Abstract
Functionalized chiral diazaphospholanes ligate to a variety of transition metals, yielding chiral, catalytically active, metal complexes. Previous work has established that amino acid derivatization of the carboxyl groups of (R,R)-N,N′-phthaloyl-2,3-(2-carboxyphenyl)-phenyl-3,4-diazaphospholane (1) yields phosphines that are excellent ligands for palladium-catalyzed asymmetric allylic alkylation reactions. Alanine functionalization is particularly effective for allylic alkylation of 1,3-dimethylallyl acetate. Standard Merrifield resins and amino acid coupling methods are used to synthesize the bead-attached phosphine having the topology bead-linker-lAla-(R,R)-1-lAla-OMe, as a 1:1 mixture of linkage isomers. Use of this supported phosphine in Pd-catalyzed asymmetric allylic alkylation yields 92% enantiomeric excess, matching prior solution-phase results. A 20-member collection of amino acid-functionalized phosphines on beads with the topology bead-linker-AA2-AA1-1-AA1-AA2 was synthesized by using parallel solid-state methods and screened for efficacy in allylic alkylation. Resulting enantioselectivities indicate that the AA1 position has the strongest effect on the reaction. Catalyst activities can vary widely with the nature of the phosphine ligand and the reaction conditions. Meaningful analysis of intrinsic catalytic activities awaits identification of the structure and abundance of the active catalyst.
The ubiquity of phosphine-ligated transition metals in homogeneous catalysis and the important role of chiral phosphines in the development of enantioselective catalysis advocates continued expansion of the diversity and applicability of novel chiral phosphines. We believe that key elements of such an expansion include (i) increasingly sophisticated catalyst–substrate interactions in the secondary coordination sphere of the metal, (ii) combinatorial elaboration of promising “template” structures, and (iii) application of solid-phase methods for both acceleration of synthesis and “heterogenization” of the chiral catalyst. This work demonstrates, on a small scale, a synthetic strategy for making resolved chiral phosphines that are rich in functional groups, their combinatorial elaboration by solid-phase synthesis, and their application to enantioselective asymmetric allylic alkylation (AAA) in both supported and solution phases.
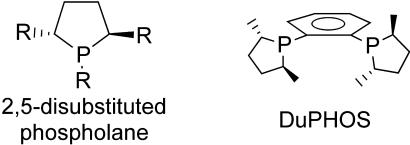
Ito and Sawamura (1) have coined the phrase “secondary interactions” to describe interactions between transition metal catalyst functional groups and complementary substrate functional groups that occur outside the primary coordination sphere. In principle, such interactions can include hydrogen bonding, Lewis acid–base, electrostatic, hydrophobic–hydrophilic, and reversible covalent attachment. Many of these motifs are thought to play important roles in nature's remarkably selective catalysts, enzymes (2). Several years ago we initiated a molecular modeling exercise to identify phosphine structures that promoted secondary interactions. Effectively, the model sought was one that enabled strong interactions of complementary substrate- and ligand-based functional groups. Consistently, structures based on 2,5-substituted phospholanes, e.g., the Du-PHOS ligand (3, 4) framework, looked promising (Fig. 1). Unfortunately, the existing syntheses of such phospholanes were not tolerant of most functional groups. This problem was overcome by creating a simple, rapid, and general synthesis of phospholane analogs, 3,4-diazaphospholanes (5–7).
Fig. 1.
Phospholane structures.
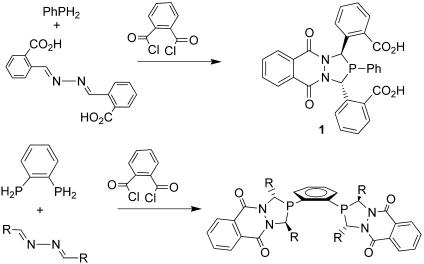
Racemic 3,4-diazaphospholanes form in high yield upon condensing a primary phosphine with azines and an acid dichloride (5) (Fig. 2). Azines are simply synthesized from aldehydes and hydrazine. The reaction generally exhibits remarkable selectivity for the rac product and tolerates the presence of functional groups in the azine (basic functional groups such as amines are the primary exception). Bis-3,4-diazaphospholanes also can be synthesized by using bis primary phosphines and can be isolated as pure rac materials, although in somewhat low overall yields (≈30%). This chemistry demonstrates that a wide variety of highly functionalized chiral phospholane structures can be synthesized rapidly from simple starting materials. However, enantioselective catalysis requires access to a wide range of resolved chiral ligands.
Fig. 2.
Synthesis of 3,4-diazaphospholanes.
The synthesis of the diazaphospholane 1 derived from 2-carboxybenzaldehyde provides an easily resolved diazaphospholane bearing a carboxylic acid that can be further derivatized by using many available methods for coupling and reducing carboxyl groups (7). Most importantly, the racemic diazaphospholane is resolved in >99% enantiomeric excess (ee) by simple crystallization of diastereomeric salts made with α-methylbenzylamine enantiomers. Furthermore, the presence of the carboxylic acid groups permits further functionalization by coupling with amines and amino acids. Attachment of several amino acids or other bifunctional fragments enables truly combinatorial ligand development from a single phosphine precursor.
Although hundreds of chiral phosphine ligands have been reported in the literature, the creation of combinatorial libraries (for a general review of combinatorial library synthesis see ref. 8; see also refs. 9–13) of phosphine ligands is a recent phenomenon (14–23). Mannich condensation reactions for the combinatorial synthesis of achiral phosphine ligand collections have been reported by LaPointe (14) and by Portnoy and coworkers (16). Most relevant to this work, Gilbertson's group has pioneered the synthesis of phosphinated polypeptides (20–22), both in solution and in the solid phase, based on unnatural phosphine-functionalized amino acids. This seminal work has demonstrated that catalysis with such libraries may be evaluated “on-bead,” and the discovery of effective new phosphine ligands based on β-hairpin structures has been reported.
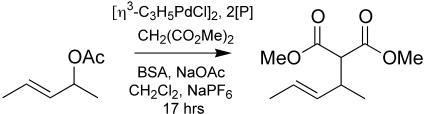
Of the many applications of chiral phosphines to enantioselective catalysis, we have focused on AAA (see Fig. 3) for testing simple monophosphine ligand libraries derived from 1 (7). In particular, we have focused on the substrate 1,3-dimethylallyl acetate, because so few ligands are capable of effecting this transformation with >85% ee at ambient or higher temperatures (24–26). Our recently reported results for this AAA reaction demonstrated several notable features for small libraries based on 1: (i) High ee (92%) is obtained with the alanine-derivatized ligand, a value that is similar to the state of the art. (ii) Chemical yields and especially enantioselectivities are exquisitely sensitive to minor variations in the amino acid structure; e.g., alanine gives very high ee but phenylglycine and phenylalanine of the same absolute configuration yield only 52% and 82% ee, respectively. (iii) The alanine derivative of 1, unique among all chiral ligands of which we are aware, is effective for AAA with both dimethyl- and diphenylallyl acetate substrates. (iv) Initial kinetic and selectivity studies suggest that the active catalyst with 1-Ala is monophosphine complex, perhaps featuring bidentate coordination by P and either N or O.
Fig. 3.
Pd-catalyzed AAA reaction.
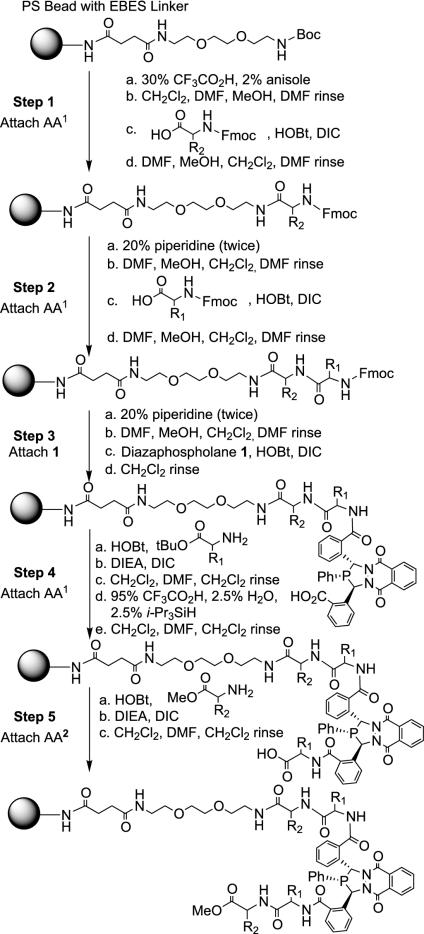
Solution-phase synthesis of ligand libraries involves extensive manual manipulations and purification steps. Solid-phase synthesis has the advantages of facilitated purification and adaptability to both parallel and split-pool combinatorial synthetic strategies (27–33). Our construction of chiral solid-supported phosphine ligands involves coupling of common cross-linked Merrifield resins, by a linker, to amino acids and phosphines with the general topology bead-linker-AAn-1-AAn (Fig. 4). The first application of this strategy is to synthesize a high-purity chiral immobilized phosphine with AA = Ala and n = 1, characterize this ligand on-bead, by means of magic-angle spinning NMR (MAS-NMR) methods, and explore the efficiency of supported-Pd AAA catalysts. A second application involves application of combinatorial techniques to synthesize a small demonstration library of supported phosphines for the purpose of screening potentially interesting candidates for further study.
Fig. 4.
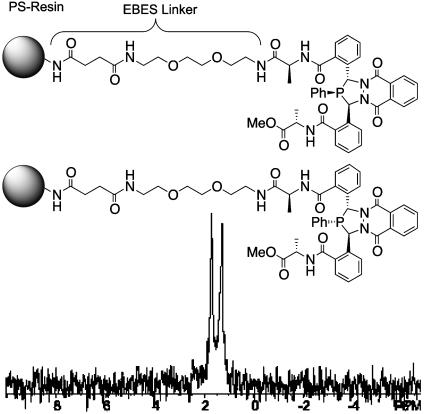
Synthesis of linkage isomers bead-linker-AA2-AA1-1-AA1-AA2. PS, polystyrene; EBES, 2,2′-(ethylenedioxy)bis(ethylamino) monosuccinimide; Boc, t-butoxycarbonyl; Fmoc, fluorenylmethoxycarbonyl; DMF, dimethylformamide; DIC, N,N′-diisopropylcarbodiimide; HOBt, hydroxybenzotriazole; DIEA, N,N-diisopropylethylamine.
Materials and Methods
Synthesis of diazaphospholane 1 followed previously reported procedures (7). Solution-phase syntheses used the general procedures reported previously. Full details of the synthesis and characterization of all newly synthesized ligands are reported in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
General Synthesis of Immobilized Array. Fig. 4 outlines the overall procedure for solid-phase synthesis of the amino acid-functionalized phosphines. Methylbenzhydrylamine (MBHA)-functionalized PS resin (75 mg) was placed in 20 PolyPrep chromatography columns (Bio-Rad). Each coupling step was performed in parallel by using the DIC/HOBt coupling protocol in 1:4 DMF/CH2Cl2, and the reactions were mixed overnight on a shaker. All reactants were added in large excess relative to the immobilized material (4–5 eq). The resins were isolated after each coupling cycle by thorough washings with DMF, CH2Cl2, and MeOH. Removal of the Fmoc protecting group was effected by adding 20% piperidine in DMF solutions to the resins and mixing for 30 min. The Boc and t-butyl ester protecting groups were removed by treatment with trifluoroacetic acid. A t-butyl cation scavenger mixture of 2.5% triisopropylsilane and 2.5% water was also used during the ester deprotection. MAS-NMR characterization of products indicated that scavengers are necessary to minimize the appearance of additional phosphine-containing impurities as identified by 31P NMR. The final products exhibit little oxygen sensitivity; 31P NMR spectroscopy reveals that moderate exclusion of oxygen during the coupling steps involving the diazaphospholane prevents oxidation.
Allylic Alkylation Procedure. Methylene chloride (2.0 ml) was added to a septum-sealed 3-dram (11-ml) vial containing [(η3-C3H5)PdCl]2 (1.8 mg, 5 μmol), phosphine (10 μmol or 15–20 mg of resin), tetra-n-butylammonium fluoride trihydrate (≈5 mg), and NaPF6 under N2. After stirring the solution at room temperature for 5 min, N,O-bis(trimethylsilyl)acetamide (0.70 ml, 3.0 mmol), dimethyl malonate (0.30 ml, 3.0 mmol), and 1,3-dimethylallyl acetate (0.15 ml, 1.0 mmol) were added. The mixture was stirred for 17 h at room temperature. The products were diluted with 6 ml of 5:1 hexane/ethyl acetate and filtered twice through plugs of silica. The enantiomeric excess of the product was determined by chiral GC (Supelco β-DEX 120 column, 30 m × 0.25 mm i.d., column temperature 70°C, flow rate 1.8 ml/min). The absolute configuration of products was determined by comparison of GC retention times with results obtained previously (7).
Results and Discussion
Solid-Phase Synthesis of Alanine Diazaphospholane (Bead-Linker-Ala-1-Ala). Incorporation of amino acid-functionalized 3,4-diazaphospholanes onto PS beads used a combination of Fmoc and Boc amino acid coupling techniques. Starting with MBHA-PS resin, chosen for stability to the trifluoroacetic acid and basic treatments, a polyethylene glycol-like linker, EBES (34), was attached to the resin. This linker enables high-quality NMR characterization by MAS-NMR spectroscopy and yields a more homogeneous environment for the immobilized phosphine sites.
Four coupling steps are necessary to synthesize the immobilized l-alanine diazaphospholane analog. Because there is little selectivity over which of the two inequivalent phospholane carboxylic acids couples with the amino acid-functionalized resin, two linkage isomers result. Correspondingly, characterization with 31P MAS-NMR reveals two resonances (Fig. 5). The 1H MAS-NMR spectra exhibit reasonably narrow lines but are less useful for detailed characterization because of overlap of the Ala-1-Ala-OMe resonances with the resin and linker resonances.
Fig. 5.
31P MAS-NMR spectrum of the two bead-linker-Ala-1-Ala linkage isomers whose structures are shown.
Asymmetric allylic alkylation of dimethylallyl acetate with dimethylmalonate in the presence of 0.5 mol % [Pd(allyl)Cl]2, ≈1 mol % immobilized PS-linker-Ala-1-Ala, N,O-bis(trimethylsilyl)acetamide, sodium hexafluorophosphate, and tetrabutylammonium fluoride in dichloromethane solution at room temperature for 17 h gives the alkylated allyl in 92% ee and 67% yield. Importantly, the immobilized monophosphine matches the enantioselectivity previously demonstrated for its homogeneous counterpart (93%). Although others have reported difficulty matching homogeneous selectivities and activities with ligands immobilized on standard Merrifield resins (27, 28), this work demonstrates that the combination of bead cross-linking, EBES linker, and amino acids yields active immobilized catalysts with solution-like properties.
Results with immobilized 1 support our previous formulation of the catalytically active species (7). Previously we postulated that the active AAA catalyst consists of a Pd center bound to one molecule of 1. In support, the crude kinetics of allylic alkylation and the enantioselectivities were independent of the ratio of 1 to Pd over the range of 1:1 to 4:1. Immobilization of 1 on resin beads provides high isolation of phosphine sites, essentially preventing formation of bis(phosphine)Pd complexes. Thus, the observation of identical enantioselectivities in solution and with immobilized 1 virtually requires that the active catalyst is a 1:1 phosphine:Pd adduct.
Design and Synthesis of 3 × 7 Array of Tetrapeptide Diazaphospholanes (Bead-Linker-AA2-AA1-1-AA1-AA2). Previous solution work found functionalization of 1 with amino acids bearing small alkyl side chains to be most effective in the AAA reaction. Therefore, we chose to explore three simple apolar residues, Ala, Leu, and Val, in the positions (AA1 in Fig. 4) adjacent to 1. With the goal of further fine-tuning the catalyst selectivity, seven different amino acids of varying bulk and functionality were attached in the AA2 position. Building the immobilized ligand in one direction requires a switch from N-protected to C-protected amino acids after the diacid phospholane is incorporated. Empirically we found the t-butyl ester to perform better than the methyl ester in the last deprotection step. Deprotection of t-butyl esters spontaneously proceeds under standard conditions for Boc-group removal. As before, each bead contains two linkage isomers. The diazaphospholane structure is quite robust under acidic conditions, and solution-phase testing shows no decomposition of the diazaphospholane under the deprotection conditions.
To minimize oxidation at phosphorus of the 20-member array, each reaction involving the phosphine was prepared and sealed while inside a glove bag. A solvent mixture of 4:1 methylene chloride/DMF provides enough polarity to dissolve all reactants and has sufficient density to suspend the resins, ensuring maximum agitation. Resins were dried overnight and stored in a glove box.
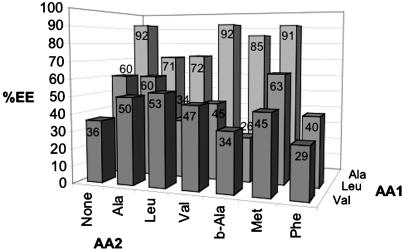
Allylic Alkylation with Solid-Phase 3 × 7 Array of Tetrapeptide Diazaphospholanes. Fig. 6 displays the enantioselectivities obtained with the small library. All products have the S configuration. As expected, the impact of the second amino acid (AA2) was much less than AA1. Large unpredictable variations in selectivities and rates accompanied changes in AA1, with Ala clearly superior. With Ala at AA1, the best selectivities were obtained with either Met or Val at AA2. Significantly poorer results were obtained with Leu or Phe at AA2. The general unpredictability of the results demonstrates the need for empirical screening.
Fig. 6.
AAA enantiomeric excess (EE) for 20-member tetrapeptide library: bead-linker-AA2-AA1-1-AA1-AA2 (no results were obtained for AA1 = Ala, AA2 = Phe; the column labeled None refers to dipeptidophospholanes).
Solution Phase Allylic Alkylation with Tetrapeptide Diazaphospholanes. Soluble versions of three of the phosphines from the screen were synthesized: Ala × Met, Ala × Ala, and Val × Ala (listed as AA1 × AA2). The purpose of this study was to compare solution and solid-phase behavior for a representative sample from the small library with a special eye toward evaluating whether poor performance of solid-phase materials originated from impurities accumulated during solid-phase synthesis. In agreement with solid-phase data, the Val × Ala diazaphospholane yielded poor enantioselectivity (47% ee solution, 50% ee solid phase). The solution diazaphospholanes containing Ala in the AA1 position, Ala × Met and Ala × Ala, catalyzed the reaction with high selectivities, 91% and 71% ee, respectively. As far as enantioselectivity is concerned, bead and solution-phase behaviors are identical.
Overall activities on-bead appear to be lower than solution activities by factors of 1.5–4. This observation does underscore the advantage of examining a reaction, such as asymmetric allylic alkylation, that is “ligand accelerated” (refs. 24 and 25; we thank one of the manuscript reviewers for pointing this out) when screening bead-based ligand libraries. Even if not all immobilized ligand sites are accessible to the catalyst precursor, nonligated metal species do not form significant product and lower the overall reaction selectivity, although the apparent activities may be lower. At this point it is unclear why the supported catalysts exhibit lower activity. It appears that effective catalysis of asymmetric allylic alkylation with amino acid-functionalized diazaphospholanes requires ionization of the catalyst precursor Pd–Cl bonds as indicated by the requirement for sodium or silver hexafluorophosphate salts. Future work should focus on broader screening with halide-free catalysts and more detailed mechanistic investigations of the catalytic process.
In summary, 3,4-diazaphospholanes are versatile ligands for asymmetric catalysis. The readily resolved diacid 1 enables synthesis of both solution and solid-phase ligands by using well developed amino acid chemistry. This work complements Gilbertson and coworkers' pioneering work in the sense that the diazaphospholane is intrinsically chiral and relies on small peptides for tuning of the catalyst environment. In contrast, Gilbertson's ligands involve rather simple phosphine fragments that rely on peptide secondary structure for the development of asymmetry. Both approaches lend themselves to combinatorial modification.
The synthesis and screening of the small library reported herein demonstrates that (i) solid-phase synthetic techniques using MBHA-functionalized PS beads are suitable for making libraries of diazaphospholanes; (ii) AAA with these ligands can lead to state-of-the-art selectivity for a difficult substrate under both homogeneous and supported catalyst conditions; and (iii) ligands derived from 1 exhibit exquisite sensitivity to amino acid modification that is difficult to predict a priori, thus validating a screening approach. Significantly, the strategy outlined here should be generalizable to a large number of metal–phosphine-promoted transformations. Continuing to explore diazaphospholane modifications and applications to asymmetric catalysis should prove fruitful. With respect to asymmetric allylations, the foci are expanding the range of substrates and nucleophiles as well as probing mechanistic features of the reactions.
Supplementary Material
Acknowledgments
Prof. Helen Blackwell and her research group and Mr. Ryan Nelson provided stimulating discussion. We thank the Department of Energy, Dow Chemical Company, and the University of Wisconsin for partial funding of this work.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AAA, asymmetric allylic alkylation; ee, enantiomeric excess; MAS-NMR, magic-angle spinning NMR; PS, polystyrene; EBES (2,2′-(ethylenedioxy)bis(ethylamino) monosuccinimide; Boc, t-butoxycarbonyl; Fmoc, fluorenylmethoxycarbonyl; DMF, dimethylformamide; HOBt, hydroxybenzotriazole; DIC, N,N′-diisopropylcarbodiimide; MBHA, methylbenzhydrylamine.
References
- 1.Sawamura, M. & Ito, Y. (1992) Chem. Rev. 92, 857–871. [Google Scholar]
- 2.Loch, J. A. & Crabtree, R. H. (2000) J. Organomet. Chem. 600, 7–11. [Google Scholar]
- 3.Burk, M. J. (1991) J. Am. Chem. Soc. 113, 8518–8519. [Google Scholar]
- 4.Burk, M. J., Feaster, J. E. & Harlow, R. L. (1991) Tetrahedron: Asymmetry 2, 569–592. [Google Scholar]
- 5.Landis, C. R., Jin, W., Owen, J. S. & Clark, T. P. (2001) Angew. Chem. Int. Ed. 40, 3432–3434. [DOI] [PubMed] [Google Scholar]
- 6.Landis, C. R., Jin, W., Owen, J. S. & Clark, T. P. (2003) International Patent WO 03/010174 A1.
- 7.Clark, T. P. & Landis, C. R. (2003) J. Am. Chem. Soc. 125, 11792–11793. [DOI] [PubMed] [Google Scholar]
- 8.Dolle, R. E. (2003) J. Comb. Chem. 5, 693–753. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu, K. D., Snapper, M. L. & Hoveyda, A. H. (1998) Chem. Eur. J. 4, 1885–1889. [Google Scholar]
- 10.Jandeleit, B., Schaefer, D. J., Powers, T. J., Turner, H. W. & Weinberg, W. H. (1999) Angew. Chem. Int. Ed. Engl. 38, 2494–2532. [PubMed] [Google Scholar]
- 11.Crabtree, R. H. (1999) Chem. Commun. 1611–1616.
- 12.Reetz, M. T. (2001) Angew. Chem. Int. Ed. 40, 284–310. [PubMed] [Google Scholar]
- 13.Shimizu, K. D., Snapper, M. L. & Hoveyda, A. H. (1999) in Comprehensive Asymmetric Catalysis, eds. Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (Springer, Berlin), Vol. 3, pp. 1389–1399. [Google Scholar]
- 14.LaPointe, A. M. (1999) J. Comb. Chem. 1, 101–104. [Google Scholar]
- 15.Loch, J. A. & Crabtree, R. H. (2001) Pure Appl. Chem. 73, 119–128. [Google Scholar]
- 16.Ben-Aroya, B. B.-N. & Portnoy, M. (2001) J. Comb. Chem. 3, 524–527. [DOI] [PubMed] [Google Scholar]
- 17.Parrish, C. A. & Buchwald, S. L. (2001) J. Org. Chem. 66, 3820–3827. [DOI] [PubMed] [Google Scholar]
- 18.Porte, A. M., Reibenspies, J. & Burgess, K. (1998) J. Am. Chem. Soc. 120, 9180–9187. [Google Scholar]
- 19.Hou, D.-R. & Burgess, K. (1999) Org. Lett. 1, 1745–1747. [Google Scholar]
- 20.Gilbertson, S. R., Collibee, S. E. & Agarkov, A. (2000) J. Am. Chem. Soc. 122, 6522–6523. [Google Scholar]
- 21.Greenfield, S. J., Agarkov, A. & Gilbertson, S. J. (2003) Org. Lett. 5, 3069–3072. [DOI] [PubMed] [Google Scholar]
- 22.Gilbertson, S. R. & Wang, X. (1996) J. Org. Chem. 61, 434–435. [DOI] [PubMed] [Google Scholar]
- 23.Agarkov, A., Uffman, E. W. & Gilbertson, S. R. (2003) Org. Lett. 5, 2091–2094. [DOI] [PubMed] [Google Scholar]
- 24.Trost, B. M. & Van Vranken, D. L. (1996) Chem. Rev. 96, 395–422. [DOI] [PubMed] [Google Scholar]
- 25.Trost, B. M. (2002) Chem. Pharm. Bull. 50, 1–14. [DOI] [PubMed] [Google Scholar]
- 26.Trost, B. M., Krueger, A. C., Bunt, R. C. & Zambrano, J. (1996) J. Am. Chem. Soc. 118, 6520–6521. [Google Scholar]
- 27.Song, C. E., Jung, W. Y., Roh, E. J., Lee, S.-g., Ahn, J. H. & Han, H. (2002) Angew. Chem. Int. Ed. 41, 3852–3854. [DOI] [PubMed] [Google Scholar]
- 28.Trost, B. M., Pan, Z., Zambrano, J. & Kujat, C. (2002) Angew. Chem. Int. Ed. 41, 4691–4693. [DOI] [PubMed] [Google Scholar]
- 29.Uozumi, Y. & Shibatomi, K. (2001) J. Am. Chem. Soc. 123, 2919–2920 (lett.). [DOI] [PubMed] [Google Scholar]
- 30.Hallman, K., Macedo, E., Nordström, K. & Moberg, C. (1999) Tetrahedron: Asymmetry 10, 4037–4046. [Google Scholar]
- 31.Uozumi, Y., Danjo, H. & Hayashi, T. (1998) Tetrahedron Lett. 39, 8303–8306. [Google Scholar]
- 32.Anson, M. S., Mirza, A. R., Tonks, L. & Williams J. M. J. (1999) Tetrahedron Lett. 40, 7147–7150. [Google Scholar]
- 33.Johnson, B. F. G., Raynor, S. A., Shephard, D. S., Mashmeyer, T., Thomas, J. M., Sankar, G., Bromley, S., Oldroyd, R., Gladden, L. & Mantle, M. D. (1999) Chem. Commun. 1167–1168.
- 34.Song, A., Zhang, J., Lebrilla, C. B. & Lam, K. S. (2003) J. Am. Chem. Soc. 125, 6180–6188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.