was used as the solvent for further optimization. Finally, the effect of reaction temperature on the selectivities was examined. As Table 1 (entries 11 and 15–18) shows, lower temperatures have a favorable effect on the selectivities. Thus, the best result to date was obtained when the reaction was carried out at 25°C, giving (S)-b-8 with 96:4 branched-to-linear ratio and 80% ee (entry 19). This result is substantially better than that obtained by using (R,S)-BINAPHOS (17) and far better than those obtained by using BINOL-based phosphoramidite ligands (17) (see above). Further optimization of the ligand design along this line should be pursued.
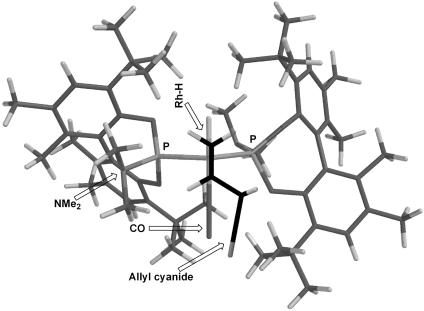
To shed light on the dramatic effects of t-Bu groups at the 3,3′ positions of ligand (S)-6e, we performed a molecular modeling study on the most plausible active-catalyst species, HRh(CO)2(L*)2 [L* = (S)-6e]. In fact, the widely accepted mechanism on the Rh-catalyzed hydroformylation of olefins with diphosphine, diphosphite, or phosphine–phosphite ligands includes a trigonal-bipyramidal configuration at the Rh metal center (25). Accordingly, it is reasonable to assume that the most active and effective Rh catalyst species in this study bears two monodentate phosphoramidite ligands on the Rh metal and has a trigonal-bipyramidal structure. Two possible phosphorus complexation modes exist for HRh(CO)2(L*)2: (i) two phosphorus atoms are in the equatorial positions and (ii) one phosphorus atom is equatorial, but the other one is apical. A molecular modeling study (Spartan program; MM2/PM3) on these two possibilities clearly indicates that the latter structure is not possible because of the serious repulsion between two bulky phosphoramidite ligands. Accordingly, we made two model complexes with a trigonal-bipyramidal configuration in which one hydrogen and one carbonyl occupy the apical positions and two phosphorus and one carbonyl are in the equatorial positions by using ligand (S)-6e. Since two arrangements for hydrogen and a carbonyl to occupy the two apical positions are still possible, we needed to minimize the energy of two model complexes and compare the values. Fig. 4 shows the energy minimized and the lowest energy structure of HRh(CO)2(L*)2 [L* = (S)-6e]. From this structure it is obvious why very bulky t-Bu groups are necessary at the 3,3′ positions to exert effective asymmetric bias to the incoming small allyl cyanide molecule. It is impressive to see how two t-Bu groups above the H-Rh moiety disfavor the formation of linear alkyl-Rh species. Also, it is obvious that the Me2N moiety in the front-left position is playing a key role in controlling the enantioface selection of allyl cyanide. Fig. 4 also illustrates the favorable approach of an allyl cyanide molecule to this complex with si-face. It is clear that the re-face approach suffers from the steric hindrance caused by the Me2N moiety. Overall, Fig. 4 can accommodate the observed enantioface selection and the high branched-to-linear ratio in this reaction.
Fig. 4.
Proposed enantioface selection and regiocontrol in the Rh-catalyzed hydroformylation of allyl cyanide by using (S)-6e.
Further mechanistic studies on the asymmetric induction and active catalyst species are needed.
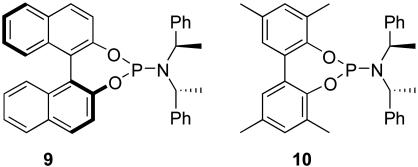
Cu-Catalyzed Conjugate Addition of Diethylzinc to Cycloalkenones. Asymmetric conjugate addition of organometallic reagents to α,β-unsaturated carbonyl compounds is an attractive enantioselective carbon–carbon bond-forming process. As one of such processes, the copper-catalyzed asymmetric conjugate addition of dialkylzinc to 2-alkenones by using monodentate phosphoramidite ligands has been extensively studied by Feringa and colleagues (26) and Alexakis et al. (7) in the past several years. The best ligand developed by Feringa and colleagues for this reaction is the BINOL-based ligand 9 bearing (R,R)-bis(phenylethyl)amine (Fig. 5) (7, 26). Also, ligand 10 consisting of a racemic biphenol and (R,R)-bis(phenylethyl)amine (Fig. 5) was able to achieve 89% ee in the Cu(OTf)2-catalyzed addition of diethylzinc to 2-cyclohexenone in toluene at –30°C (7).
Fig. 5.
Representative phosphoramidite ligands used for the Cu-catalyzed conjugate addition of R2Zn.
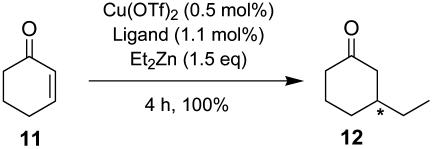
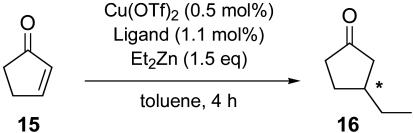
We selected 2-cyclohexenone (11), 2-cycloheptenone (13), and 2-cyclopentenone (15) as cycloalkenones to evaluate the efficacy of the phosphoramidite ligands 6 in the Cu(OTf)2-catalyzed diethylzinc conjugate addition, giving the corresponding 3-ethylcycloalkanones 12, 14, and 16, respectively.
The results of the Cu-catalyzed diethylzinc reaction with 2-cyclohexenone are summarized in Table 2. As Table 2 shows, the bulkiness of the substituents (R2) at the 3,3′ positions and of the amine moiety (R3) of ligands 6a–6k exerts marked influence on the enantioselectivity of the reaction. For the ligands bearing a dimethylamine moiety (6a–6e), the enantioselectivity decreases in the order H, Me, Ph, Br, and t-Bu (52.7% ee → 23.5% ee) (entries 1–5). When a diisopropylamine moiety is introduced, this trend becomes more drastic, i.e., whereas (S)-6f (R2 = H) gives 12 with better enantiopurity (57.7% ee) than (R)-6a (entry 6), (S)-6g (R2 = t-Bu) affords 12 with very poor enantiopurity (7.9% ee) (entry 7). The introduction of (R,R)-bis(phenylethyl)amine moiety [(S,R,R)-6h–j] brings about a dramatic increase in the enantioselectivity of the reaction to 93.2–97.3% ee (entries 8–10). However, when R2 is a phenyl group [(S,R,R)-6k], the enantioselectivity drops to 68.7% ee (entry 11). Because the best ligand in this screening was (S,R,R)-6i (R2 = Me), further optimization of the reaction conditions was carried out with this ligand. For comparison purposes, the reaction with the BINOL-based ligand 9 (see above) was also carried out under the same reaction conditions, which gave 12 with 95.5% ee (entry 12).
Table 2.
| Entry | Ligand | Solvent | Temperature, °C | % ee* |
|---|---|---|---|---|
| 1 | (R)-6a | Toluene | 23 | 52.7 (R) |
| 2 | (S)-6b | Toluene | 23 | 46.0 (S) |
| 3 | (S)-6c | Toluene | 23 | 27.4 (S) |
| 4 | (S)-6d | Toluene | 23 | 30.4 (S) |
| 5 | (R)-6e | Toluene | 23 | 23.5 (R) |
| 6 | (S)-6f | Toluene | 23 | 57.7 (S) |
| 7 | (S)-6g | Toluene | 23 | 7.9 (S) |
| 8 | (S,R,R)-6h | Toluene | 23 | 94.2 (S) |
| 9 | (S,R,R)-6i | Toluene | 23 | 97.3 (S) |
| 10 | (S,R,R)-6j | Toluene | 23 | 93.2 (S) |
| 11 | (S,R,R)-6k | Toluene | 23 | 68.7 (S) |
| 12 | 9 | Toluene | 23 | 95.5 (S) |
| 13 | (S,R,R)-6i | Toluene | -30 | 98.4 (S) |
| 14 | (S,R,R)-6i | Et2O | -30 | 98.8 (S) |
The reactions were carried out in 1.0-mmol scale in a solvent (5 ml) under nitrogen with Miniblock XT reactor (reactions at room temperature). Conversion was determined by 1H NMR and GC analysis. All reactions gave complete conversion in 4 h.
The enantiopurity was determined by GC analysis with a Supelco Beta Dex-225 column. The absolute configuration was determined by GC analysis in comparison with authentic samples.
When the reaction using (S,R,R)-6i was carried out in toluene at –30°C, the enantioselectivity was increased to 98.4% ee (entry 13) and the use of ether as the solvent further increased the enantioselectivity to 98.8% ee (entry 14).
These results clearly indicate the importance of the fine-tuning capability of the phosphoramidite ligands to achieve extremely high enantioselectivity in this reaction.
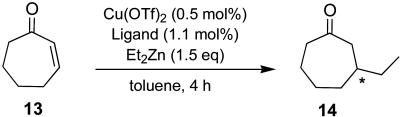
In a similar manner, the Cu-catalyzed diethylzinc reactions with 2-cycloheptenone (13) using the ligands (S,R,R)-6h–6k were performed. Results are listed in Table 3.
Table 3.
| Entry | Ligand | Temperature, °C | Conversion, % | % ee |
|---|---|---|---|---|
| 1 | (S,R,R)-6h | 23 | 100 | 91.9 (S) |
| 2 | (S,R,R)-6i | 23 | 100 | 89.4 (S) |
| 3 | (S,R,R)-6j | 23 | 99 | 81.5 (S) |
| 4 | (S,R,R)-6k | 23 | 48 | 59.6 (S) |
| 5 | (S,R,R)-6h | -30 | 100 | 95.3 (S) |
| 6 | (S,R,R)-6i | -30 | 100 | 97.5 (S) |
| 7 | 9 | -30 | 100 | 95.4 (S) |
See the legend and footnote of Table 2.
As Table 3 shows, the substituents (R2) at the 3,3′ positions have a significant effect on the enantioselectivity and the reaction rate (entries 1–4). When the reactions were carried out at room temperature (23°C), the best result (91.9% ee) was obtained by using (S,R,R)-6h (R2 = H) (entry 1). Thus, the best ligand for this reaction was different from that [(S,R,R)-6i] for the reaction of 2-cyclohexenone (see above) in this screening.
The enantioselectivity decreases (91.9% ee → 59.6% ee) in the order H, Me, Br, and Ph [(S,R,R)-6h–6k] (entries 1–4). In the reaction using (S,R,R)-6k, both the conversion (48% ee) and the enantioselectivity (59.6% ee) are significantly lower than other ligands (entry 4). The enantioselectivity increases when the reactions are carried out at –30°C (entries 5 and 6). At a low temperatures the crossover of ligand efficacy as compared with that at room temperature is observed, i.e., at –30°C (S,R,R)-6i gives higher enantioselectivity (97.5% ee) than (S,R,R)-6h (95.3% ee). This result suggests that (S,R,R)-6i (R2 = Me) has a larger entropy term than (S,R,R)-6h (R2 = H) in the enantioselectivity-determining step of the reaction.
Next, we examined the efficacy of the ligands (S,R,R)-6h–6k for the same reaction with 2-cyclopentenone (15). It has been shown that, in general, a metal-catalyzed conjugate addition to 2-cyclopetenone suffers from substantially lower enantioselectivity than that for 2-cyclohexenone using the same ligand (12), with a few exceptions (27, 28). Also, no monodentate ligand had been successfully applied to this challenging substrate when we started this investigation. The Cu(OTf)2-catalyzed reactions of 2-cyclopentenone (15) were carried out at –30°C in the same manner as those for 2-cyclohexenone (11) and 2-cycloheptanone (13). BINOL-based ligand 9 was also used for comparison. Results are listed in Table 4.
Table 4.
| Entry | Ligand | Temperature, °C | Conversion, % | %ee |
|---|---|---|---|---|
| 1 | (S,R,R)-6b | -30 | 100 | 29 (S) |
| 2 | (S,R,R)-6i | -30 | 100 | 52 (S) |
| 3 | (S,R,R)-6j | -30 | 100 | 8 (S) |
| 4 | (S,R,R)-6k | -30 | 100 | 46 (S) |
| 5 | 9 | -30 | 100 | 15 (S) |
See the legend and footnote of Table 2.
As Table 4 shows, the enantioselectivity of this reaction again highly depends on the size and nature of the substituents (R2) at the 3,3′ positions of the ligand 6. The best result (52% ee) so far was obtained with the use of (S,R,R)-6i (R2 = Me) (entry 2). The ligand (S,R,R)-6k bearing a phenyl group as R2 gives 46% ee (entry 4). These results are much better than those obtained by using the BINOL-based ligand 9, which gives only 15% ee (entry 5). Introduction of the bulky bromine substituent as R2 is detrimental to the selectivity (entry 3). Although the enantioselectivity attained to date is far from satisfactory, these results demonstrate again the merit of the fine-tuning capability of the biphenol-based phosphoramidite ligands to obtain clues for achieving high enantioselectivity in this reaction. We believe the next generation library of these phosphoramidite ligands, which will be designed and created by taking into account these clues, can achieve much higher enantioselectivity.
In conclusion, a series of monodentate phosphoramidite ligands has been developed based on enantiopure biphenols with axial chirality. These chiral ligands are easy to prepare in short steps and are flexible for modifications. The fine-tuning capability of these ligands has been proven to achieve high enantioselectivity in the asymmetric hydroformylation and the conjugate addition of diethylzinc to cycloalkenones. Further applications of these monodentate phosphorus ligands to a variety of catalytic asymmetric transformations and studies on the active catalyst species and their asymmetric induction mechanisms are needed.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institute of General Medical Sciences and, in part, by a grant from and the National Science Foundation and by generous funding from the Mitsubishi Chemical Corporation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: ee, enantiomeric excess.
References
- 1.Alexakis, A., Vastra, J., Burton, J., Benhaim, C. & Mangeney, P. (1998) Tetrahedron Lett. 39, 7869–7872. [Google Scholar]
- 2.Reetz, M. T. & Sell, T. (2000) Tetrahedron Lett. 41, 6333–6336. [Google Scholar]
- 3.Reetz, M. T. & Mehler, G. (2000) Angew. Chem. Int. Ed. Engl. 39, 3889–3890. [DOI] [PubMed] [Google Scholar]
- 4.Feringa, B. L. (2000) Acc. Chem. Res. 33, 346–353. [DOI] [PubMed] [Google Scholar]
- 5.Hu, A.-G., Fu, Y., Xie, J.-H., Zhou, H., Wang, L.-X. & Zhou, Q.-L. (2002) Angew. Chem. Int. Ed. Engl. 41, 2348–2350. [DOI] [PubMed] [Google Scholar]
- 6.Alexakis, A., Rosset, S., Allamand, J., March, S., Guillen, F. & Benhaim, C. (2001) Synlett, 1375–1378.
- 7.Alexakis, A., Benhaim, C., Rosset, S. & Humam, M. (2002) J. Am. Chem. Soc. 124, 5262–5263. [DOI] [PubMed] [Google Scholar]
- 8.Komarov, I. V. & Borner, A. (2001) Angew. Chem. Int. Ed. Engl. 40, 1197–1200. [DOI] [PubMed] [Google Scholar]
- 9.Pena, D., Minnaard, A. J., de Vries, J. G. & Feringa, B. L. (2002) J. Am. Chem. Soc. 124, 14552–14553. [DOI] [PubMed] [Google Scholar]
- 10.Reetz, M. T., Sell, T., Meiswinkel, A. & Mehler, G. (2003) Angew. Chem. Int. Ed. Engl. 42, 790–793. [DOI] [PubMed] [Google Scholar]
- 11.Feringa, B. L., Naasz, R., Imbos, R. & Arnold, L. A. (2002) in Modern Organocopper Chemistry, ed. Krause, N. (Wiley-VCH, Weinheim), pp. 224–258.
- 12.Alexakis, A. & Benhaim, C. (2002) Eur. J. Org. Chem. 3221–3236.
- 13.Boiteau, J.-G., Imbos, R., Minnaard, A. J. & Feringa, B. L. (2003) Org. Lett. 5, 681–684. [DOI] [PubMed]
- 14.Francio, G., Faraone, F. & Leitner, W. (2002) J. Am. Chem. Soc. 124, 736–737. [DOI] [PubMed] [Google Scholar]
- 15.Jensen, J. F., Svendsen, B. Y., La Cour, T. V., Pedersen, H. L. & Johannsen, M. (2002) J. Am. Chem. Soc. 124, 4558–4559. [DOI] [PubMed] [Google Scholar]
- 16.Imbos, R., Minnaard, A. J. & Feringa, B. L. (2002) J. Am. Chem. Soc. 124, 184–185. [DOI] [PubMed] [Google Scholar]
- 17.Lambers-Verstappen, M. M. H. & de Vries, J. G. (2003) Adv. Synth. Catal. 345, 478–482. [Google Scholar]
- 18.Bartels, B. & Helmchen, G. (1999) Chem. Commun. 741–742.
- 19.Malda, H., van Zijl, A. W., Arnold, L. A. & Feringa, B. L. (2001) Org. Lett. 3, 1169–1171. [DOI] [PubMed] [Google Scholar]
- 20.Ohmura, T. & Hartwig, J. F. (2002) J. Am. Chem. Soc. 124, 15164–15165. [DOI] [PubMed] [Google Scholar]
- 21.Lopez, F., Ohmura, T. & Hartwig, J. F. (2003) J. Am. Chem. Soc. 125, 3426–3427. [DOI] [PubMed] [Google Scholar]
- 22.Hua, Z., Vassar, V. C. & Ojima, I. (2003) Org. Lett. 5, 3831–3834. [DOI] [PubMed] [Google Scholar]
- 23.Hulst, R., Koen de Vries, N. & Feringa, B. L. (1994) Tetrahedron: Asymmetry 5, 699–708. [Google Scholar]
- 24.Mori, K. (1983) Tetrahedron 39, 3107–3109. [Google Scholar]
- 25.Nozaki, K. & Ojima, I. (2000) in Catalytic Asymmetric Synthesis, ed. Ojima, I. (Wiley-VCH, New York), 2nd Ed., pp 429–463.
- 26.Arnold, L. A., Imbos, R., Mandoli, A., de Vries, A. H. M., Naasz, R. & Feringa, B. L. (2000) Tetrahedron 56, 2865–2878. [Google Scholar]
- 27.Degrado, S. J., Mizutani, H. & Hoveyda, A. H. (2001) J. Am. Chem. Soc. 123, 755–756. [DOI] [PubMed] [Google Scholar]
- 28.Escher, I. H. & Pfaltz, A. (2000) Tetrahedron 56, 2879–2888. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.