Abstract
Tanycytes are highly specialized ependymal cells that form a blood–cerebrospinal fluid (CSF) barrier at the level of the median eminence (ME), a circumventricular organ (CVO) located in the tuberal region of the hypothalamus. This ependymal layer harbors well-organized tight junctions, a hallmark of central nervous system barriers that is lacking in the fenestrated portal vessels of the ME. The displacement of barrier properties from the vascular to the ventricular side allows the diffusion of blood-borne molecules into the parenchyma of the ME while tanycyte tight junctions control their diffusion into the CSF, thus maintaining brain homeostasis. In the present work, we combined immunohistochemical and permeability studies to investigate the presence of tanycyte barriers along the ventricular walls of other brain CVOs. Our data indicate that, unlike cuboidal ependymal cells, ependymal cells bordering the CVOs possess long processes that project into the parenchyma of the CVOs to reach the fenestrated capillary network. Remarkably, these tanycyte-like cells display well-organized tight junctions around their cell bodies. Consistent with these observations, permeability studies show that this ependymal layer acts as a diffusion barrier. Together, our results suggest that tanycytes are a characteristic feature of all CVOs and yield potential new insights into their involvement in regulating the exchange between the blood, the brain, and the CSF within these “brain windows.”
Keywords: ependymocytes, tight junction protein, area postrema, subfornical organ, subcommissural organ, organum vasculosum laminae terminalis
The circumventricular organs (CVOs) are atypical brain structures lining the third and fourth ventricles, and are found in the brain of all vertebrates. They play a role in regulating body homeostasis based on blood–brain communication (Gross and Weindl, 1987). In the CVOs, the blood–brain interface is formed by a rich capillary plexus harboring a fenestrated endothelium (Duvernoy and Risold, 2007; Ciofi et al., 2009; Ciofi, 2011) and lacking tight junction complexes, the hallmark of central nervous system (CNS) barriers (Schulz and Engelhardt, 2005). This permits the two-way exchange of metabolic information: the delivery of neurohormones into the bloodstream by secretory organs, and the sensing of blood-borne molecules by neurons in sensory organs (Johnson and Gross, 1993; Prevot et al., 1998, 2010; Fry et al., 2007). This circumventing of the blood–brain barrier (BBB) by fenestrated vessels is why CVOs are described as “brain windows” (Weindl and Sofroniew, 1981; Gross and Weindl, 1987). However, several authors have noted that in the CVOs the tight junction protein zonula occludens-1 (ZO-1) is expressed by ependymal as opposed to fenestrated endothelial cells (Smith and Shine, 1992; Petrov et al., 1994), suggesting that the barrier has been shifted from the vascular to the ventricular side. Recently, we carried out complementary immunohistochemical and permeability studies in the median eminence (ME), the neurohemal organ located in the tuberal region of the hypothalamus (Mullier et al., 2010; Langlet et al., 2013; Myers, 2013). This study revealed the barrier properties of a class of highly specialized ependymal cells called tanycytes, which line the third ventricle and are apposed to the fenestrated portal vessels of the ME. Unlike multiciliated ependymal cells, tanycytes are devoid of cilia but possess long processes that extend up to the perivascular space of the capillary network of the ME that they border with specialized end-feet (Prevot, 2002; De Seranno et al., 2004). Thus, the tanycyte ependymal domain links the ventricular and vascular compartments, forming a blood/cerebrospinal fluid (CSF) interface in the tuberal region of the hypothalamus.
In the ME, adhesion between adjacent tanycytes is mediated by a complex of various tight junction proteins, including ZO-1, occludin, claudin 5, and claudin 1, that prevent the free passage of molecules through the paracellular pathway. The resultant displacement of the barrier from the vascular to the ventricular surface of the CVOs allows the diffusion of blood-borne molecules through the permeable vasculature into the parenchyma of the ME, while tanycyte tight junctions control their diffusion into the CSF, thus maintaining brain homeostasis (Mullier et al., 2010; Langlet et al., 2013).
Intriguingly, the anatomical structure controlling blood–brain exchanges in other brain CVOs (i.e., sensory and secretory structures lining the third and fourth ventricles) remains unknown. Based on our recent findings (Mullier et al., 2010), the present work combines immunohistochemical and permeability studies to test for the presence of tanycyte barriers along the ventricular walls of the organum vasculosum laminae terminalis (OVLT), the subfornical organ (SFO), the area postrema (AP), and the subcommissural organ (SCO).
MATERIALS AND METHODS
Animals and tissue preparation
Twelve 2–3 months male C57BL/6 mice (Charles River, France) were used in this study. Animals were housed in a temperature-controlled room (21−22°C) under a controlled light cycle (12 hours on / 12 hours off) and provided with food and water ad libitum (Special Diet Services, RM3, 801180). All experiments were carried out in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC) regarding mammalian research and in compliance with INSERM guidelines for the care and the use of laboratory animals. Moreover, animal experimentation protocols were approved by the Directorate of Veterinary Departments of the North Region.
Mice (n = 6) were anesthetized with an intraperitoneal (i.p.) injection of a ketamine/xylazine solution (100 mg/kg and 20 mg/kg, respectively). For most immunohistochemical studies, three animals were perfused transcardially with 0.9% saline and their brains quickly removed, embedded in ice-cold OCT medium (optimal cutting temperature embedding medium, Tissue Tek, Sakura, France, Villeneuve d’Ascq), frozen in isopentane (−55°C), and stored at −80°C until use. For the detection of detyrosinated tubulin, three animals were perfused transcardially with 0.9% saline followed by an ice-cold solution of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were quickly removed, postfixed in the same fixative for 2 hours at 4°C, and immersed in 20% sucrose in 0.02 M phosphate buffered saline (PBS) at 4°C overnight. The brains were finally embedded in ice-cold OCT and frozen on dry ice.
Immunohistochemistry
Brains were cut into 20-μm-thick coronal or sagittal sections and processed for immunohistochemistry as described previously (Mullier et al., 2010). Briefly, slide-mounted sections were 1) fixed by immersion for 1 minute in methanol/acetone (vol/vol) at −20°C or for 10 minutes in 2% paraformaldehyde at 4°C for HuC/D neuronal immunolabeling; 2) blocked for 30 minutes using a solution containing 4% normal goat serum and 0.3% Triton X-100; 3) incubated overnight at 4°C with primary antibodies followed by 1 hour at room temperature with a cocktail of secondary Alexa Fluor-conjugated antibodies (1:500, Molecular Probes, Invitrogen, San Diego, CA); 4) counterstained with Hoechst (1:10,000, Molecular Probes, Invitrogen), and coverslipped using Mowiol (Calbiochem, La Jolla, CA). For triple immunofluorescence labeling experiments, anti-vimentin antibodies were visualized using a biotinylated goat antichicken antibody (1:500, 1 hour at room temperature; Vector Laboratories, Burlingame, CA) and AMCA-conjugated streptavidin (1:500, 1 hour at room temperature; Vector Laboratories).
Antibody characterization
All primary antibodies used are listed in Table 1. The zonula occludens-1 (ZO-1) antiserum stained the expected band of 225 kDa molecular weight on western blots of mouse brain (Stamatovic et al., 2005; Beauchesne et al., 2009) and produced a pattern of staining in endothelial cells (Fig. 3, open arrow), choroids plexus (Figs. 3E, 8C), and tanycytes of the median eminence (Figs. 3B, 8A), similar to that described elsewhere in the literature (Smith and Shine, 1992; Wolburg et al., 2001; Mullier et al., 2010).
TABLE 1.
Primary Antibodies Used in This Study
| Antigen | Immunogen | Manufacturer, species, type, catalog number |
Dilution used |
|---|---|---|---|
| Occludin | GST fusion protein consisting of aa 372–522 from C-terminus of human occludin fused to GST |
Invitrogen, rabbit polyclonal, 404700 |
1:500 |
| Zonula occludens-1 | 69 kD fusion protein corresponding to aa 463–1109 of human ZO-1 |
Invitrogen, rabbit polyclonal, 617300 |
1:500 |
| Claudin 1 | N-CRK TTS YPT PRP YPK PAP SSG KDY V- C synthetic peptide in the C-terminal sequence of human claudin 1 |
Invitrogen, rabbit polyclonal, 519000 |
1:100 |
| Detyrosinated tubulin | N-GEEEGEE-C synthetic peptide corresponding to the seven C- terminal aa |
Millipore, rabbit polyclonal, AB3201 |
1:500 |
| Vimentin | Recombinant Golden Syrian hamster vimentin |
Millipore, chicken polyclonal, AB5733 |
1:2,000 |
| MECA 32 | Murine lymph node stroma | Gift from Prof. Britta Engelhardt (Switzerland), rat monoclonal |
1:200 |
| HuC/D | Elav family members HuC, HuD and Hel-N1 |
Molecular probes, Invitrogen, mouse monoclonal, A21271 |
1:200 |
| GFAP | GFAP isolated from cow spinal cord |
DakoCytomation, rabbit polyclonal, Z 0334 |
1:2,000 |
| GFAP | Purified bovine GFAP | Millipore, chicken polyclonal, AB5541 |
1:600 |
Figure 3.
Expression of the tight junction protein ZO-1 in vimentin-positive ependymal cells bearing processes in sagittal sections of mouse CVOs (section adjacent to that shown in Fig. 1). A: Low-magnification photomontage of Hoechst counterstaining (blue) showing the location of CVOs in the mouse brain. B,C,E,F,H: High-magnification images showing the distribution of vimentin (red) and ZO-1 (green) immunoreactivity in each CVO (B, ME; C, OVLT; E, SFO; F, SCO; H, AP). Insets in B,E,H: High-magnification images corresponding to the areas indicated. D,G: High-magnification images corresponding to the areas indicated in C,F, respectively. All sections were counterstained with Hoechst (blue). ZO-1 is expressed in brain capillaries (green, open arrows in B,C,E,F,H) and in the choroid plexus (CP in E), which are known to display well-differentiated tight junction complexes. Notably, in the ME (inset in B), the OVLT (D), the SFO (inset in E), the AP (inset in H), and SCO (G), vimentin-positive ependymal cells (red) express ZO-1 (green) in a distinct honeycomb pattern around their cell bodies (arrow). 3V, third ventricle; 4V, fourth ventricle; ME, median eminence; SCO, subcommissural organ; SFO, subfornical organ; OVLT, organum vasculosum laminae terminalis; AP, area postrema; CP, choroid plexus; PC, posterior commissure. Scale bars = 1,000 μm in A; 100 μm in B,C,E,F,H; 10 μm in D,G and inset in B (applies also to E,H).
Figure 8.
Photomicrographs showing the distribution GFAP and ZO-1 immunoreactivity in coronal sections of each CVO. A: ME, B: OVLT. C: SFO. D: AP. E: SCO. Insets in A−E: High-magnification images of the areas indicated. The tight junction protein ZO-1 is expressed in blood-brain barrier capillaries (green, open arrow in A−E) and in the choroid plexus (CP in C,D). Notably, ZO-1 expression displays a typical honeycomb pattern at the ventricular wall (green, arrow in A−E). GFAP immunoreactivity is observed throughout the nervous tissue, with an increase in the signal around ZO-1-positive brain capillaries (white, open arrow in A−E), which are known to display well-differentiated tight junction complexes. Importantly, at the level of the CVOs, although GFAP-positive cells were found throughout the parenchyma of the CVOs, predominant GFAP immunoreactivity was observed close to the ventricular surface forming a dense ribbon associated with the honeycomb pattern of tight junction proteins displayed by ependymal cell bodies (arrows, insets in A−E). In the SCO, GFAP is also expressed in some cell bodies (inset in E). ME, median eminence; OVLT, organum vasculosum laminae terminalis; SFO, subfornical organ; AP, area postrema; SCO, subcommissural organ; 3V, third ventricle; 4V, fourth ventricle; CP, choroid plexus. Coordinates relative to bregma for coronal sections: ME (−1,70), OVLT (+0.35), SFO (−0.50), AP (−7,20), SCO (−2.55). Scale bars = 100 μm in A–E; 20 μm in insets.
The occludin antiserum stained the expected band of 65 kDa molecular weight on western blots of mouse brain (Koedel et al., 2002; Beauchesne et al., 2009), and stained a pattern of membrane-associated structures in endothelial cells (Fig. 4A, open arrow) and choroid plexus (Fig. 5A) that is identical to previous reports (Hirase et al., 1997; Mullier et al., 2010).
Figure 4.
Expression pattern of tight junction proteins in vimentin-positive ependymal cells and their association with MECA 32-immunoreactive fenestrated capillaries in coronal sections of the mouse OVLT. A,B: Photomicrographs showing the distribution of vimentin (red) and occludin (green) immunoreactivity. C,D: Photomicrographs showing the distribution of ZO-1 (green) and MECA 32 (white) immunoreactivity. E,F: Photomicrographs showing the distribution of vimentin (red), claudin 1 (green), and MECA 32 (white) immunoreactivity. B,D,F: High-magnification images corresponding to areas indicated in A,C,E, respectively. A–D: Sections are counterstained with Hoechst (blue). Vimentin is expressed in both brain capillaries (red, open arrow in A,E) and ependymal cells. In the OVLT, vimentin-positive ependymal cells extend processes into the brain parenchyma (asterisks, A,B,E,F) and contact (arrowheads in F) MECA 32-positive fenestrated vessels (white) localized in the parenchyma of the CVO. Importantly, these ependymal cells display immunoreactivity for the tight junction proteins occludin (A,B), ZO-1 (C,D), and claudin 1 (E,F) (green). Tight junction proteins are expressed in a honeycomb pattern around ependymal cell bodies (arrows). Notably, brain vessels in neighboring structures are MECA 32-negative, ZO-1- and occludin-positive, and claudin 1-negative (open arrows in A,C,E). 3V, third ventricle; VmPO, ventromedial preoptic nucleus. Coordinates relative to bregma for coronal sections: A (+0.50), C (+0,35), E (+0.40). Scale bars = 100 μm in A,C,E; 10 μm in F (applies also to B,D).
Figure 5.
Expression pattern of tight junction proteins in vimentin-positive ependymal cells and their association with MECA 32-immunoreactive fenestrated capillaries in coronal sections of the mouse SFO. A,B: Photomicrographs showing the distribution of vimentin (red) and occludin (green) immunoreactivity. C,D: Photomicrographs showing the distribution of ZO-1 (green) and MECA 32 (white) immunoreactivity. E,F: Photomicrographs showing the distribution of vimentin (red), claudin 1 (green), and MECA 32 (white) immunoreactivity. B,D,F: High-magnification images corresponding to areas indicated in A,C,E, respectively. A–D: Sections were counterstained with Hoechst (blue). Vimentin is expressed in both brain capillaries (red, open arrow in A,E) and ependymal cells. In the SFO, vimentin-positive ependymal cells extend processes into the brain parenchyma (asterisks, A,B,E,F) and contact (arrowheads in F) MECA 32-positive fenestrated vessels (white) localized in the parenchyma of the CVO. Importantly, these ependymal cells display immunoreactivity for the tight junction proteins occludin (A,B), ZO-1 (C,D), and claudin 1 (E,F) (green). Tight junction proteins are expressed in a honeycomb pattern around ependymal cell bodies (arrows). Notably, brain vessels in neighboring structures are MECA 32-negative, ZO-1- and occludin-positive, and claudin 1-negative (open arrows in A,C,E). 3V, third ventricle; CP, choroid plexus; SFO, subfornical organ; vhc, ventral hippocampal commissure. Coordinates relative to bregma for coronal sections: A (−0.60), C (−0,50), E (−0.50). Scale bars = 100 μm in A,C,E; 10 μm in F (applies also to B,D).
The claudin 1 antiserum had been previously characterized for immunocytochemical use by others in claudin 1 knockout mice (Furuse et al., 2002), and stained apical membrane-associated structures that exhibit the classical morphology of tight junction protein complexes (Fig. 4F, arrow) that is identical with previous reports (Wolburg et al., 2001; Mullier et al., 2010).
The detyrosinated tubulin antiserum (Gundersen et al., 1984) was shown to stain microtubules in neural tissue (Paturle-Lafanechere et al., 1994), ependymal cilia (Mullier et al., 2010), and primary cilia in vitro (Gundersen and Bulinski, 1986).
The vimentin antiserum produced a pattern of staining similar to that described elsewhere by others (Prevot, 2002; Kameda et al., 2003; Sanchez et al., 2009; Mullier et al., 2010) (Fig. 1).
Figure 1.
Photomicrographs showing the association of vimentin-positive cells bearing processes with MECA 32-immunoreactive fenestrated capillaries in sagittal sections of mouse CVOs. A: Low-magnification photomontage of Hoechst counterstaining (blue) showing the location of CVOs in the mouse brain. B,C,E,F,H: High-magnification images showing the distribution of vimentin (red) and MECA 32 (white) immunoreactivity in each CVO (B, ME; C, OVLT; E, SFO; F, AP; H, SCO). Insets in B,E: High-magnification images of the areas indicated. D,G: High-magnification images corresponding to the areas indicated in C,F. All sections were counterstained using Hoechst (blue) to visualize cell nuclei and determine the morphological limits of each brain structure. Vimentin immunoreactivity (red) is distributed throughout the cells lining the third (3V in B–E,H) and fourth ventricles (4V in F,G). Notably, vimentin-positive ependymal cells send processes into the parenchyma of the CVOs (asterisks in B–H). MECA 32-positive vessels (white) are observed in the choroid plexus (CP in E) and CVOs (B–G), but not in the SCO (H). Remarkably, high-magnification images show the association between vimentin-positive cells and MECA 32-immunoreactive fenestrated capillaries via vimentin-positive processes (arrowheads in B,D,E,G). 3V, third ventricle; 4V, fourth ventricle; ME, median eminence; SCO, subcommissural organ; SFO, subfornical organ; OVLT, organum vasculosum laminae terminalis; AP, area postrema; CP, choroid plexus; PC, posterior commissure. Scale bars = 1,000 μm in A; 100 μm in B,C,E,F,H; 10 μm in D,G and inserts in B,E.
The MECA 32 antiserum (Leppink et al., 1989) was raised against a mouse endothelial cell surface antigen as described previously (Streeter et al., 1988). This antibody has been shown to selectively recognize fenestrated capillaries in the circumventricular organs (Fig. 1) and the choroid plexus (Fig. 1E) (Hallmann et al., 1995; Schulz and Engelhardt, 2005). It was a generous gift from Professor Britta Engelhardt (University of Bern, Switzerland).
The mouse monoclonal anti-HuC/D antibody was prepared against human peptide QAQRFRLDNLN-C-KLH conjugate. The antiserum recognized the Elav family members HuC, HuD, and Hel-N1, which are all neuronal proteins. The antibody labeled neuronal cell nuclei and perikarya (Ciofi et al., 2009; Caron et al., 2010). The expression pattern of HuC/D proteins was similar to that of reported mRNA (Allen Brain Atlas).
Rabbit anti-glial fibrillary acidic protein (GFAP, Dako, Glostrup, Denmark, Z0334) was produced from purified bovine spinal cord isolate (manufacturer’s information). Specificity of this antibody in mouse brain has been confirmed by immunohistochemistry in GAFP knockout mice (Hanbury et al., 2003). The antibody produced a pattern of staining similar to that described elsewhere (Castellano et al., 1991). Chicken polyclonal antibody against GFAP (Millipore, Bedford, MA, AB5541) was produced from purified bovine GFAP and recognizes bands of 50 and 55 kDa on mouse brain homogenates in western blot analysis (manufacturer’s information). The staining pattern of this antibody was identical to that of rabbit polyclonal antibody against GFAP (Dako, Z0334).
Permeability assays
Mice were subjected to intravenous (i.v.) (n = 3) or intracerebroventricular (i.c.v.) (n = 3) injections of Evans blue (Sigma, St. Louis, MO), as described previously (Mullier et al., 2010). Briefly, unanesthetized animals received an injection of Evans blue solution (50 μl; 1% in saline) into the tail vein and were killed by decapitation 20 minutes later. For i.c.v. injections, 2 μl of 0.1% Evans blue solution was injected over 2 minutes into the left lateral ventricle (1 mm lateral and 0.3 mm posterior to the Bregma, according to the mouse brain atlas of Paxinos and Franklin, 2001) using an infusion pump (KD Scientific, Holliston, MA). The cisterna magna was then exposed to the atmosphere and the mice killed by decapitation. Brains were quickly removed, frozen in OCT, and stored at −80°C until use. Serial coronal and sagittal sections, 20-μm thick, were cut and mounted onto slides. Evans blue staining was directly visualized in these sections and imaged using a fluorescent microscope. The sections were then fixed with methanol/acetone (vol/vol) at −20°C for 1 minute, dried at room temperature, and processed immediately for immunohistochemistry.
Microscopic analysis
Sections were analyzed using an Axio Imager.Z1 Apo-Tome microscope, equipped with a motorized stage and an AxioCam MRm camera (Zeiss, Germany). Specific filter cubes were used for the visualization of green (EX: 475/40 nm, DM: 500 nm, BA: 530/50 nm), red (EX: 550/25 nm, DM: 570 nm, BA: 605/70 nm), and blue (Hoechst or AMCA, amino-methyl-coumarin-acetate) fluorescence (EX: 365 nm, DM: 395 nm, BA: 445/50 nm).
To create photomontages, single-plane images were captured using the MosaiX module of the AxioVision 4.6 system (Zeiss) and a Zeiss 20× objective (N.A.0.8) for each fluorophore sequentially, or using a Zeiss 5× objective (N.A.0.16) for Hoechst counterstaining. High-magnification microphotographs represent maximal intensity projections derived from 18–22 triple-ApoTome images collected using the z-stack module of the Axio-Vision 4.6 system and a Zeiss 40× or 63× oil-immersion objective (N.A. 1.3 and N.A. 1.4, respectively). All images were captured in a stepwise fashion over a defined z-focus range corresponding to all visible fluorescence within the section and consistent with the optimum step size for the corresponding objective and the wavelength (500 nm).
Adobe Photoshop (Adobe Systems, San Jose, CA) was used to process (i.e., adjust brightness and contrast) and merge images.
RESULTS
Organum vasculosum laminae terminalis (OVLT), subfornical organ (SFO), area postrema (AP), and median eminence (ME)
First, we investigated the presence of tanycyte-like cells in the CVOs by examining the morphology of vimentin-positive ependymal cells lining their ventricular walls, and the association of these cells with fenestrated capillaries (Fig. 1). Intense vimentin immunoreactivity was seen throughout the lining of the third and fourth ventricles, including the ependymal cells bordering the CVOs. In the parenchyma of the CVOs, vimentin immunolabeling revealed long and slender fibers extending from the ependymal cell bodies towards MECA 32-positive fenestrated capillaries, where they formed a dense network surrounding these capillaries. These results suggest that the ventricular walls of the OVLT, SFO, and AP is formed by process-bearing ependymal cells that link the ventricular and fenestrated-blood-vessel compartments, as described in the ME (Fig. 1B). Furthermore, unlike multiciliated cuboidal ependymal cells, ependymal cells of the CVOs do not appear to possess any cilia on their apical surfaces, as no immunoreactivity for detyrosinated tubulin was observed along the ventricular wall of the CVOs (Fig. 2).
Figure 2.
Photomicrographs showing the distribution of vimentin and detyrosinated-tubulin immunoreactivity in coronal sections of mouse CVOs. A,D,G,J: Low-magnification photomontage of detyrosinated tubulin (green) and vimentin (red) immunofluorescence in each CVO, with Hoechst counterstaining (blue) (A, OVLT; D, SFO; G, AP; J, SCO). B,C,E,F,H,I,K,L: High-magnification images of areas indicated in A,D,G,J, respectively. Vimentin immunoreactivity is distributed throughout the cells lining the third (3V in A–F,J–L) and fourth ventricles (4V in G–I). Note that detyrosinated-tubulin-immunoreactive cilia (green) observed along the ventricular wall (open arrowhead in B,E,H,K) are absent in ependymal cells bordering the OVLT, SFO, and AP (C,F,I). Interestingly, ependymal cells lining the SCO display numerous detyrosinated-tubulin-positive structures (green) that coat the ventricular wall and project into the ventricular lumen (L). Coordinates relative to bregma for coronal sections of CVOs: OVLT (+0.35), SFO (−0.60), AP (−7,20), SCO (−2.55). 3V, third ventricle; 4V, fourth ventricle; SCO, subcommissural organ; SFO, subfornical organ; OVLT, organum vasculosum laminae terminalis; AP, area postrema. Scale bars = 100 μm in A,D,G,J; 20 μm in B,C,E,F,H,I,K,L.
The characterization of these tanycyte-like cells was extended by an analysis of tight junction complexes along the ventricular walls of the CVOs (Figs. 3-6; Table 2). The expression and distribution of three tight junction proteins known to play key functional roles in CNS diffusion barriers, namely, ZO-1, occludin, and claudin 1, were examined (Abbott et al., 2010; Mullier et al., 2010). ZO-1 and occludin immunolabeling was observed at the level of cells lining the third and fourth ventricles, including the specialized ependymal cells that form the ventricular wall of the CVOs. High-magnification images showed that ZO-1 and occludin immunoreactivity formed a continuous belt around the cell bodies of vimentin-positive ependymal cells, giving rise to a honeycomb-like shape. Although this honeycomb pattern was observed along the entire ventricular wall, there was a distinct increase in signal strength at the level of the tanycyte-like cells forming the ventricular wall of the CVOs, when compared to cuboidal ependymal cells lining adjacent structures (Table 2). The distinction between these two cell types, tanycyte-like cells versus multiciliated cuboidal ependymal cells, was also revealed by claudin 1 immunoreactivity, which was restricted to the ependymal cells lining the CVOs (Fig. 4-6). Claudin 1 immunoreactivity also exhibited a honeycomb pattern, similar to that of ZO-1 and occludin. These data demonstrate the differential expression patterns of tight junction proteins in the tanycyte-like cells bordering the CVOs versus cuboidal ependymal cells.
Figure 6.
Expression pattern of tight junction proteins in vimentin-positive ependymal cells and their association with MECA 32-immunore-active fenestrated capillaries in coronal sections of the mouse AP. A,D: Photomicrographs showing the distribution of vimentin (red) and occludin (green) immunoreactivity. B,E: Photomicrographs showing the distribution of ZO-1 (green) and MECA 32 (white) immunoreactivity. C,F: Photomicrographs showing the distribution of vimentin (red), claudin 1 (green) and MECA 32 (white) immunoreactivity. D–F: High-magnification images corresponding to areas indicated in A–C, respectively. A,B,D,E: Sections were counterstained with Hoechst (blue). Vimentin is expressed in both brain capillaries (red, open arrow in A,C) and ependymal cells. In the AP, vimentin-positive ependymal cells extend processes into the brain parenchyma (asterisks, A,C,D,F) and contact (arrowheads in F) MECA 32-positive fenestrated vessels (white) localized in the parenchyma of the CVO. Importantly, these ependymal cells display immunoreactivity for the tight junction proteins occludin (A,D), ZO-1 (B,E), and claudin 1 (C,F) (green). Tight junction proteins are expressed in a honeycomb pattern around ependymal cell bodies (arrows). Notably, brain vessels in neighboring structures are MECA 32-negative, ZO-1- and occludin-positive, and claudin 1-negative (open arrows in A–C). AP, area postrema; NTS, nucleus of the solitary tract. Coordinates relative to bregma for coronal sections: A (−7,20), B (−7,35), C (−7,30). Scale bars = 100 μm in A–C; 20 μm in F (applies also to B,D).
TABLE 2.
Tight Junction Protein Expression in Blood-Brain Barrier Capillaries, Classic Ependymal Cells, Choroid Plexus, and Each of the CVOs of the Mouse Brain
| Blood-brain barrier capillaries |
Ependymal cells |
Choroid plexus |
Tanycyte-like cells in CVOs |
|||||
|---|---|---|---|---|---|---|---|---|
| ME | OVLT | SFO | AP | SCO | ||||
| Occludin | +++ | + | +++ | ++ | ++ | ++ | ++ | ++++ |
| ZO-1 | +++ | + | +++ | +++ | ++ | ++ | +++ | ++++ |
| Claudin 1 | − | − | ++++ | ++ | ++ | + | ++ | ++++ |
The density of protein expression is indicated as very high (++++), high (+++), moderate (++), low (+) or undetectable (−) based on signal strength. ZO-1 and occludin are found in blood-brain barrier capillaries, ependymal cells and in the ependyma of CVOs. Interestingly, claudin 1 immunolabeling is found exclusively in CVO ependymal cells and the choroid plexus (considered a CVO by some authors) but not in brain capillaries or ependymal cells located outside the CVOs. The SCO and the choroid plexus express the most dense immunolabeling for tight junction proteins, whereas the SFO possesses the least dense immunolabeling. ME: median eminence; OVLT, organum vasculosum laminae terminalis; SFO, subfornical organ; AP, area postrema; SCO, subcommissural organ.
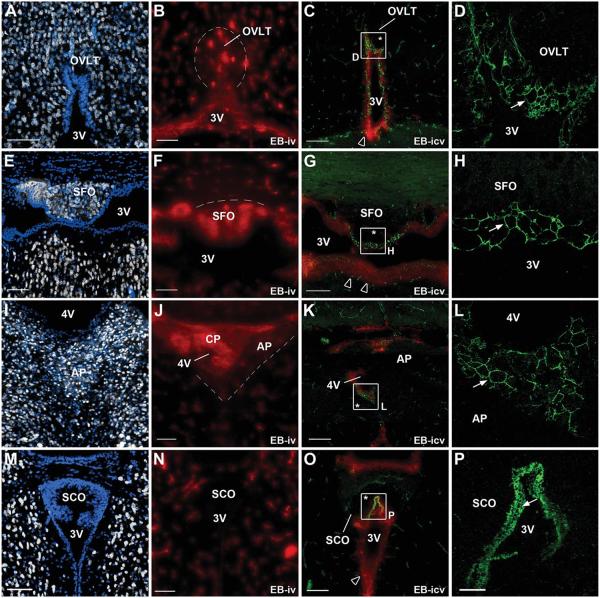
To determine whether tight junction protein expression patterns reflect diffusion barriers, mice were injected with Evans blue dye into either the tail vein or the lateral ventricle of the brain, as described in the Materials and Methods (Fig. 7). After i.v. injection, the dye was restricted in most brain regions to the vascular bed of BBB capillaries, known to possess highly efficient tight junctions, and to the endothelium of the choroid plexi, known to be surrounded by an epithelial barrier. Interestingly, the dye was also found surrounding the fenestrated vascular bed and throughout the parenchyma of the CVOs, where HuC/D-immunoreactive neurons reside (Fig. 7). Importantly, the Evans blue dye had diffused up to the ventricular walls of the CVOs, reaching the tanycyte-like cell bodies that display the honeycomb pattern of ZO-1, occludin, and claudin 1 immunoreactivity. In contrast, when the dye was injected into the lateral ventricle both the ventricular walls and the parenchyma of the CVOs remained dye-free, whereas the Evans blue dye was seen in ependyma lining the third and fourth ventricles outside of the CVOs. These data suggest that the highly specialized ependymal cell domain that forms the ventricular wall of the OVLT, SFO, and AP displays barrier properties.
Figure 7.
Evans blue dye permeability studies associated with HuC/D and ZO-1 immunolabeling in mouse CVOs. A,E,I,M: Low-magnification photomontages showing HuC/D immunoreactivity in coronal sections of each CVO. All CVOs (OVLT, A; SFO, E; AP, I) except the SCO (M) display HuC/D-positive neuronal cell bodies (gray). B,F,J,N: Low-magnification photomontages showing fluorescence after an intravenous injection of Evans blue dye (red). When injected into the blood, Evans blue reaches the parenchyma of the OVLT, SFO, and AP, but is confined to brain vessels in neighboring structures. Evans blue diffusion is limited to the parenchyma of the CVOs (dotted line), delineating the CVOs and matching the distribution of HuC/D immunoreactivity. Note that the parenchyma of the SCO is free of the blood-borne dye. C,G,K,O: Low-magnification photomontages showing fluorescence after an i.c.v. injection of Evans blue dye (red) in association with ZO-1 immunoreactivity (green). D,H,L,P: High-magnification images corresponding to the areas indicated in C,G,K,O, respectively. Evans blue reaches and crosses the walls of the third and fourth ventricles (open arrowheads), except at the level of the CVOs, where both the ventricular wall and parenchyma (asterisk) remain dye-free. Importantly, the honeycomb pattern of ZO-1 expression (green, arrow in D,H,L,P) corresponds to the nondiffusion of the dye across the ventricular wall. OVLT, organum vasculosum laminae terminalis; SFO, subfornical organ; AP, area postrema; SCO, subcommissural organ; 3V, third ventricle; 4V, fourth ventricle; CP, choroid plexus; iv, intravenous injection; icv, intracerebroventricular injection. Coordinates relative to bregma for coronal sections: OVLT (+0.35), SFO (−0.60), AP (−7,30), SCO (−2.55). Scale bars = 100 μm in A−C,E−G,I−K,M−O; 20 μm in P (applies also to D,H,L).
We next investigated the distribution of astrocytes, known to mediate BBB properties in the CNS, by studying GFAP immunoreactivity (Fig. 8). As expected, GFAP immunoreactivity was observed throughout the nervous tissue, with a denser signal around brain capillaries. GFAP immunoreactivity was also detected throughout the parenchyma of the CVOs. However, this labeling was more intense close to the ventricular surface of the CVOs, where it formed a dense ribbon associated with the cell bodies of tanycyte-like cells displaying a honeycomb pattern of tight junction proteins (Fig. 8).
Subcommissural organ (SCO)
As described in the literature, immunolabeling for HuC/D revealed the absence of neural cells in the parenchyma of the SCO (Fig. 7). Another known characteristic of the SCO is the absence of MECA 32 immunoreactivity (Figs. 1, 9). Consequently, following the permeability study, no blood-derived Evans blue dye was noted in the SCO parenchyma (Fig. 7). The vimentin immunoreactivity revealed tall elongated cells delimiting the SCO in the nervous tissue (Figs. 1-3, 9). Their long cytoplasms extended towards the ventricular lumen and lined the ventricular wall (Fig. 9). These cells also display vimentin-positive basal processes extending into brain parenchyma (Figs. 1-9) and for a few of them projecting to BBB capillaries (Fig. 9). Interestingly, detyrosinated-tubulin-positive structures were associated with the apical poles coating the ventricular wall of the SCO. An examination of tight junction protein expression showed intense ZO-1, occludin, and claudin 1 immunolabeling at the wall of the third ventricle connecting the vimentin-positive protruded apical poles (Figs. 2,9). Tight junction proteins exhibited a similar pattern of expression when compared to the other CVOs: indeed, high-magnification images reveal the honeycomb pattern of tight junction protein immunoreactivity bordering the third ventricle (Figs. 2,9). Ependymal cells displaying this honeycomb pattern were associated with the distribution of GFAP-positive cells, since GFAP immunolabeling formed a dense ribbon around SCO ependymal cell bodies. Moreover, GFAP is also present in some SCO ependymal cells (Fig. 8).
Figure 9.
Expression pattern of tight junction proteins in vimentin-positive cells in coronal sections of the mouse SCO. A,D: Photomicrographs showing the distribution of vimentin (red) and occludin (green) immunoreactivity. B,E: Photomicrographs showing the distribution of ZO-1 (green) and MECA 32 (white) immunoreactivity. C,F: Images showing the distribution of vimentin (red), claudin 1 (green), and MECA 32 (white) immunoreactivity. D–F: High-magnification images corresponding to areas indicated in A–C respectively. A,B,D,E: Sections were counterstained with Hoechst (blue). No MECA 32 immunoreactivity was observed in SCO and neighboring structures. Vimentin immunoreactivity is distributed throughout the cells lining the third ventricle (3V in A,C,D,F). Remarkably, vimentin-positive elongated cell bodies (red in A,C,D,F) delimit the SCO in the brain parenchyma and line the ventricular wall. High-magnification images of the ventricular wall of the SCO showing occludin (A,D), ZO-1 (B,E), and claudin 1 (C,F) immunoreactivity organized in a continuous belt around vimentin-positive cell bodies, giving rise to the characteristic honeycomb pattern (arrows in D–F). These cells display also vimentin-positive basal processes extending into brain parenchyma (asterisks in A,C) and for a few of them projecting to occludin-positive capillaries (open arrow in A). Notably, brain vessels in neighboring structures are ZO-1- and occludin-positive and claudin 1-negative (open arrows in A–C). 3V, third ventricle; PC, posterior commissure; PAG, periaqueductal gray. Coordinates relative to bregma for coronal sections: A–C (−2.55). Scale bars = 100 μm in A–C; 20 μm in D–F.
Figure 10 summarizes the localization and features of the tanycyte-like cells in the CVOs.
Figure 10.
Representative figure summarizing the type of ependymal cells and the distribution of tight junction proteins in each CVO (A, OVLT; B, SCO; C, SFO; D, AP) of the mouse brain. OVLT, organum vasculosum laminae terminalis; SCO, subcommissural organ; SFO, subfornical organ; AP, area postrema; 3V, third ventricle; PO, preoptic nucleus; OC, optic chiasma; MPA, medial preoptic area; PC, posterior commissure; PrC, precommissural nucleus; PAG, periaqueductal gray; vhc, ventral hippocampal commissure; CP, choroid plexus; sm, stria medullaris; 4V, fourth ventricle; NTS, nucleus of the solitary tract; TJ: tight junction.
DISCUSSION
Brain homeostasis requires the maintenance of barriers between the brain and the periphery, enforced by brain microvessels in the BBB and epithelial cells in the choroid plexi. In the present study we provide evidence that CVOs, often described as “brain windows,” also possess a blood-CSF barrier at the ventricular wall composed of tanycyte-like cells. This barrier facing the fenestrated vessels of the CVOs may confine blood-borne molecules to these “brain windows” and prevent their diffusion into the CSF, thus controlling their diffusion to the rest of the brain.
Tanycytes are highly specialized ependymal cells that have mainly been described in the ME, the CVO located in the tuberal region of the hypothalamus (Akmayev et al., 1973; Akmayev and Fidelina, 1976, 1981; Akmayev and Popov, 1977; Rodriguez et al., 2005). The presence of tanycytes has been also suggested in the AP and the OVLT (Gotow and Hashimoto, 1979; Del Brio et al., 1990; Maolood and Meister, 2009). Here, our observations confirm that ependymal cells that form the ventricular wall of the OVLT, SFO, and AP share some morphological and functional features with tanycytes that have been described in the ME. In contrast with multiciliated cuboidal ependymal cells, these tanycyte-like cells are devoid of cilia, and possess long processes that extend into the parenchyma of the CVOs to reach the fenestrated capillary network (Weindl and Joynt, 1972; Mullier et al., 2010). Moreover, we have shown that these tanycyte-like cells are immunoreactive for occludin, ZO-1, and claudin 1, three tight junction proteins known to play key functional roles in CNS diffusion barriers, and that have already been described in the blood-CSF barrier of the ME (Abbott et al., 2010; Mullier et al., 2010). Remarkably, claudin 1 expression is restricted to the ependymal cells of the CVOs, as no immunoreactivity is detected in BBB capillaries or in ciliated ependymal cells. These results are consistent with our previous data showing that claudin 1 expression characterizes ependymal cells in close contact with fenestrated vessels, e.g., tanycytes in the ME and epithelial cells in the choroid plexi. Occludin, ZO-1, and claudin 1 proteins encircle each tanycyte-like cell in the ependymal layer, giving rise to the honeycomb pattern of tight junction proteins (Petrov et al., 1994; Mullier et al., 2010) associated with barrier properties (Wolburg et al., 2001; Coisne et al., 2005; Mullier et al., 2010). Consistent with these neuroanatomical observations, the diffusion marker Evans blue, when injected directly into the CSF, reaches the ventricular wall of the CVOs without penetrating the ependymal layer composed of tanycyte-like cell bodies, demonstrating that these specialized ependymoglial cells are joined at their apices by functional tight junctions. In contrast, the Evans blue dye was seen in the ependyma, which cover the ventricular walls outside of the CVOs. This diffusion is associated with weak and discontinuous ZO-1 and occludin immunoreactivity (Petrov et al., 1994; Del Bigio, 1995). These findings are consistent with those from the ME (Mullier et al., 2010). Importantly, this highly specialized ependymal layer with barrier properties is composed of tanycyte-like cells that are in contact with the fenestrated vessels of the CVOs. The vasculature of the CVOs differs from typical brain vessels in that they harbor a fenestrated endothelium that lacks tight junction complexes (Maolood and Meister, 2009). This structural characteristic and the presence of various blood-borne molecules in the parenchyma of the CVOs indicate the enhanced permeability of this type of vasculature (Broadwell et al., 1983; Ciofi, 2011; Morita and Miyata, 2012). In this study, we combined both neuroanatomical investigations, using antibodies to the neuronal marker HuC/D and to the marker for fenestrated capillaries, MECA 32, and the assessment of vascular permeability. After injection into the blood, the diffusion marker Evans blue was found in the parenchyma of the OVLT, SFO, and AP, matching the localization of fenestrated vessels and the distribution of neuronal cell bodies. These results confirm the permeability of the fenestrated vessels (Morita and Miyata, 2012) and are consistent with the proposed role of these OCVs in sensing blood-borne molecules and conveying the information they contain to other brain regions (Johnson and Gross, 1993). However, our findings show that in the CVOs, the blood-CNS barrier subverted by the fenestrated endothelium is in fact shifted from the vascular to the ventricular side, where it takes the form of a tanycyte-like barrier. This displacement allows the diffusion of blood-borne molecules into the parenchyma of the CVOs, while controlling the access of these peripheral molecules to the rest of the brain by preventing their diffusion into the CSF.
Tight junctions, described here as complexes that prevent diffusion through the paracellular cleft, are also known to contribute to cell polarity by restricting the migration of apical and basolateral membrane components. In this way, tight junctions maintain the spatial cues necessary for the establishment of receptor-mediated transcytosis across the BBB and the epithelium of the choroid plexi (Cereijido et al., 1998; Shin et al., 2006). The presence of effective tight junctions together with neuroanatomical studies showing the presence of microvilli (Klara and Brizzee, 1975, 1977), bulbous protrusions at the ependymal surface of CVOs (Mestres and Rascher, 1994), as well as of vesicles within the ependymal cells bordering the CVOs (Akmayev and Popov, 1977; Gotow and Hashimoto, 1979; Peruzzo et al., 2004; Rodriguez et al., 2005) suggest that, like other CNS barriers, the tanycyte barrier is also capable of transporting macromolecules between the blood and CSF compartments via transcytosis.
Since the CNS barrier in the CVOs is composed of tanycyte-like cells that directly contact permeable fenestrated capillaries, it is possible that its barrier properties are related to this distinct anatomical feature. In addition, our study indicates that the CVOs also contain many GFAP-immunoreactive cells, as reported in the literature (Bennett et al., 2009). The rich astrocyte population of the CVOs could also participate to the establishment of the tanycyte barrier. Indeed, studies show that astroglial cells play an important role in the integrity of the BBB (Goldstein, 1988; Dehouck et al., 1994; Abbott et al., 2006). Moreover, astrocytes may play a key role in the functioning of the CVOs because they are implicated in the generation and regulation of the flow of information within the brain through their control of both synaptic transmission and neurosecretion (Prevot, 2002; Theodosis et al., 2008; Prevot et al., 2010).
Our study included the SCO, considered a CVO in spite of its lack of fenestrated vessels. We show that the SCO displays vimentin-positive elongated cells as described in the literature (Chouaf et al., 1989; Didier-Bazes et al., 2001). These cells display apical long cytoplasm forming the ventricular wall (Meiniel, 2007) and basal processes extending into brain parenchyma and for a few of them projecting to BBB capillaries (Rodriguez et al., 1984, 1992, 1998). Tight junctions are located at the apical pole of the ependymal cells with the typical honeycomb pattern. Our study supports the view that the SCO is sequestered within a double-barrier system (Rodriguez et al., 1992, 1998) and supplements the work of Szathmari et al. (2013), who demonstrated claudin 3 immunoreactivity in ependymal cells of the SCO. Importantly, in contrast with the ependymal cells of other CVOs, ependymal cells in the SCO display numerous cilia that coat the ventricular wall and project into the ventricular lumen (Meiniel, 2007). These results corroborate the view that this CVO is involved in the secretion of substances into the CSF (Rodriguez et al., 1986; Vio et al., 2008).
To our knowledge, this is the first detailed study that combined both anatomical investigation and the assessment of vascular/ependymal permeability to demonstrate the barrier properties of CVOs in the adult mouse brain. Our data are consistent with the description of CVOs as “brain windows,” but extend this description to the potential role of tanycytes at these ventricular interfaces, where they regulate the exchange between the blood, brain, and CSF. The tanycyte barrier could thus contribute to the delivery of blood-borne molecules conveying metabolic information to the sensory neurons of the CVOs while ensuring brain homeostasis.
ACKNOWLEDGMENTS
NEUROBESE International Associated Laboratory (INSERM, SABAN, University of Lille2, to VP and SGB)
“Centre National de la Recherche Scientifique” (CNRS, to SGB)
the “Agence Nationale de la Recherche” (ANR, to VP: GLIODIABESITY, and to SGB: PROGRALEP and LIPOBRAIN)
National Institutes of Health
DK84142
EU FP7 integrated project (grant agreement no. 266408, “Full4Health”)
FL is a Ph.D. student supported by a fellowship from the French Department for Research and New Technologies.
MECA 32 antibodies were kindly provided by Professor Britta Engelhardt (Switzerland). We thank Doctor. S. Rasika for editing the article.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ROLE OF AUTHORS
Study concept and design: BD and VP. Acquisition of data: FL and AM. Analysis and interpretation of data: FL and BD. Drafting of the manuscript: FL and BD. Critical revision of the manuscript for important intellectual content: SGB and VP. Obtained funding: SGB and VP. Study supervision: BD.
LITERATURE CITED
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Akmayev IG, Fidelina OV. Morphological aspects of the hypothalamic-hypophyseal system. VI. The tanycytes: their relation to the sexual differentiation of the hypothalamus. An enzyme-histochemical study. Cell Tissue Res. 1976;173:407–416. doi: 10.1007/BF00220328. [DOI] [PubMed] [Google Scholar]
- Akmayev IG, Fidelina OV. Tanycytes and their relation to the hypophyseal gonadotrophic function. Brain Res. 1981;210:253–260. doi: 10.1016/0006-8993(81)90898-2. [DOI] [PubMed] [Google Scholar]
- Akmayev IG, Popov AP. Morphological aspects of the hypothalamic-hypophyseal system. VII. The tanycytes: their relation to the hypophyseal adrenocorticotrophic function. An ultrastructural study. Cell Tissue Res. 1977;180:263–282. doi: 10.1007/BF00231958. [DOI] [PubMed] [Google Scholar]
- Akmayev IG, Fidelina OV, Kabolova ZA, Popov AP, Schitkova TA. Morphological aspects of the hypothalamic-hypophyseal system. IV. Medial basal hypothalamus. An experimental morphological study. Z Zellforsch Mikrosk Anat. 1973;137:493–512. doi: 10.1007/BF00307226. [DOI] [PubMed] [Google Scholar]
- Beauchesne E, Desjardins P, Hazell AS, Butterworth RF. Altered expression of tight junction proteins and matrix metalloproteinases in thiamine-deficient mouse brain. Neurochem Int. 2009;55:275–281. doi: 10.1016/j.neuint.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Bennett L, Yang M, Enikolopov G, Iacovitti L. Circumventricular organs: a novel site of neural stem cells in the adult brain. Mol Cell Neurosci. 2009;41:337–347. doi: 10.1016/j.mcn.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell RD, Balin BJ, Salcman M, Kaplan RS. Brain-blood barrier? Yes and no. Proc Natl Acad Sci U S A. 1983;80:7352–7356. doi: 10.1073/pnas.80.23.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol. 2010;518:459–476. doi: 10.1002/cne.22219. [DOI] [PubMed] [Google Scholar]
- Castellano B, Gonzalez B, Dalmau I, Vela JM. Identification and distribution of microglial cells in the cerebral cortex of the lizard: a histochemical study. J Comp Neurol. 1991;311:434–444. doi: 10.1002/cne.903110312. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Valdes J, Shoshani L, Contreras RG. Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol. 1998;60:161–177. doi: 10.1146/annurev.physiol.60.1.161. [DOI] [PubMed] [Google Scholar]
- Chouaf L, Didier-Bazes M, Aguera M, Tardy M, Sallanon M, Kitahama K, Belin MF. Comparative marker analysis of the ependymocytes of the subcommissural organ in four different mammalian species. Cell Tissue Res. 1989;257:255–262. doi: 10.1007/BF00261828. [DOI] [PubMed] [Google Scholar]
- Ciofi P. The arcuate nucleus as a circumventricular organ in the mouse. Neurosci Lett. 2011;487:187–190. doi: 10.1016/j.neulet.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Ciofi P, Garret M, Lapirot O, Lafon P, Loyens A, Prevot V, Levine JE. Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus. Endocrinology. 2009;150:5509–5519. doi: 10.1210/en.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coisne C, Dehouck L, Faveeuw C, Delplace Y, Miller F, Landry C, Morissette C, Fenart L, Cecchelli R, Tremblay P, Dehouck B. Mouse syngenic in vitro blood-brain barrier model: a new tool to examine inflammatory events in cerebral endothelium. Lab Invest. 2005;85:734–746. doi: 10.1038/labinvest.3700281. [DOI] [PubMed] [Google Scholar]
- De Seranno S, Estrella C, Loyens A, Cornea A, Ojeda SR, Beauvillain JC, Prevot V. Vascular endothelial cells promote acute plasticity in ependymoglial cells of the neuroendocrine brain. J Neurosci. 2004;24:10353–10363. doi: 10.1523/JNEUROSCI.3228-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehouck B, Dehouck MP, Fruchart JC, Cecchelli R. Up-regulation of the low density lipoprotein receptor at the blood-brain barrier: intercommunications between brain capillary endothelial cells and astrocytes. J Cell Biol. 1994;126:465–473. doi: 10.1083/jcb.126.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR. The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia. 1995;14:1–13. doi: 10.1002/glia.440140102. [DOI] [PubMed] [Google Scholar]
- Del Brio MA, Riera P, Garcia JM, Alvarez-Uria M. Ultrastructural study of the cellular components of the organum vasculosum lamina terminalis of the rabbit (Oryctolagus cuniculus) J Submicrosc Cytol Pathol. 1990;22:303–309. [PubMed] [Google Scholar]
- Didier-Bazes M, Chouaf-Lakhdar L, Dutuit M, Aguera M, Belin MF. Cell lineage of the subcommissural organ secretory ependymocytes: differentiating role of the environment. Microsc Res Tech. 2001;52:461–467. doi: 10.1002/1097-0029(20010301)52:5<461::AID-JEMT1032>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Risold PY. The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Rev. 2007;56:119–147. doi: 10.1016/j.brainresrev.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Fry M, Hoyda TD, Ferguson AV. Making sense of it: roles of the sensory circumventricular organs in feeding and regulation of energy homeostasis. Exp Biol Med (Maywood) 2007;232:14–26. [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein GW. Endothelial cell-astrocyte interactions. A cellular model of the blood-brain barrier. Ann N Y Acad Sci. 1988;529:31–39. doi: 10.1111/j.1749-6632.1988.tb51417.x. [DOI] [PubMed] [Google Scholar]
- Gotow T, Hashimoto PH. Fine structure of the ependyma and intercellular junctions in the area postrema of the rat. Cell Tissue Res. 1979;201:207–225. doi: 10.1007/BF00235058. [DOI] [PubMed] [Google Scholar]
- Gross PM, Weindl A. Peering through the windows of the brain. J Cereb Blood Flow Metab. 1987;7:663–672. doi: 10.1038/jcbfm.1987.120. [DOI] [PubMed] [Google Scholar]
- Gundersen GG, Bulinski JC. Microtubule arrays in differentiated cells contain elevated levels of a post-translationally modified form of tubulin. Eur J Cell Biol. 1986;42:288–294. [PubMed] [Google Scholar]
- Gundersen GG, Kalnoski MH, Bulinski JC. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984;38:779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Hallmann R, Mayer DN, Berg EL, Broermann R, Butcher EC. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev Dyn. 1995;202:325–332. doi: 10.1002/aja.1002020402. [DOI] [PubMed] [Google Scholar]
- Hanbury R, Ling ZD, Wuu J, Kordower JH. GFAP knockout mice have increased levels of GDNF that protect striatal neurons from metabolic and excitotoxic insults. J Comp Neurol. 2003;461:307–316. doi: 10.1002/cne.10667. [DOI] [PubMed] [Google Scholar]
- Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110(Pt 14):1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- Kameda Y, Arai Y, Nishimaki T. Ultrastructural localization of vimentin immunoreactivity and gene expression in tanycytes and their alterations in hamsters kept under different photoperiods. Cell Tissue Res. 2003;314:251–262. doi: 10.1007/s00441-003-0789-y. [DOI] [PubMed] [Google Scholar]
- Klara PM, Brizzee KR. The ultrastructural morphology of the squirrel monkey area postrema. Cell Tissue Res. 1975;160:315–326. doi: 10.1007/BF00222042. [DOI] [PubMed] [Google Scholar]
- Klara PM, Brizzee KR. Ultrastructure of the feline area postrema. J Comp Neurol. 1977;72:409–431. doi: 10.1002/cne.901710307. [DOI] [PubMed] [Google Scholar]
- Koedel U, Winkler F, Angele B, Fontana A, Pfister HW. Meningitis-associated central nervous system complications are mediated by the activation of poly(ADP-ribose) polymerase. J Cereb Blood Flow Metab. 2002;22:39–49. doi: 10.1097/00004647-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, Balland E, Lacombe A, Mazur D, Carmeliet P, Bouret SG, Prevot V, Dehouck B. Tanycytic VEGF-A Boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 2013;17:607–617. doi: 10.1016/j.cmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppink DM, Bishop DK, Sedmak DD, Henry ML, Ferguson RM, Streeter PR, Butcher EC, Orosz CG. Inducible expression of an endothelial cell antigen on murine myocardial vasculature in association with interstitial cellular infiltration. Transplantation. 1989;48:874–877. doi: 10.1097/00007890-198911000-00032. [DOI] [PubMed] [Google Scholar]
- Maolood N, Meister B. Protein components of the blood-brain barrier (BBB) in the brainstem area postrema-nucleus tractus solitarius region. J Chem Neuroanat. 2009;37:182–195. doi: 10.1016/j.jchemneu.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Meiniel A. The secretory ependymal cells of the subcommissural organ: which role in hydrocephalus? Int J Biochem Cell Biol. 2007;39:463–468. doi: 10.1016/j.biocel.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Mestres P, Rascher K. The ventricular system of the pigeon brain: a scanning electron microscope study. J Anat. 1994;184(Pt 1):35–58. [PMC free article] [PubMed] [Google Scholar]
- Morita S, Miyata S. Different vascular permeability between the sensory and secretory circumventricular organs of adult mouse brain. Cell Tissue Res. 2012;349:589–603. doi: 10.1007/s00441-012-1421-9. [DOI] [PubMed] [Google Scholar]
- Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol. 2010;518:943–962. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG., Jr How is the hungry brain like a sieve? Cell Metab. 2013;17:467–468. doi: 10.1016/j.cmet.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paturle-Lafanechere L, Manier M, Trigault N, Pirollet F, Mazarguil H, Job D. Accumulation of delta 2-tubulin, a major tubulin variant that cannot be tyrosinated, in neuronal tissues and in stable microtubule assemblies. J Cell Sci. 1994;107(Pt 6):1529–1543. doi: 10.1242/jcs.107.6.1529. [DOI] [PubMed] [Google Scholar]
- Peruzzo B, Pastor FE, Blazquez JL, Amat P, Rodriguez EM. Polarized endocytosis and transcytosis in the hypothalamic tanycytes of the rat. Cell Tissue Res. 2004;317:147–164. doi: 10.1007/s00441-004-0899-1. [DOI] [PubMed] [Google Scholar]
- Petrov T, Howarth AG, Krukoff TL, Stevenson BR. Distribution of the tight junction-associated protein ZO-1 in circumventricular organs of the CNS. Brain Res Mol Brain Res. 1994;21:235–246. doi: 10.1016/0169-328x(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Prevot V. Glial-neuronal-endothelial interactions are involved in the control of GnRH secretion. J Neuroendocrinol. 2002;14:247–255. doi: 10.1046/j.0007-1331.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- Prevot V, Dutoit S, Croix D, Tramu G, Beauvillain JC. Semi-quantitative ultrastructural analysis of the localization and neuropeptide content of gonadotropin releasing hormone nerve terminals in the median eminence throughout the estrous cycle of the rat. Neuroscience. 1998;84:177–191. doi: 10.1016/s0306-4522(97)00537-x. [DOI] [PubMed] [Google Scholar]
- Prevot V, Hanchate NK, Bellefontaine N, Sharif A, Parkash J, Estrella C, Allet C, de Seranno S, Campagne C, de Tassigny X, Baroncini M. Function-related structural plasticity of the GnRH system: a role for neuronal-glial-endothelial interactions. Front Neuroendocrinol. 2010;31:241–258. doi: 10.1016/j.yfrne.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Oksche A, Hein S, Rodriguez S, Yulis R. Spatial and structural interrelationships between secretory cells of the subcommissural organ and blood vessels. An immunocytochemical study. Cell Tissue Res. 1984;237:443–449. doi: 10.1007/BF00228428. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Herrera H, Peruzzo B, Rodriguez S, Hein S, Oksche A. Light- and electron-microscopic immunocytochemistry and lectin histochemistry of the subcommissural organ: evidence for processing of the secretory material. Cell Tissue Res. 1986;243:545–559. doi: 10.1007/BF00218061. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Oksche A, Hein S, Yulis CR. Cell biology of the subcommissural organ. Int Rev Cytol. 1992;135:39–121. doi: 10.1016/s0074-7696(08)62038-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Rodriguez S, Hein S. The subcommissural organ. Microsc Res Tech. 1998;41:98–123. doi: 10.1002/(SICI)1097-0029(19980415)41:2<98::AID-JEMT2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Blazquez JL, Pastor FE, Pelaez B, Pena P, Peruzzo B, Amat P. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- Sanchez E, Vargas MA, Singru PS, Pascual I, Romero F, Fekete C, Charli JL, Lechan RM. Tanycyte pyroglutamyl peptidase II contributes to regulation of the hypothalamic-pituitary-thyroid axis through glial-axonal associations in the median eminence. Endocrinology. 2009;150:2283–2291. doi: 10.1210/en.2008-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M, Engelhardt B. The circumventricular organs participate in the immunopathogenesis of experimental autoimmune encephalomyelitis. Cerebrospinal Fluid Res. 2005;2:8. doi: 10.1186/1743-8454-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Smith GM, Shine HD. Immunofluorescent labeling of tight junctions in the rat brain and spinal cord. Int J Dev Neurosci. 1992;10:387–392. doi: 10.1016/0736-5748(92)90028-x. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Streeter PR, Rouse BT, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szathmari A, Champier J, Ghersi-Egea JF, Jouvet A, Watrin C, Wierinckx A, Fevre Montange M. Molecular characterization of circumventricular organs and third ventricle ependyma in the rat: potential markers for periventricular tumors. Neuropathology. 2013;33:17–19. doi: 10.1111/j.1440-1789.2012.01321.x. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- Vio K, Rodriguez S, Yulis CR, Oliver C, Rodriguez EM. The subcommissural organ of the rat secretes Reissner’s fiber glycoproteins and CSF-soluble proteins reaching the internal and external CSF compartments. Cerebrospinal Fluid Res. 2008;5:3. doi: 10.1186/1743-8454-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindl A, Joynt RJ. Ultrastructure of the ventricular walls. Three-dimensional study of regional specialization. Arch Neurol. 1972;26:420–427. doi: 10.1001/archneur.1972.00490110054005. [DOI] [PubMed] [Google Scholar]
- Weindl A, Sofroniew MV. Relation of neuropeptides to mammalian circumventricular organs. Adv Biochem Psychopharmacol. 1981;28:303–320. [PubMed] [Google Scholar]
- Wolburg H, Wolburg-Buchholz K, Liebner S, Engelhardt B. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci Lett. 2001;307:77–80. doi: 10.1016/s0304-3940(01)01927-9. [DOI] [PubMed] [Google Scholar]