Abstract
It is found that the addition of hexamethylphosphoramide to the solution of an alkyne, Et2Zn, and (S)-1,1′-bi-2-naphthol in methylene chloride allows the generation of an alkynylzinc at room temperature and shows highly enantioselective additions to aldehydes. The mild condition for the formation of the alkynylzinc reagent enables the use of functional alkynes in this asymmetric reaction with excellent enantioselectivity. It avoids the reflux of the toluene solutions of the alkynes and Et2Zn as previously reported.
Keywords: asymmetric catalysis, alkynylzinc additions, propargylic alcohols
Enantiomerically pure propargylic alcohols are very useful precursors to many organic targets (1–11). Two general methods exist for the asymmetric synthesis of propargylic alcohols, including the asymmetric reduction of ynones (12–18) and the nucleophilic addition of metal acetylides to aldehydes (19–29). The addition of metal acetylides to aldehydes is particularly useful since it simultaneously produces a carbon–carbon bond and a chiral alcohol center. Only until recently have highly enantioselective and catalytic alkynylmetal additions been developed. For example, Corey's laboratory reported the use of an oxazaborolidine to catalyze the reaction of alkynylboranes with aldehydes (21). It showed excellent enantioselectivity for the reactions of a variety of alkynes with aldehydes. This method required the preparation of alkynylstannanes first, which were then converted to the alkynylboranes for the addition to aldehydes.
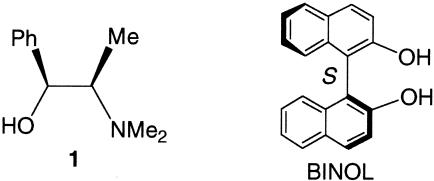
Among the asymmetric alkyne additions to aldehydes the use of alkynylzincs is the most studied. The greater use of alkynylzincs is because alkynylzincs can be directly prepared in situ from the reaction of terminal alkynes with the commercially available alkylzincs or Zn(OTf)2. In addition, alkynylzincs can also tolerate many functional groups, such as ketones, esters, amides, nitro groups, and nitriles. Two highly enantioselective catalytic systems have recently been developed for the alkynylzinc addition to aldehydes. One involves the use of N-methyl ephedrine (1; Scheme 1), Zn(OTf)2, and Et3N discovered by Carreira and colleagues (22, 23). This catalytic method shows high enantioselectivity for the reaction of alkynes with mostly aliphatic aldehydes. The reactions can be even conducted in air by using reagent-grade solvents. Another system uses 1,1′-bi-2-naphthol (BINOL), Ti(OiPr)4, and Et2Zn or Me2Zn discovered as described (24–29). In particular, the method using BINOL/Ti(OiPr)4/Et2Zn is highly enantioselective for the reaction of terminal alkynes with a broad range of substrates, including alkyl, aryl, and α,β-unsaturated aldehydes (24, 25). It also shows high stereocontrol for the reaction of chiral aldehydes (26). This method is useful for the asymmetric synthesis of various propargylic alcohols.
Scheme 1.
In the reaction catalyzed by BINOL/Ti(OiPr)4/Et2Zn, refluxing of a terminal alkyne and Et2Zn in toluene was required in the first step to prepare the corresponding alkynylzinc. The high temperature of this step caused the decomposition of certain functional alkynes. It is therefore desirable to reduce the reaction temperature for the preparation of the alkynylzinc reagents. Although using Me2Zn can reduce the reaction temperature, it also limits the scope of the enantioselectivity in comparison with the use of Et2Zn. Herein, we report that with the addition of hexamethylphosphoramide (HMPA), the BINOL-catalyzed alkynylzinc addition to aldehydes can be conducted at room temperature without refluxing in toluene in the first step and still shows high enantioselectivity even for the reactions of functional alkynes.
Experimental Procedures
General Data. All reactions were carried out under nitrogen. Unless otherwise specified, all reagents were purchased from Aldrich and used directly. Diethylzinc (95%) was purchased from Strem (Newburyport, MA). Deuterated chloroform was purchased from Cambridge Isotope Laboratories (Cambridge, MA). Anhydrous N,N-dimethylformamide and methyl sulfoxide were used directly from Sure/Seal bottles as purchased (water, <0.005%). Hexamethylphosphoramide (HMPA) was first stirred with calcium hydride for 24 h under nitrogen at room temperature. It was then distilled under vacuum and stored over 4-Å molecular sieves under nitrogen before use. Methylene chloride and tetrahydrofuran were dried by passing through an activated alumina column under nitrogen and were stored over 4-Å molecular sieves before use.
1H NMR spectra were obtained by using the Varian-300 MHz spectrometer. HPLC analyses were conducted on the Waters 600 with the Daicel Chiracel OD column eluted with 10% iPrOH in hexane at 1.0 ml/min, detected at 254 nm by Waters 486, unless otherwise indicated.
General Procedure for the Preparation of the Racemic Propargylic Alcohols. All the racemic propargylic alcohols used for the HPLC analysis were prepared according to the following procedure unless otherwise indicated. Under nitrogen, n-BuLi in hexanes (1.6 M, 0.32 mmol, 0.2 ml) was added into a solution of an alkyne (0.35 mmol) in tetrahydrofuran (3 ml) in a 15-ml flask. After the mixture was stirred for 3 h, an aldehyde (0.25 mmol) was added and the stirring continued for 8 h. The reaction was quenched with ice and extracted with methylene chloride. The extract was dried over magnesium sulfate. After rotoevaporation, the residue was passed through a short silica gel column to afford the desired product.
Analysis of the Propargylic Alcohols Produced from the Asymmetric Alkyne Additions to Aldehydes. 1,3-Diphenyl-prop-2-yn-1-ol. 72% yield. 93% enantiomeric excess (ee) determined by HPLC analysis. Retention time: tmajor = 13.6 min, and tminor = 24.2 min. 1H NMR (300 MHz, CDCl3) δ 7.63–7.60 (m, 2H), 7.48–7.28 (m, 8H), 5.69 (br, 1H), 2.26 (br, 1H) (21, 30).
3-Phenyl-1-o-tolyl-prop-2-yn-1-ol. 77% yield. 93% ee determined by HPLC analysis. Retention time: tmajor = 12.0 min, and tminor = 27.1 min. 1H NMR (300 MHz, CDCl3) δ 7.72 (m, 1H), 7.47–7.43 (m, 2H), 7.32–7.18 (m, 6H), 5.83 (d, J = 5.4 Hz, 1H), 2.49 (s, 3H), 2.18 (d, J = 5.7, 1H) (31).
3-Phenyl-1-m-tolyl-prop-2-yn-1-ol. 75% yield. 93% ee determined by HPLC analysis. Retention time: tmajor = 13.9 min, and tminor = 34.3 min. 1H NMR (300 MHz, CDCl3) δ 7.48–7.45 (m, 2H), 7.41–7.39 (m, 2H), 7.33–7.26 (m, 4H), 7.15 (d, J = 7.5, 1H), 5.64 (br, 1H), 2.38 (s, 3H), 2.24 (br, 1H).
3-Phenyl-1-p-tolyl-prop-2-yn-1-ol. 69% yield. 93% ee determined by HPLC analysis. Retention time: tmajor = 11.8 min, and tminor = 25.3 min. 1H NMR (300 MHz, CDCl3) δ 7.51–7.44 (m, 4H), 7.32–7.29 (m, 3H), 7.21–7.19 (d, J = 7.5 Hz, 1H), 5.64 (br, 1H), 2.36 (s, 3H), 2.21 (br, 1H).
1-(3-Chloro-phenyl)-3-phenyl-prop-2-yn-1-ol. 57% yield. 93% ee determined by HPLC analysis. Retention time: tmajor = 12.6 min, and tminor = 45.1 min. 1H NMR (300 MHz, CDCl3) δ 7.60 (s, 1H), 7.49–7.44 (m, 3H), 7.34–7.30 (m, 5H), 5.66 (d, J = 5.7 Hz, 1H), 2.32 (d, J = 6.0 Hz, 1H).
1-(4-Chloro-phenyl)-3-phenyl-prop-2-yn-1-ol. 57% yield. 93% ee determined by HPLC analysis. Retention time: tmajor = 11.9 min, and tminor = 38.3 min. 1H NMR (300 MHz, CDCl3) δ 7.55–7.52 (m, 2H), 7.47–7.43 (m, 2H), 7.38-7.29 (m, 5H), 5.65 (d, J = 5.1 Hz, 1H), 2.32 (d, J = 5.7 Hz, 1H).
1-(4-Fluoro-phenyl)-3-phenyl-prop-2-yn-1-ol. 67% yield. 93% ee determined by HPLC analysis. Retention time: tmajor = 11.6 min, and tminor = 35.6 min. 1H NMR (300 MHz, CDCl3) δ 7.60–7.56 (m, 2H), 7.47–7.44 (m, 2H), 7.34–7.29 (m, 3H), 7.10–7.04 (tm, J = 8.4 Hz, 2H), 5.66 (d, J = 5.1 Hz, 1H), 2.27 (d, J = 6.0 Hz, 1H).
1-(4-Bromo-phenyl)-3-phenyl-prop-2-yn-1-ol. 72% yield. 93% ee determined by HPLC analysis. Retention time: tmajor = 12.2 min, and tminor = 40.7 min. 1H NMR (300 MHz, CDCl3) δ 7.53–7.43 (m, 6H), 7.34–7.29 (m, 3H), 5.64 (br, 1H), 2.27 (br, 1H) (29).
1-(4-Methoxy-phenyl)-3-phenyl-prop-2-yn-1-ol. 56% yield. 93% ee determined by HPLC analysis. Retention time: tmajor = 16.7 min, and tminor = 37.9 min. 1H NMR (300 MHz, CDCl3) δ 7.55–7.50 (m, 2H), 7.47–7.44 (m, 2H), 7.33–7.28 (m, 3H), 6.94–6.89 (dm, J = 8.7 Hz, 2H), 5.63 (d, J = 5.7 Hz, 1H), 2.18 (d, J = 5.7 Hz, 1H) (32, 33).
1-Pentafluorophenyl-3-phenyl-prop-2-yn-1-ol. 66% yield. 88% ee determined by HPLC analysis. Retention time: tmajor = 7.5 min, and tminor = 16.6 min. 1H NMR (300 MHz, CDCl3) δ 7.44–7.41 (m, 2H), 7.33–7.30 (m, 3H), 5.96 (d, J = 7.8 Hz, 1H), 2.66 (d, J = 8.1, 1H) (34).
1,5-Diphenyl-pent-1-en-4-yn-3-ol. 56% yield. 92% ee determined by HPLC analysis. Retention time: tmajor = 21.1 min, and tminor = 69.1 min. 1H NMR (300 MHz, CDCl3) δ 7.48–7.41 (m, 4H), 7.35–7.24 (m, 6H), 6.83 (d, J = 16.2 Hz, 1H), 6.38 (dd, J = 15.9, 6.0 Hz, 1H), 5.27 (br, t, J = 6.0 Hz, 1H), 2.07 (d, J = 6.3 Hz, 1H) (35).
1-Naphthalen-1-yl-3-phenyl-prop-2-yn-1-ol. 86% yield. 95% ee determined by HPLC analysis. Retention time: tmajor = 19.3 min, and tminor = 42.8 min. 1H NMR (300 MHz, CDCl3) δ 8.38 (d, J = 8.1 Hz, 1H), 7.93–7.84 (m, 3H), 7.60–7.44 (m, 5H), 7.33–7.28 (m, 3H), 6.50 (d, J = 5.1 Hz, 1H), 2.38 (d, J = 6.0 Hz, 1H).
1-Furan-2-yl-3-phenyl-prop-2-yn-1-ol. 69% yield. 88% ee determined by HPLC analysis. Retention time: tmajor = 12.8 min, and tminor = 23.3 min. 1H NMR (300 MHz, CDCl3) δ 7.49–7.44 (m, 2H), 7.434–7.425 (m, 1H), 7.34–7.29 (m, 3H), 6.52–6.51 (dm, J = 3.3 Hz, 1H), 6.37–6.36 (dd, J = 3.3, 1.5 Hz, 1H), 5.68 (br, 1H), 2.38 (br, 1H).
4,4-Diethoxy-1-phenyl-but-2-yn-1-ol. 51% yield. 91% ee determined by HPLC analysis. Retention time: tmajor = 10.5 min, and tminor = 12.8 min. 1H NMR (300 MHz, CDCl3) δ 7.53–7.50 (m, 2H), 7.40–7.29 (m, 3H), 5.51 (br, 1H), 5.34 (d, J = 1.2 Hz, 1H), 3.79–3.68 (m, 2H), 3.64–3.53 (m, 2H), 2.23 (br, 1H), 1.221 (t, J = 6.9 Hz, 3H), 1.215 (t, J = 6.9 Hz, 3H) (36).
Acetic acid 4-hydroxy-4-phenyl-but-2-ynyl ester. 52% yield. 88% ee determined by HPLC analysis. Retention time: tmajor = 18.2 min, and tminor = 16.3 min. 1H NMR (300 MHz, CDCl3) δ 7.53–7.50 (m, 2H), 7.41–7.32 (m, 3H), 5.50 (br, 1H), 4.75 (d, J = 1.8 Hz, 2H), 2.21 (br, 1H), 2.09 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 170.3, 140.0, 128.6, 128.5, 126.6, 86.3, 880.5, 64.5, 52.3, 20.7. The racemic compound was prepared by replacing (S)-BINOL with racemic BINOL in the alkyne addition to benzaldehyde (37).
6-Chloro-1-phenyl-hex-2-yn-1-ol. 53% yield. 93% ee determined by HPLC analysis (2% i-PrOH in hexane at 1.0 ml/min). Retention time: tmajor = 40.0 min, and tminor = 30.4 min. 1H NMR (300 MHz, CDCl3) δ 7.53–7.50 (m, 2H), 7.40–7.31 (m, 3H), 5.44 (br, 1H), 3.63 (t, J = 6.3 Hz, 2H), 2.49–2.44 (dt, J = 5.1, 6.9 Hz, 2H), 2.10 (br, 1H), 2.02–1.94 (p, J = 6.6 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 141.0, 128.6, 128.3, 126.5, 85.4, 81.0, 64.7, 43.6, 31.1, 16.2 (38).
Results and Discussion
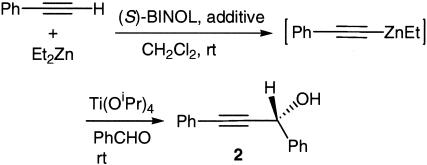
Previously, it was reported that, in solvents such as DMSO, dimethylformamide, and HMPA, Et2Zn reacted rapidly with phenylacetylene at room temperature to generate the corresponding alkynylzinc complex (39). We therefore tested the use of these compounds as additives for the (S)-BINOL-catalyzed reaction of phenylacetylene with benzaldehyde for the synthesis of the propargylic alcohol 2 (Scheme 2). The results are summarized in Table 1. Unless otherwise indicated, the reactions in Table 1 were conducted by stirring phenylacetylene, Et2Zn, (S)-BINOL, and an additive in a solvent at room temperature for 1 h, which was then mixed with Ti(OiPr)4 for 1 h followed by the addition of benzaldehyde. Entry 1 shows that without an additive, the room-temperature reaction gave mainly the Et2Zn addition product with the formation of a very small amount of 2. This result occurred because the alkynylzinc reagent cannot be generated from phenylacetylene and Et2Zn at room temperature. Addition of 2–4 equivalents (equiv) of dimethylformamide or DMSO improved the yield of the propargylic alcohol but gave lower ee's (entries 2–5). When 2 equiv of HMPA was added to facilitate the reaction of phenylacetylene with Et2Zn at room temperature, the yield of 2 was greatly increased (88%) with a small reduction in enantioselectivity (83% ee) (entry 6). Reducing the amount of HMPA to 1 equiv decreased the reaction rate and also slightly reduced the enantioselectivity (entry 7). When phenylacetylene, Et2Zn, and HMPA were mixed in methylene chloride in the first step and (S)-BINOL and Ti(OiPr)4 were added later, the enantioselectivity was significantly reduced (entry 8). Increasing the amount of (S)-BINOL and Ti(OiPr)4 led to a small increase in ee (entry 9). Decreasing the reaction temperature significantly improved the enantioselectivity but it slowed down the reaction and reduced the yield of the product (entries 10 and 11). Changing the solvent from methylene chloride to THF, diethyl ether, or toluene greatly reduced the ee (entries 12–14). Use of the redistilled HMPA slightly increased the ee (entry 15). Increasing the amount of Ti(OiPr)4 to 1 equiv also slightly improved the ee (entry 16), but the further increase of Ti(OiPr)4 led to ee reduction (entry 17). In entry 18, (S)-BINOL, HMPA, phenylacetylene, Et2Zn, and Ti(OiPr)4 were stirred together in methylene chloride for 1 h before the addition of benzaldehyde. This procedure led to a small reduction in enantioselectivity but greatly reduced the yield. When Me2Zn was used in place of Et2Zn, a small ee reduction was observed (entry 19). Reducing the amount of the solvent reduced the enantioselectivity (entry 20). Increasing the amount of the solvent did not change the enantioselectivity (entry 21). Increasing the amount of (S)-BINOL, phenylacetylene, Et2Zn, and Ti(OiPr)4 boosted the enantioselectivity to 93% ee (entry 22). Decreasing the concentration of entry 22 did not change the enantioselectivity (entry 23). Increasing the amount of HMPA from 2 to 4 equiv in entry 24 reduced the ee. Thus, entry 22 is identified as the optimized procedure for this reaction because of its high enantioselectivity. The configuration of the propargylic alcohol product is R as determined by comparing the HPLC and optical rotation data with the data in the literature (21).
Scheme 2.
The asymmetric reaction of phenylacetylene with benzaldehyde.
Table 1. Reactions of phenylacetylene with benzaldehyde in the presence of (S)-BINOL, Ti(OiPr)4, Et2Zn, and an additive.
| Entry | BINOL, mol% | Additive, equiv | PhCCH +Et2Zn, equiv | Ti(OiPr)4, mol% | Solvent | T, °C | Yield, % | ee, % |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | None | 2 | 50 | CH2Cl2 (3 ml) | rt | —* | 88 |
| 2 | 20 | DMF, 2 | 2 | 50 | CH2Cl2 (3 ml) | rt | 51 | 75 |
| 3 | 20 | DMSO, 2 | 2 | 50 | CH2Cl2 (3 ml) | rt | 43 | 71 |
| 4 | 20 | DMF, 4 | 2 | 50 | CH2Cl2 (3 ml) | rt | 51 | 72 |
| 5 | 20 | DMSO, 4 | 2 | 50 | CH2Cl2 (3 ml) | rt | ≈60 | 68 |
| 6 | 20 | HMPA, 2 | 2 | 50 | CH2Cl2 (3 ml) | rt | 88 | 83 |
| 7 | 20 | HMPA, 1 | 2 | 50 | CH2Cl2 (3 ml) | rt | 88 | 82 |
| 8† | 20 | HMPA, 2 | 2 | 50 | CH2Cl2 (3 ml) | rt | 73 | 69 |
| 9 | 40 | HMPA, 2 | 2 | 100 | CH2Cl2 (3 ml) | rt | 77 | 86 |
| 10 | 20 | HMPA, 2 | 2 | 50 | CH2Cl2 (3 ml) | 0 | 73 | 85 |
| 11 | 20 | HMPA,‡ 2 | 2 | 50 | CH2Cl2 (3 ml) | -40∼-50 | 46 | 93 |
| 12 | 20 | HMPA, 2 | 2 | 50 | THF (3 ml) | rt | 49 | 76 |
| 13 | 20 | HMPA, 2 | 2 | 50 | Ether (3 ml) | rt | — | 52 |
| 14 | 20 | HMPA,‡ 2 | 2 | 50 | Toluene (3 ml) | rt | 67 | 76 |
| 15 | 20 | HMPA,‡ 2 | 2 | 50 | CH2Cl2 (3 ml) | rt | 90 | 86 |
| 16 | 20 | HMPA,‡ 2 | 2 | 100 | CH2Cl2 (3 ml) | rt | 76 | 87 |
| 17 | 20 | HMPA,‡ 2 | 2 | 150 | CH2Cl2 (3 ml) | rt | 83 | 83 |
| 18§ | 20 | HMPA,‡ 2 | 2 | 50 | CH2Cl2 (3 ml) | rt | 37 | 82 |
| 19¶ | 20 | HMPA,‡ 2 | 2 | 50 | CH2Cl2 (3 ml) | rt | 90 | 85 |
| 20 | 20 | HMPA,‡ 2 | 2 | 50 | CH2Cl2 (1 ml) | rt | 98 | 76 |
| 21 | 20 | HMPA,‡ 2 | 2 | 50 | CH2Cl2 (5 ml) | rt | 85 | 86 |
| 22 | 40 | HMPA,‡ 2 | 4 | 100 | CH2Cl2 (3 ml) | rt | 72 | 93 |
| 23 | 40 | HMPA,‡ 2 | 4 | 100 | CH2Cl2 (6 ml) | rt | 75 | 93 |
| 24 | 40 | HMPA,‡ 4 | 4 | 100 | CH2Cl2 (6 ml) | rt | ≈80 | 89 |
rt, room temperature.
The main product was that of the Et2Zn addition.
Phenylacetylene, Et2Zn, and HMPA in methylene chloride were stirred for 1 h, and then (S)-BINOL and Ti(OiPr)4 were added. After an additional hour, benzaldehyde was added.
Redistilled HMPA was used.
(S)-BINOL, HMPA, phenylacetylene, Et2Zn, and Ti(OiPr)4 in methylene chloride were stirred for 1 h, then benzaldehyde was added.
Me2Zn was used in place of Et2Zn.
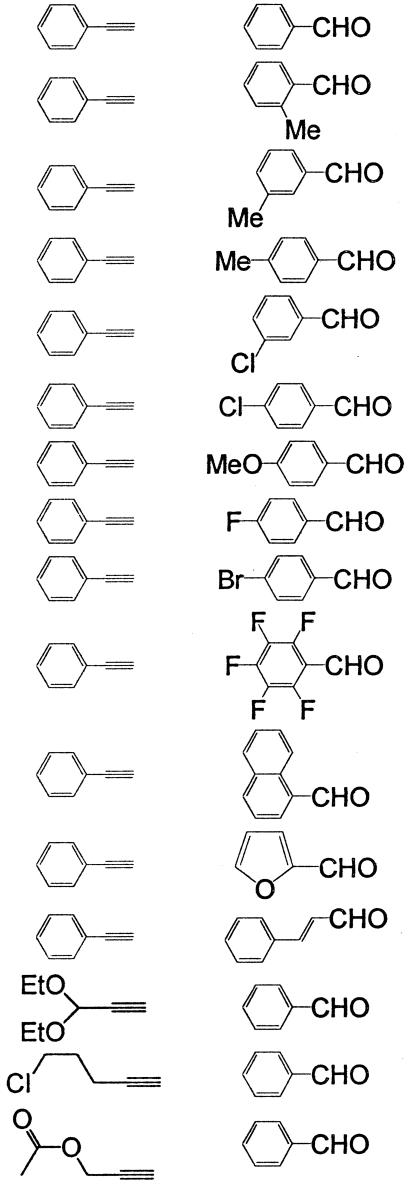
The conditions of entry 22 were applied to the reaction of several alkynes with various aldehydes. The results are summarized in Table 2. High enantioselectivities were observed for the reactions of various alkynes with aromatic aldehydes. Entries 14–16 also demonstrate that the addition of HMPA makes it possible to conduct the highly enantioselective reaction of benzaldehyde with functional alkynes. These substrates underwent decomposition when heated under reflux in toluene in the presence of Et2Zn. The addition of HMPA greatly reduced the temperature for the formation of the alkynylzinc reagents and significantly improved the BINOL/Et2Zn/Ti(OiPr)4 method for the asymmetric functional alkyne addition to aldehydes.
Table 2. Asymmetric reactions of various alkynes with aromatic aldehydes in the presence of (S)-BINOL, HMPA, Et2Zn, and Ti(OiPr)4 at room temperature.
A general procedure for the asymmetric reactions is given below. Under nitrogen, to a 10-ml flask containing (S)-BINOL (>99% ee, 28.6 mg, 0.1 mmol) in methylene chloride (dried with activated alumina, 3 ml) was sequentially added HMPA (0.5 mmol, 88 μl), phenylacetylene (1.0 mmol, 120 μl), and Et2Zn (1.0 mmol, 110 μl). After the solution was stirred at room temperature for 1 h, Ti(OiPr)4 (0.25 mmol, 74 μl) was added and the solution was stirred for another hour. Then, an aldehyde (0.25 mmol) was added and the reaction was completed in 3–4 h. Saturated ammonium chloride solution was added to quench the reaction, and methylene chloride was used for extraction. After rotoevaporation, the residue was passed through a short silica gel column eluted with hexane/ethyl acetate (98:2–90:10) to afford the propargylic alcohol product.
Acknowledgments
This work was supported, in part, by the Petroleum Research Fund, administered by the American Chemical Society and the National Institutes of Health (Grant R01GM58454).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BINOL, 1,1′-bi-2-naphthol; HMPA, hexamethylphosphoramide; equiv, equivalents; ee, enantiomeric excess.
References
- 1.Marshall, J. A. & Wang, X. J. (1992) J. Org. Chem. 57, 1242–1252. [Google Scholar]
- 2.Henderson, M. A. & Heathcock, C. H. (1988) J. Org. Chem. 53, 4736–4745. [Google Scholar]
- 3.Fox, M. E., Li, C., Marino, J. P., Jr., & Overman, L. E. (1999) J. Am. Chem. Soc. 121, 5467–5480. [Google Scholar]
- 4.Nicolaou, K. C. & Webber, S. E. (1984) J. Am. Chem. Soc. 106, 5734–5736. [Google Scholar]
- 5.Chemin, D. & Linstrumelle, G. (1992) Tetrahedron 48, 1943–1952. [Google Scholar]
- 6.Corey, E. J., Niimura, K., Konishi, Y., Hashimoto, S. & Hamada, Y. (1986) Tetrahedron Lett. 27, 2199–2202. [Google Scholar]
- 7.Vourloumis, D., Kim, K. D., Petersen, J. L. & Magriotis, P. A. (1996) J. Org. Chem. 61, 4848–4852. [DOI] [PubMed] [Google Scholar]
- 8.Evans, D. A., Halstead, D. P. & Allison, B. D. (1999) Tetrahedron Lett. 40, 4461–4462. [Google Scholar]
- 9.Trost, B. & Krische, M. J. (1999) J. Am. Chem. Soc. 121, 6131–6141. [Google Scholar]
- 10.Roush, W. R. & Sciotti, R. J. (1994) J. Am. Chem. Soc. 116, 6457–6458. [Google Scholar]
- 11.Burgess, K. & Jennings, L. D. (1991) J. Am. Chem. Soc. 113, 6129–6139. [Google Scholar]
- 12.Brinkmeyer, R. S. & Kapoor, V. M. (1977) J. Am. Chem. Soc. 99, 8339–8341. [DOI] [PubMed] [Google Scholar]
- 13.Midland, M. M., McLoughlin, J. I. & Gabriel, J. (1989) J. Org. Chem. 54, 159–165. [Google Scholar]
- 14.Ramachandran, P. V., Teodorovic, A. V., Rangaishenvi, M. V. & Brown, H. C. (1992) J. Org. Chem. 57, 2379–2386. [Google Scholar]
- 15.Noyori, R., Tomino, I., Yamada, M. & Nishizawa, M. (1984) J. Am. Chem. Soc. 105, 6717–6725. [Google Scholar]
- 16.Bach, J., Berenguer, R., Garcia, J., Loscertales, T. & Vilarrasa, J. (1996) J. Org. Chem. 61, 9021–9025. [DOI] [PubMed] [Google Scholar]
- 17.Helal, C. J., Margriotis, P. A. & Corey, E. J. (1996) J. Am. Chem. Soc. 118, 10938–10939. [Google Scholar]
- 18.Matsumura, K., Hashiguchi, S., Ikariya, T & Noyori, R. (1997) J. Am. Chem. Soc. 119, 8738–8739. [Google Scholar]
- 19.Mukaiyama, T., Suzuki, K., Soai, K. & Sato, T. (1997) Chem. Lett. 447–448.
- 20.Tan, L., Chen, C., Tillyer, R. D., Grabowski, E. J. J. & Reider, P. J. (1999) Angew. Chem. Int. Ed. Engl. 38, 711–713. [DOI] [PubMed] [Google Scholar]
- 21.Corey, E. J. & Cimprich, K. A. (1994) J. Am. Chem. Soc. 116, 3151–3152. [Google Scholar]
- 22.Frantz, D. E., Fässler, R., Tomooka, C. S. & Carreira, E. M. (2000) Acc. Chem. Res. 33, 373–381. [DOI] [PubMed] [Google Scholar]
- 23.Anand, N. K. & Carreira, E. M. (2001) J. Am. Chem. Soc. 123, 9687–9688. [DOI] [PubMed] [Google Scholar]
- 24.Moore, D. & Pu, L. (2002) Org. Lett. 4, 1855–1857. [DOI] [PubMed] [Google Scholar]
- 25.Gao, G., Moore, D., Xie, R.-G. & Pu, L. (2002) Org. Lett. 4, 4143–4146. [DOI] [PubMed] [Google Scholar]
- 26.Mashall, J. A. & Bourbeau, M. P. (2003) Org. Lett. 5, 3197–3199. [DOI] [PubMed] [Google Scholar]
- 27.Pu, L. (2003) Tetrahedron 59, 9873–9886. [Google Scholar]
- 28.Lu, G., Li, X., Chan, W. L. & Chan, A. S. C. (2002) J. Chem. Soc. Chem. Commun. 2, 172–173. [DOI] [PubMed] [Google Scholar]
- 29.Li, X., Lu, G., Kwok, W. H. & Chan, A. S. C. (2002) J. Am. Chem. Soc. 124, 12636–12637. [DOI] [PubMed] [Google Scholar]
- 30.Tombo, G. M. R., Didier, E. & Loubinoux, B. (1990) Synlett 1, 547–548. [Google Scholar]
- 31.Li, Z., Upadhyay, V., DeCamp, A. E., DiMichele, L. & Reider, P. J. (1999) Synthesis 1453–1448.
- 32.Lappin, G. R. (1951) J. Org. Chem. 16, 419–423. [Google Scholar]
- 33.Kundu, N. G., Pal, M. & Chowdhury, C. (1995) J. Chem. Res. Miniprint 1, 101–123. [Google Scholar]
- 34.Ooi, T., Miura, T., Takaya, K., Ichikawa, H. & Maruoka, K. (2001) Tetrahedron 57, 867–873. [Google Scholar]
- 35.Frantz, D. E., Fassler, R. & Carreira, E. M. (2000) J. Am. Chem. Soc. 122, 1806–1807. [Google Scholar]
- 36.Obrecht, D. (1989) Helv. Chim. Acta 72, 447–456. [Google Scholar]
- 37.El-Sayed, E., Anand, N. K. & Carreira, E. M. (2001) Org. Lett. 3, 3017–3020. [DOI] [PubMed] [Google Scholar]
- 38.Duchon d'Engenieres, M. (1968) Bull Soc. Chim. Fr. 201–204.
- 39.Okhlobystin, O. Y. & Zakharkin, L. I. (1965) J. Organomet. Chem. 3, 257–258. [Google Scholar]