Abstract
Background:
High levels of free fatty acids (FFA) have been suggested to be one of the underlying mechanisms for adipose tissue (AT) inflammation and dysfunction in obesity. Human AT produces several adipokines including monocyte chemoattractant protein-1 (MCP-1), which are involved in the pathogenesis of obesity-mediated inflammation.
Objective:
In this study, we investigated the effects of lipopolysaccharide (LPS) and a panel of dietary FFA on MCP-1 gene and protein expression in adipocytes and macrophages. Furthermore, we investigated whether the effect of LPS and FFA were mediated through the toll-like receptor 4 (TLR4).
Methods:
3T3-L1 adipocytes and THP-1 macrophages were incubated for 24 h with the following FFA: monounsaturated fatty acid (oleic acid), saturated fatty acid (palmitic acid) and trans fatty acid (elaidic acid; 500 μM) with and without LPS (2 ng ml−1), and MCP-1 and TLR4 mRNA expression and MCP-1 protein secretion was determined.
Results:
The results showed that LPS significantly increased MCP-1 and TLR4 expression and MCP-1 secretion in 3T3-L1 adipocytes, and that the MCP-1 expression was blocked by a TLR4 inhibitor (CLI095). The effects of the various FFA on MCP-1 mRNA expression and protein secretion in the adipocytes showed no significant changes either alone or in combination with LPS. In macrophages, palmitic acid increased MCP-1 mRNA expression by 1.8-fold (P<0.05), but oleic acid and elaidic acid had no effects.
Conclusions:
In conclusion, in 3T3-L1 adipocyte, the TLR4-agonist, LPS, stimulates the proinflammatory chemokine MCP-1. The different classes of FFA did not induce MCP-1 mRNA expression or protein secretion in the adipocytes, but the saturated FFA, palmitic acid, induced MCP-1 mRNA expression in macrophages, possibly because of the higher expression level of TLR4 in the macrophages than the adipocytes. Our results indicate that FFA may induce AT inflammation through proinflammatory stimulation of macrophages.
Introduction
Inflammation in adipose tissue is increasingly considered to be of importance for the development of diseases associated with obesity such as type 2 diabetes and the metabolic syndrome.1 The mechanistic basis for the inflammatory response in the expanding adipose tissue is still unknown but suggestions include: dysregulation in free fatty acid (FFA) fluxes, oxidative stress, endoplasmatic reticulum stress, adipocyte cell death, inhibition of adipogenesis, fibrosis and hypoxia.1, 2, 3 It has long been recognized that plasma FFA concentrations are commonly elevated in obese individuals, mainly due to increased FFA release associated with the expansion in fat mass.4 It has been found that FFA can serve as an agonist of the toll-like receptor 4 (TLR4) complex.5 Stimulation of TLR4 activates proinflammatory pathways and induces cytokine expression in a variety of cell types, which ultimately drives macrophage accumulation and thereby inflammation.6, 7, 8, 9, 10 During sepsis and endotoxemia, TLR4 is pronouncedly activated by lipopolysacharide (LPS), but recent studies indicate that small amounts of LPS in the circulation might also have a role for the ‘low-grade' inflammation characterizing metabolic syndrome/obesity.11, 12 In adipocytes, stimulation with LPS induces lipolysis, insulin resistance13 and secretion of proinflammatory adipocytokines like monocyte chemoattractant protein-1 (MCP-1).14 The chemokine MCP-1 is a key regulator of macrophage recruitment, and studies have shown how mice fed with a high-fat diet have an increased expression of adipose tissue MCP-1 mRNA and elevated MCP-1 plasma protein levels.15 These animals had also more widespread macrophage infiltration in the adipose tissue compared with control animals. Furthermore, MCP-1 release from human adipose tissue is higher in visceral than the subcutaneous depots16 and is increased in obesity, indicating that MCP-1 may be involved in obesity-related health complications.17, 18
Recently Erridge and Samani18 have shown that saturated fatty acid-mediated TLR2/4 activation is mainly due to LPS and other lipopeptide contamination present in the bovine serum albumin (BSA) used for the experimental setup. This creates doubt in the understanding of fatty acid-mediated inflammation in different cell lines and animal models.
Therefore, in this present study, we aimed to analyze the effect of common dietary FFA on two important adipose tissue cell types, namely the adipocytes and the macrophages. We investigated the effect of the monounsaturated fatty acid, oleic acid, and the saturated fatty acids, palmitic acid. Moreover, the trans fatty acid, elaidic acid, was investigated, as the trans fatty acids have particularly been implicated in worsening markers of metabolic syndrome and obesity. We focused particularly on MCP-1 mRNA and protein expression, a target of the TLR-pathway and a key regulator of the initial macrophage infiltration and adipose tissue inflammation. Finally, we investigated whether the effect of LPS and FFA was mediated through TLR4 by using a specific TLR4 intracellular receptor antagonist. Thus, our overall aim was to elucidate the importance of FFA for inducing adipose tissue inflammation in obesity and investigate the cell types involved.
Materials and methods
Reagents
Palmitic acid (Cat. No. P0500, Sigma, Sigma chemicals, St Louis, MO, USA), Oleic acid (Cat. No. O1008, Sigma, Sigma chemicals), Elaidic acid (Cat. No. E4637, Sigma, Sigma chemicals), LPS (Cat. No. L8274, Sigma, Sigma chemicals), CLI095 (intracellular TLR4 antagonist; Cat. No. tlrl-cli95, InvivoGen, San Diego, CA, USA), fatty acid free (FAF) BSA, FAF-BSA; Cat. No. 10775835001, Roche, Mannheim, Germany) and BSA (Cat. No. A7888 Sigma, Sigma chemicals).
3T3-L1 murine adipocytes
3T3-L1 preadipocytes were obtained from the American Type Culture Collection (Manassas, VA, USA) and were grown in 24-well plates according to standard conditions. Briefly, cells were grown in 5% CO2 in Dulbecco's modified eagle's medium (DMEM; Gibco BRL, Life Technologies, Roskilde, Denmark) containing 10% calf serum (Gibco BRL, Life Technologies) in the presence of a 1% penicillin/streptomycin (P/S) mixture (Invitrogen, Carlsbad, CA, USA). At 2 days post confluence (day 0), cells were induced to differentiate with a medium containing 10% fetal bovine serum (FBS; Gibco BRL, Life Technologies), 175 nM insulin, 1 μM dexamethasone and 0.5 mM isobutyl-1-methylxanthine for 48 h. At day 3, fresh DMEM medium containing 10% FBS, 1% P/S and 175 nM insulin was added and the cells were incubated another 48 h. At day 6, the medium was changed to an insulin-free DMEM medium containing 10% FBS and 1% P/S. Adipocytes were used in the experiments at days 7–9. Before experiments, cells were kept in DMEM containing 0.2% FAF-BSA overnight. The cells were then treated with DMEM containing 2% FAF-BSA and the various fatty acids complexed with FAF-BSA (final concentration of added FFA was 500 μM) for 24 h or in addition with LPS and the TLR4-inhibitor, CLI095.19
THP-1 human macrophages
The human monocytic leukemia cell line THP-1 was obtained from the American Type Culture Collection. THP-1 cells were maintained in RPMI-1640 growth medium (Invitrogen) supplemented with 10% FBS and 1% P/S at 37 °C under 5% CO2. Differentiation of THP-1 cells was induced by treatment with 10 ng ml−1 12-O-tetradecanoylphorbol-13-acetate (Sigma) in RPMI-1640 supplemented with 10% FBS and 1% P/S for 72 h. After 72 h of differentiation, the cells were used for the experiments. Before the incubations were performed, cells were kept in RPMI-1640 media containing 0.2% FAF-BSA overnight, and during the experiment, in RPMI-1640 media containing 2% FAF-BSA. The cells were then treated with RPMI-1640 media containing 2% FAF-BSA and 500 μM of BSA-complexed fatty acid for 24 h.
Preparation of fatty acid–albumin complexes
Saturated palmitic acid, trans fatty elaidic acid and monounsaturated oleic acid were used in this study. Lipid-containing media were prepared by conjugation of FFA with BSA using a modified method described by Svedberg et al.20 Briefly, the complex of fatty acid and FAF-BSA was prepared by dissolving and heating (90 °C) to equal molar amounts of NaOH and fatty acid, supplemented with distilled water to a concentration of 500 mM. The FFA complex was further diluted to 50 mM, with a 5% FAF-BSA solution. The stock solution was sterile filtered and stored in dark at −20 °C. The stock solution was heated before use in the cellular experiment.
Isolation of RNA from adipocytes and macrophages
Total RNA was isolated from the cells using TriZol reagent (Gibco BRL, Life Technologies); RNA was quantified by measuring absorbance at 260 and 280 nm, and the inclusion criteria was a ratio ⩾2. Finally, the integrity of the RNA was checked by visual inspection of the two ribosomal RNAs, 18S and 28S, on an agarose gel.
Real-time reverse transcriptase PCR
For real-time reverse transcriptase PCR, complementary DNA was constructed using random hexamer primers as described by the manufacturer (GeneAmp RNA PCR Kit from Perkin-Elmer Cetus, Norwalk, CT, USA). PCR-mastermix, containing the specific primers, HotStar Taq DNA polymerase (Qiagen, Valencia, CA, USA) and SYBR-Green PCR buffer were added. The following primer pairs were used: Murine MCP-1 sense primer 5′-GCTTGAGGTGGTTGTGGAAAA-3′ and antisense primer 5′-CTCACCTGCTGCTACTCATTC-3′. Murine TLR4 sense primer5′-TGTCATCAGGGACTTTGCTG-3′ and antisense primer 5′-TGTTCTTCTCCTGCCTGACA -3′. Human MCP-1 sense primer 5′-ACTCTCGCCTCCAGCATGAAAGTC-3′ and antisense primer 5′-TGCAAAGACCCTCAAAACATCCCA-3′. The housekeeping genes, ABL1 (3T3-L1) and glyceraldehyde-3-phosphate dehydrogenase (THP-1), were amplified using murine ABL1 sense primer 5′-CTGTTTGAAGTTGGTGGGCT-3′ and antisense primer 5′-CTGGGGCTCAAAGTCAGATG-3′. GADPH sense primer 5′-CTGGGGCTCAAAGTCAGATG-3′ and antisense primer 5′-CTGTTTGAAGTTGGTGGGCT-3′. Real-time quantification of genes was performed by SYBR-green real-time reverse transcription PCR assay (Qiagen) using an ICycler from Bio-Rad (Bio-Rad Laboratories, Hercules, CA, USA). cDNA with specific primers was amplified in separate tubes, and the increase in fluorescence was measured in real time. The threshold cycle was calculated, and the relative gene expression was calculated essentially as described in the User Bulletin no. 2, 1997, from Perkin-Elmer. All samples were amplified in duplicate. A similar setup was used for negative controls, except that the reverse transcriptase was omitted and no PCR products were detected under these conditions.
The levels of mRNA in this study were calculated relative to a housekeeping gene and expressed as relative values vs control=1 (FAF-BSA=1). We tested several different housekeeping genes and in 3T3-L1 adipocytes, ABL1, and in THP-1 macrophages, glyceraldehyde-3-phosphate dehydrogenase, were stably expressed in the different study groups. Therefore, these two genes were chosen as the reference in the present study.
Determination of proteins in cell culture media
Media concentrations of MCP-1 was measured using ELISA kits from Quantikine DY479, R&D Systems Europe Ltd, Abingdon, UK, according to the manufacturer's protocols.
Statistical analysis
Differences between group means were determined using one analysis of variance with Tukey post hoc test. The level of significance was P<0.05. Data represent mean±s.e.m. All analyses were performed with Sigma Stat (Systat software, Richmond, CA, USA) statistical software.
Results
Effect of BSA on MCP-1 mRNA expression in 3T3-L1 adipocytes
In initial studies, we found that the ordinary BSA used elicited a 14.7-fold increase in MCP-1 expression compared with media without BSA (data not shown). However, FAF-BSA was not found to stimulate MCP-1 expression in 3T3-L1 adipocytes (data not shown). These findings indicate that ordinary BSA is contaminated with LPS or LPS-like materials as previously described.18 Therefore, FAF-BSA was used throughout the present work.
Effect of LPS on MCP-1 in 3T3-L1 adipocytes
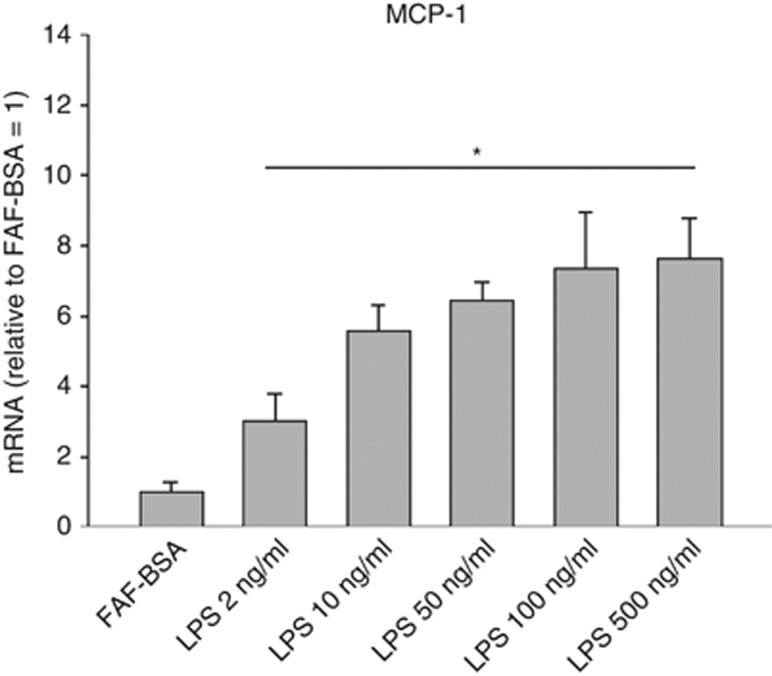
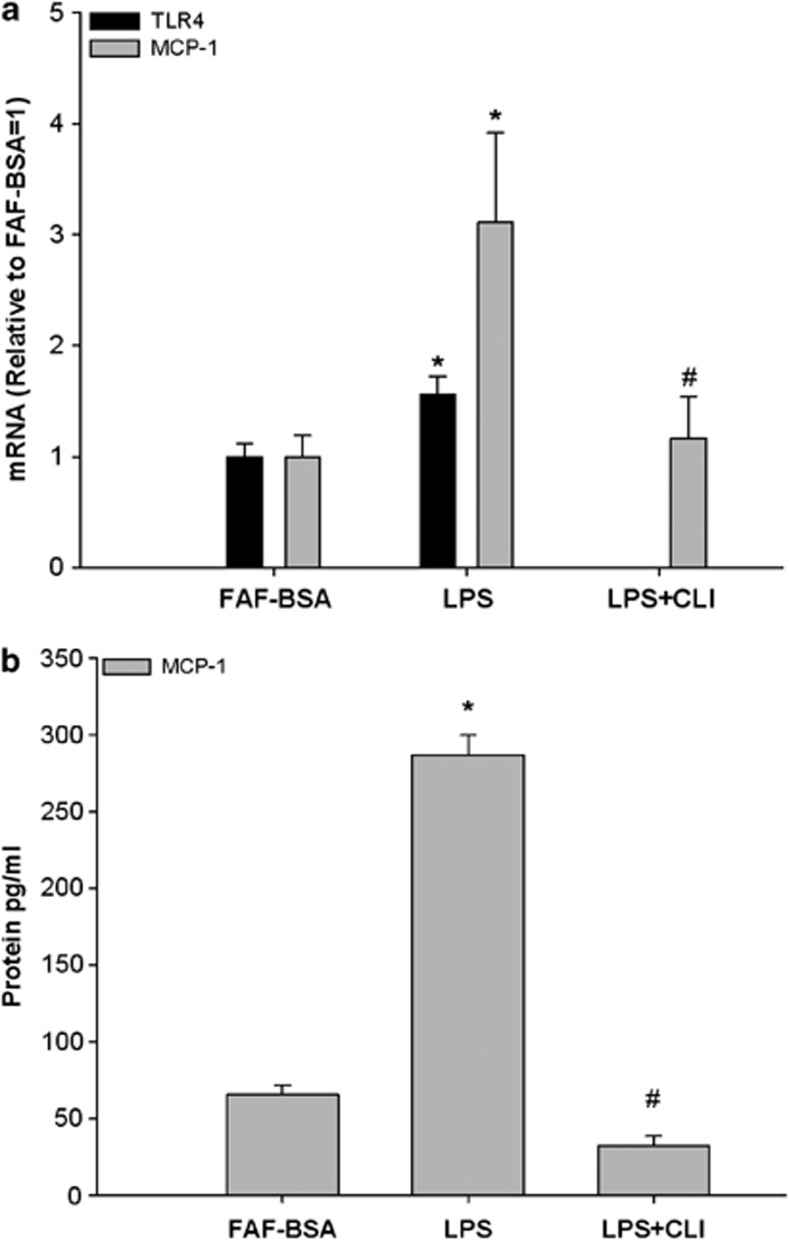
LPS dose-dependently stimulated the MCP-1 mRNA expression and it reached significance at a concentration of 2 ng ml−1 (Figure 1; P<0.05). This concentration was chosen for all further incubations in this study to stimulate MCP-1 and to demonstrate possible inhibitory effects of coincubated FFA. LPS stimulation (2 ng ml−1) for 24 h increased the MCP-1 expression by threefold and TLR4 expression by 1.56-fold (Figure 2a; P<0.05). As shown in Figure 2a, incubation with the specific TLR4-inhibitor CLI095 (3 μM) completely inhibited the LPS-induced MCP-1 expression (inhibition with 93%, P<0.05). Concerning the protein secretion, LPS increased MCP-1 protein secretion by 4.4-fold (Figure 2b; P<0.05) and this response was also completely inhibited by the TLR4-inhibitor CLI095 (3 μM) (inhibition with 115%, P<0.05; Figure 2b; P<0.05).
Figure 1.
Dose-dependent effects of LPS on MCP-1 mRNA expression in 3T3-L1 adipocytes. 3T3-L1 adipocytes were incubated with DMEM growth media containing 2% FAF-BSA and LPS (2 ng ml−1 to 500 ng ml−1) for 24 h. Results were expressed as ±s.e.m., relative to FAF-BSA=1 (n=9), *P<0.05 vs FAF-BSA.
Figure 2.
Effect of LPS and a TLR4-inhibitor on (a) TLR4 and MCP-1 mRNA expression and (b) MCP-1 protein secretion in 3T3-L1 adipocytes. 3T3-L1 adipocytes were incubated with DMEM growth media containing 2% FAF-BSA and LPS (2 ng ml−1) alone or in combination with the TLR4-inhibitor CLI095 (CLI, 3 μM) for 24 h. Results were expressed as ±s.e.m., relative to FAF-BSA=1 (n=9), *P<0.05 vs FAF-BSA, #P<0.05 vs LPS.
Effects of various FFA on MCP-1mRNA expression and protein secretion in 3T3-L1 adipocytes
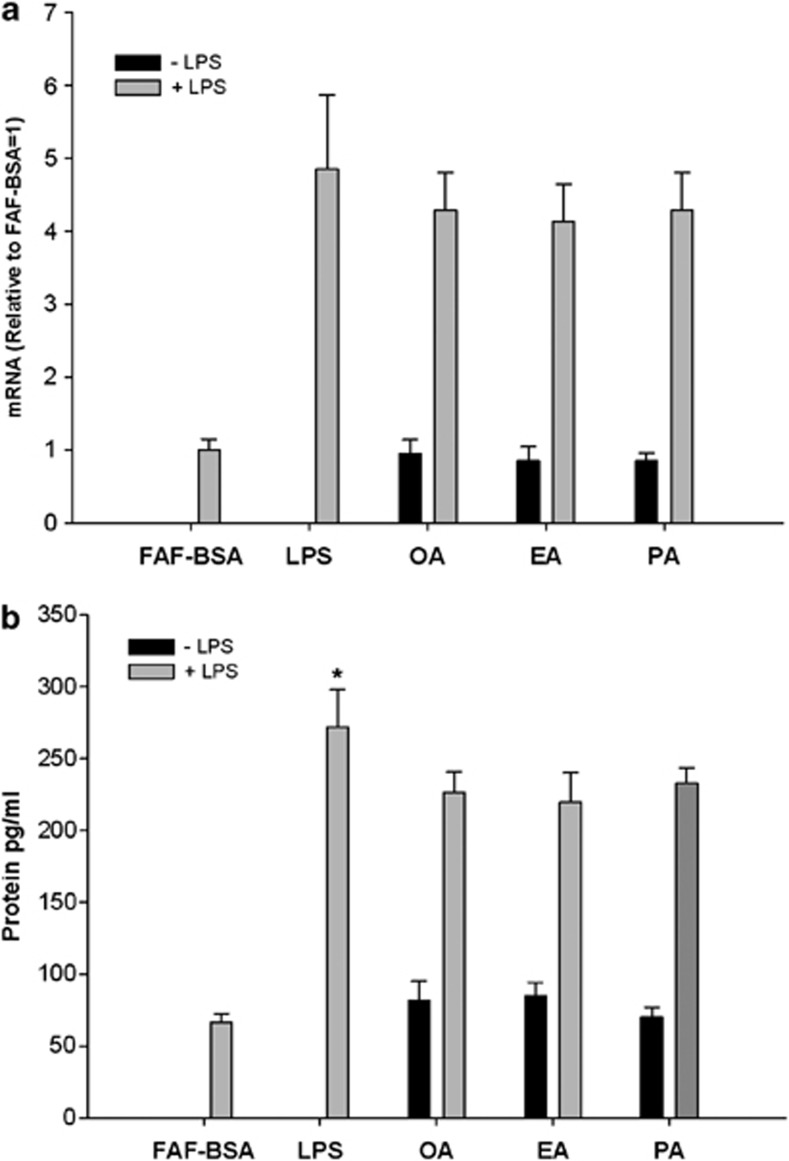
The adipocytes were incubated with the various FFA at a final concentration of 500 μM for 24 h. As shown in Figure 3, no effects were found of the various FFA either on MCP-1 expression or on the release of MCP-1 to the medium. In order to examine whether the investigated FFA may have additive or antiinflammatory effects in adipocytes, the adipocytes were also stimulated by LPS (2 ng ml−1) and coincubated with the various FFA at a concentration of 500 μM and again the various FFA had no effects on the LPS-induced MCP-1 expression/release (Figure 3).
Figure 3.
Effects of (a) FFA and LPS stimulation on MCP-1 mRNA expression and (b) MCP-1 protein secretion in 3T3-L1 adipocytes. 3T3-L1 adipocytes were incubated with DMEM growth media containing 2% FAF-BSA and 500 μM of BSA-complexed fatty acid for 24 h. The fatty acids investigated are: monounsaturated fatty acid (oleic acid/OA), saturated fatty acid (palmitic acid/PA) and trans fatty acid (elaidic acid/EA). Results were expressed as±s.e.m., relative to FAF-BSA=1 (n=9), ns=non-significant vs FAF-BSA.
We tested the effects of the three FFA (oleic, elaidic and palmitic acid) at other time points (3 and 9 h) with similar result (data not shown). Choosing a relatively high FFA concentration (500 μM) in the present study was based on previous studies.31 However, we did also perform incubations with lower FFA concentrations (250 μM) also showing similar negative results (data not shown).
Effect of FFA on MCP-1 expression in THP-1 macrophages
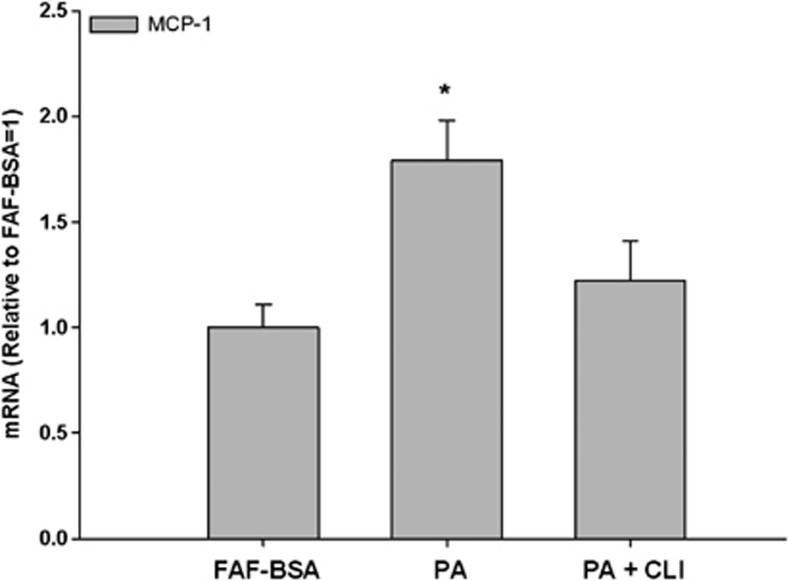
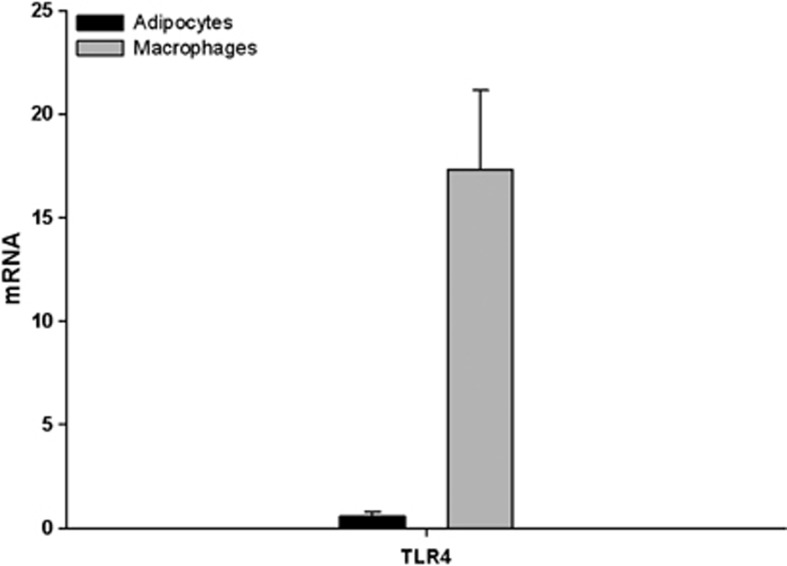
As macrophages are a very important cell type in adipose tissue particularly related to inflammation, we also investigated the effect of FFA on macrophages in vitro. This investigation was performed with THP-1 macrophages, a human cell line, with the same panel of FFA as used in the 3T3-L1 cell experiments. Incubation with palmitic acid (500 μM) for 24 h increased MCP-1 mRNA 1.8-fold times (Figure 4; P<0.05). In parallel incubation with the TLR4-inhibitor, CLI095 (3 μM), the increments were reduced by 33% (P=0.09), although not significantly. Incubation with oleic acid and elaidic acid had no effects on the MCP-1 expression in these macrophages (data not shown). We investigated the expression of TLR4 in both cell lines, and showed a 96.6% higher expression of TLR4 in macrophages than in the adipocytes (Figure 5).
Figure 4.
Effect of palmitic acid on MCP-1 mRNA expression in THP-1 macrophages. THP-1 macrophages were incubated with RPMI-1640 growth media containing 2% FAF-BSA and palmitic acid (PA, 500 μM) alone or in combination with the TLR4-inhibitor CLI095 (CLI, 3 μM) for 24 h. Results were expressed as±s.e.m., relative to FAF-BSA=1 (n=9), *P<0.05 vs FAF-BSA.
Figure 5.
Expression of TLR4 in 3T3-L1 adipocytes and THP-1 macrophages. Unstimulated 3T3-L1 adipocytes and THP-1 macrophages were analyzed for TLR4 gene expression.
Testing for cell damage during the incubation conditions
In order to investigate whether the levels of FFA used in this study induces apoptosis/cell death in the adipocytes and macrophages, we measured lactate dehydrogenase in the media of each group and found no change in lactate dehydrogenase levels between control and FFA-treated groups (data not shown).
Discussion
Triglycerides in adipose tissue consist primarily of saturated fatty acids and monounsaturated fatty acids (mainly palmitic and oleic acid), and some studies indicate that saturated fatty acids have the potential to modulate AT inflammation through TLR-dependent pathways.5, 21, 22, 23 Excess intake and endogenous release (lipolysis) of saturated fatty acids might therefore enhance expression of TLR target genes like MCP-1. Indeed, TLR4-deficient knockout mice fed with a diet rich in saturated fatty acid had a lower grade of macrophage infiltration and MCP-1 expression in their visceral adipose tissue compared with wild-type mice.24
In the present study, we confirmed that TLR4 is expressed in 3T3-L1 adipocytes, but to a much lower degree than in macrophages. Furthermore, it was shown how LPS (a TLR4 ligand) potently induced MCP-1 mRNA expression and protein secretion in the 3T3-L1 adipocytes, an effect that was inhibited by a TLR4 inhibitor.
To investigate the effects of FFA on MCP-1 production in 3T3-L1 adipocytes, we chose a panel of three dietary FFA consisting of the monounsaturated fatty acid (oleic acid)-naturally present in food, the trans fatty acid (elaidic acid)-a major component in industrially produced trans fatty acid and the saturated fatty acid (palmitic acid). Elaidic acid is the predominant isomer of the trans fatty acids in human food. Oleic acid and palmitic acid represent the two most highly abundant FFA in the diet, in the blood and in the white adipose tissue.25
The study showed that elaidic acid had no detectable effect on MCP-1 expression and secretion in 3T3-L1 adipocytes. Furthermore, we found no effect of oleic acid on MCP-1 expression, which is in agreement with previous studies.22, 26 In contrast to some studies showing increased MCP-1 secretion in adipocytes in response to palmitic acid stimulation, we did not observe any effects on MCP-1 production in 3T3-L1 adipocytes.22, 26 Thus, it can be suggested that several of the previous studies finding stimulatory effects of FFA on cytokine production may be due to LPS-like contamination of the BSA used to conjugate the fatty acids as showed by Erridge and Samani.18 In contrast to the latter authors, we found that palmitic acid was able to stimulate MCP-1 in macrophages, which we do not believe was due to LPS contamination of our BSA as we used FAF-BSA and, moreover, had the same FAF-BSA in the control incubation. Thus, the effect in macrophages on MCP-1 may be a direct effect of palmitic acid but whether this effect is primarily mediated through TLR4 is unknown.
Thus, we found no pro or antiinflammatory effect of the various FFA in adipocytes which may be somehow logical as the primary function of the adipocytes is to handle fatty acids (storage/release) and the adipocytes may in some way therefore be protected from the negative effect (i.e., lipotoxicity) during the FFA overload seen in obesity. Furthermore, the subcutaneous adipose tissue of obese individuals maintains its ability to store FFA from plasma to the same degree as normal-weight individuals.27
In contrast to the adipocyte, we found a proinflammatory effect of palmitic acid in the macrophages but the other tested FFA had no effects. This could be due to an increased expression of TLR4 in macrophages compared with adipocytes as shown in Figure 5. Furthermore, a new study supports these findings by showing that neither saturated fatty acid nor polyunsaturated fatty acids have a proinflammatory effect on human adipose tissue and adipocytes as they are not able to activate TLR2 and TLR4.28 However, it has recently been shown that TLR2 and TLR4 were activated by saturated fatty acids in THP-1 macrophages.29 That TLR4 may be involved in the effect of palmitic acid is supported by our finding that the effect of palmitic acid was partly blocked by the TL4R antagonist, CLI095.
FFA can, however, also activate signaling pathways in the adipocytes via other receptors than the TLRs. Recently, it has been shown that other receptors that affect inflammation in the adipose tissue when activated by FFA are the gene coupled receptors GPR40-43 and GPR120.30 Besides binding of FFA to its receptors, FFA can also act as a signaling molecule by cellular uptake mediated by a number of proteins, including CD36, fetuin A and various fatty acid transporters.31, 32
In conclusion, we confirmed that the 3T3-L1 adipocyte expresses the proinflammatory chemokine MCP-1 when stimulated with the TLR4 ligand LPS. Our present study in adipocytes suggests that a panel of common dietary fatty acids did not have any effects on MCP-1 production. In macrophages, the saturated fatty acid palmitic acid was found to stimulate macrophage MCP-1 gene expression. We suggest that high levels of some saturated FFA in obesity do not affect the inflammatory response in adipocytes but may affect the inflammatory response in other cell types present in the adipose tissue, predominantly macrophages.
Acknowledgments
We thank Lenette Pedersen and Pia Hornbek for their skillful technical assistance.
The authors declare no conflict of interest.
Footnotes
The study was part of the DanORC consortium. DanORC is supported by the Danish Council for Strategic Research.
References
- Bluher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009;117:241–250. doi: 10.1055/s-0029-1192044. [DOI] [PubMed] [Google Scholar]
- Wood IS, Wang B, Lorente-Cebrian S, Trayhurn P. Hypoxia increases expression of selective facilitative glucose transporters (GLUT) and 2-deoxy-D-glucose uptake in human adipocytes. Biochem Biophys Res Commun. 2007;361:468–473. doi: 10.1016/j.bbrc.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 2009;33:54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian M, Wang Y, Sul HS. Lipolysis in adipocytes. Int J Biochem Cell Biol. 2010;42:555–559. doi: 10.1016/j.biocel.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MT, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- Thompson BR, Lobo S, Bernlohr DA. Fatty acid flux in adipocytes: the in's and out's of fat cell lipid trafficking. Mol Cell Endocrinol. 2010;318:24–33. doi: 10.1016/j.mce.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Troseid M, Nestvold TK, Rudi K, Thoresen H, Nielsen EW, Lappegard KT. Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: evidence from bariatric surgery. Diabetes Care. 2013;36:3627–3632. doi: 10.2337/dc13-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. The c-Jun N-terminal kinase mediates the induction of oxidative stress and insulin resistance by palmitate and toll-like receptor 2 and 4 ligands in 3T3-L1 adipocytes. Horm Metab Res. 2009;41:523–530. doi: 10.1055/s-0029-1202852. [DOI] [PubMed] [Google Scholar]
- Kopp A, Buechler C, Neumeier M, Weigert J, Aslanidis C, Scholmerich J, et al. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring) 2009;17:648–656. doi: 10.1038/oby.2008.607. [DOI] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90:2282–2289. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond) 2005;29:146–150. doi: 10.1038/sj.ijo.0802839. [DOI] [PubMed] [Google Scholar]
- Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29:1944–1949. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- Svedberg J, Bjorntorp P, Smith U, Lonnroth P. Free-fatty acid inhibition of insulin binding, degradation, and action in isolated rat hepatocytes. Diabetes. 1990;39:570–574. doi: 10.2337/diab.39.5.570. [DOI] [PubMed] [Google Scholar]
- Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, et al. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233–245. doi: 10.1111/j.1365-2567.2008.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, et al. Role of the toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 2008;16:1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- Kien CL. Dietary interventions for metabolic syndrome: role of modifying dietary fats. Curr Diab Rep. 2009;9:43–50. doi: 10.1007/s11892-009-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeop HC, Kargi AY, Omer M, Chan CK, Wabitsch M, O'Brien KD, et al. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59:386–396. doi: 10.2337/db09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsari C, Ali AH, Mundi MS, Jensen MD. Storage of circulating free fatty acid in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes. 2011;60:2032–2040. doi: 10.2337/db11-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murumalla RK, Gunasekaran MK, Padhan JK, Bencharif K, Gence L, Festy F, et al. Fatty acids do not pay the toll: effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis. 2012;11:175. doi: 10.1186/1476-511X-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53:2002–2013. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Lagakos WS. The role of G-protein-coupled receptors in mediating the effect of fatty acids on inflammation and insulin sensitivity. Curr Opin Clin Nutr Metab Care. 2011;14:322–327. doi: 10.1097/MCO.0b013e3283479230. [DOI] [PubMed] [Google Scholar]
- Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- Georgiadi A, Kersten S. Mechanisms of gene regulation by fatty acids. Adv Nutr. 2012;3:127–134. doi: 10.3945/an.111.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]