Abstract
Catalyst and substrate studies have been performed on the rhodium-catalyzed asymmetric ring opening reaction. A working model is advanced that involves oxidative insertion with retention to form an organorhodium intermediate that then undergoes nucleophilic attack with inversion. Kinetic and competition experiments have uncovered evidence for a proton transfer step in the catalytic cycle that may activate both the allylrhodium intermediate and the nucleophile. We have also conducted experiments designed to understand which properties of the PPF-PtBu2 ligand contribute to the high reactivities and enantioselectivities.
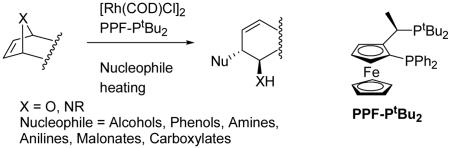
We have previously reported that chiral rhodium(I) catalysts induce ring opening oxa- and azabicyclic alkenes with a variety of soft nucleophiles such as alcohols, phenols, amines, anilines, malonates, and carboxylates in high yield and enantioselectivity (1–8). The products are generated by an SN2′ nucleophilic displacement of the bridgehead leaving group with inversion providing the 1,2-trans product as one regio- and diastereomer. The formation of the 1,2-trans products is atypical for ring opening reactions of oxabicyclic alkenes, which usually generate the syn-1,2 diastereomers via exo nucleophilic attack (1, 2). Furthermore, regio- and stereochemical results diverge from previously documented rhodium-catalyzed reactions with allylic carbonates (9–18). The observations obtained with oxabicyclic alkenes find little precedent when compared to other allylic functionalizations including those catalyzed by Pd (refs. 19 and 20 and references therein and ref. 21), Mo (22–26), Ni (27–33), Pt (ref. 34 and references therein and ref. 35), Co (36), Ir (37–43), Fe (44), Ru (45–49), and W (50).
In this report we describe catalyst and substrate studies and advance a mechanistic working model that rationalizes the stereochemical outcome and other experimental observations (Scheme 1). We have also conducted experiments designed to understand the properties of the PPF-PtBu2 ligand that contribute to high reactivities and enantioselectivities.
Scheme 1.
Key aspects are as follows: (i) products are obtained in high yield (> 90% ee and as one regio- and diastereomer); (ii) very low catalyst loadings can be employed (as low as 0.01% mol %); and (iii) the reaction outcome is SN2′ displacement of the bridgehead leaving group with inversion.
Experimental procedures and characterization data can be found in Supporting Text, which is published as supporting information on the PNAS web site.
Ligand Studies: Variations on the Josiphos Template and Other Ligand Motifs
Several structural elements are present on the PPF scaffold that could contribute to its successful application in these reactions (51–53). PPF has elements of central and planar chirality as well as two phosphine atoms that differ both sterically and electronically. Two methods were used to determine the influence of the ligand on the rate. In cases where a significant difference in rate was observed, percent conversion vs. time, as determined by crude 1H NMR, was used to quantify the reactivity of the catalyst. In cases where a more precise measurement of rate was desired, kinetic runs were performed.
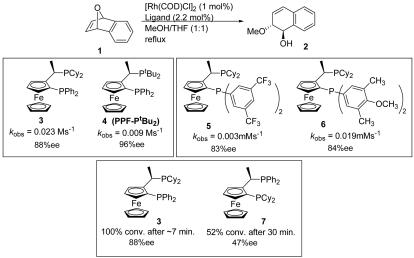
By comparing the reactivity and enantioselectivity of ligands 3 and 4, two trends emerge (Scheme 2). First, by increasing the steric bulk (tBu > Cy) the enantioselectivity is increased from 88% with 3 to 96% enantiomeric excess (ee) with the bulkier tBu2P ligand 4. This increase in enantioselectivity comes at the expense of reactivity, however, because the di-t-butyl ligand 4 produces a catalyst that is roughly half as active as that generated from the Cy2P ligand 3 (kobs 0.023 vs. 0.009 M·s–1).
Scheme 2.
To probe the impact of phosphine electronic effects, ligands 5 and 6 were compared (Scheme 2). We assumed a minimal steric difference because the methoxy substituents of 6 are at the para positions. The major difference in these ligands is electronic because the (3,5-(CF3)2-C6H3)2P group is electron-deficient, whereas (3,5-(Me)2-4-(OMe)-C6H2)2P is electron-rich. When reaction outcomes are compared, very little difference in product ee is observed (83% ee with 5 vs. 84% ee with 6). A dramatic impact on reactivity is observed, however. From kinetic runs, it was determined that the more electron-rich ligand 6 produces a catalyst that is 6.8 times more reactive than one generated from ligand 5.
To examine the importance of the relative size of the phosphines at the benzylic and ferrocenyl positions, ligands 3 and 7 were compared (Scheme 2). Inferior results were obtained with ligand 7 compared to 3 both in terms of reactivity and enantioselectivity.
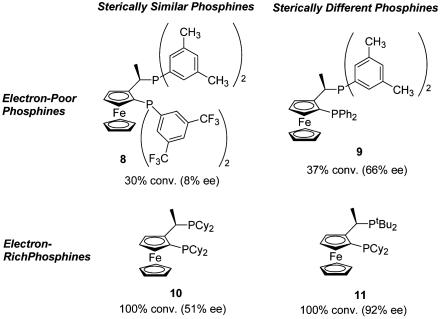
Ligands 8–11 were also studied to gain further insight into the effect of substituents on the ligand (Scheme 3). Phosphine 8, which is electron-deficient and possesses two phosphines of similar size, produces the poorest outcome, giving only 30% conversion after 30 min and 8% ee. The best result was obtained with electron-rich ligand 11 bearing a large benzylic phosphine and a smaller ferrocenyl phosphine. With ligand 11, 100% conversion and 92% ee was obtained. Results obtained with ligands 9 and 10 lie in the other two quadrants of Scheme 3, supporting the notion that electron-rich ligands produce more reactive catalysts (ligands 10 and 11 vs. ligands 8 and 9) and that sterically larger phosphines at the benzylic position give improved ee (ligands 9 and 11 vs. ligands 8 and 10).
Scheme 3.
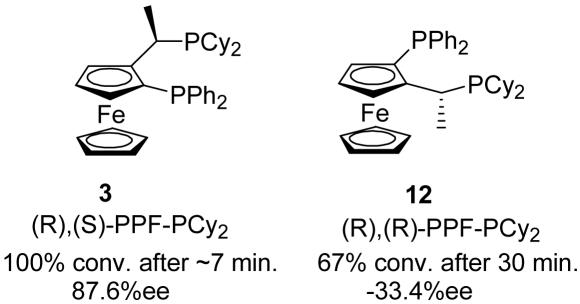
To probe the relative importance of the planar and central elements of chirality within the Josiphos skeleton, ligands 3 and 12 were studied. These two diastereomeric ligands bear the same (R)-central chirality but have the opposite planar chirality. When the reaction outcomes are compared, significant differences appear. The (R),(S)-ligand 3 gives 100% conversion after ≈7 min, whereas the (R),(R)-diastereomer 12 gives 67% conversion after 30 min. The enantioselectivity is also influenced by both elements of chirality. By changing only the planar chirality, the sense of induction is reversed, giving the opposite enantiomer in 33.4% ee. This finding indicates that the planar chirality plays the largest role in determining the absolute sense of induction but that the central chirality must be matched with the planar chirality for high ee to be obtained. The highest ee is obtained when the (R),(S)-diastereomer is used (87.6% ee). Conveniently, this is also the diastereomer that results in the highest reactivity (Scheme 4).
Scheme 4.
These results delineate the important aspects of the PPF-PtBu2 framework and lay down guidelines that may be used in the search for more reactive and selective catalysts. To maximize reactivity, both phosphines must be electron-rich. For maximal enantioselectivity, the two phosphines must be of different size with the larger phosphine located at the benzylic position. Finally, the optimal diastereomers contain (R) central and (S) planar chirality [or the enantiomeric (S,R)].
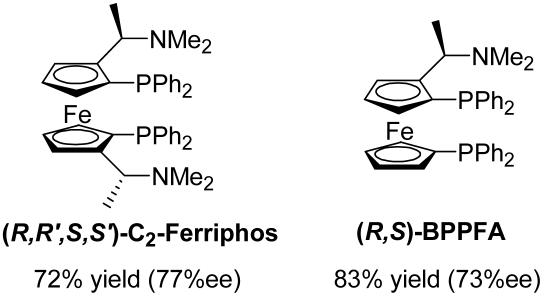
Other ferrocenyl ligands have been used in catalytic asymmetric reactions, including C2-Ferriphos and BPPFA (Scheme 5). Although they are moderately effective in the ARO of oxabicyclic alkenes, their reactivity is inferior to PPF-PtBu2. For example, use of C2-Ferriphos generates 2 in 72% yield and 77% ee after 30 min, whereas BPPFA gives 2 in 83% yield and 73% ee.
Scheme 5.
Catalyst Studies
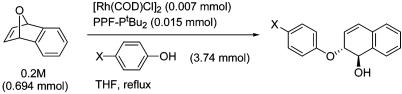
Effect of Ligand to Rhodium Ratio. If incomplete ligand binding occurs with a catalyst that is active in the absence of ligand, a mixture of chiral and achiral catalysts will be present that can potentially erode the enantioselectivity. When 1 was treated with [Rh(COD)Cl]2 in the absence of chiral ligand in a THF/MeOH (1:1) mixture, no reaction was observed after 30 min. When the reaction is performed with a 2:1 rhodium to chiral ligand ratio, 2 is obtained quantitatively in 95% ee. These results indicate that no background reaction occurs with the metal in the absence of a bound phosphine. In contrast, the addition of excess ligand appears to have no effect on the reaction outcome. Consequently, this reaction is technically very simple to perform because imprecise measurements with respect to rhodium and ligand do not negatively impact the outcome.
Enantioselectivity vs. Percent Conversion. Studying the relationship between percent conversion vs. enantioselectivity can indicate whether there is an induction period for the catalyst or whether the catalyst is changing over the course of the reaction. Such changes could occur as a result of prolonged exposure to the reagents or to new metal complexes being formed by interaction with the newly formed products. Three different runs were performed by using 0.5 mol % [Rh(COD)Cl]2, 1.1 mol % PPF-PtBu2, and 7.5 eq of PhOH in refluxing THF. In all three cases, the product was formed in 98% ee with no variation of more than ±1% ee between 1% and 100% conversion. It therefore appears that no catalyst induction is required on the time scale studied and that if changes in catalyst structure occur, they have no impact on the enantioselectivity.
Nonlinear Effects. Nonlinear effects were first quantified by Kagan and coworkers and have since become a valuable tool in the study of reaction mechanisms (54–57). The presence of nonlinear effects was investigated in the asymmetric ring-opening reactions with methanol as the nucleophile. In a typical run, oxabenzonorbornadiene 6 was treated with 2 mol % [Rh(COD)Cl]2 and 5 mol % PPF-PtBu2 of varying enantioenrichment in a refluxing THF/MeOH mixture (1:1). These experiments revealed the presence of a small negative nonlinear effect reaching a maximum of 6% with a ligand of 50% ee.
In another set of experiments, [Rh(CO)2Cl]2 was used as the rhodium source instead of [Rh(COD)Cl]2. Oxabenzonorbornadiene was treated with 2 mol % [Rh(CO)2Cl]2 and 5 mol % PPF-PtBu2 of varying enantiopurity in a refluxing THF/MeOH mixture (1:1). In contrast to [Rh(COD)Cl]2, the use of [Rh(CO)2Cl]2 in these reactions gives a linear relationship between ligand and product enantioenrichment.
Substrate and Nucleophile Studies
Nucleophile Acidity/Nucleophilicity on Reaction Rate and Outcome. While conducting scope studies with phenol nucleophiles, it was noted qualitatively that more acidic phenols reacted faster than basic phenols (5). To quantify these observations, we conducted kinetic and competition experiments by using isosteric alcohols and phenols of differing acidity. A plot of the appearance of product vs. time for five different phenol nucleophiles of varying pKa reveals that the reaction rate is faster when more acidic phenols are used (Table 1). Thus, 4-hydroxyacetophenone resulted in the fastest reaction (kobs = 0.0033 M·s–1) and para-cresol gave the slowest reaction (kobs = 0.0016 M·s–1). By plotting the log of [kobs(X)/kobs(H)] vs. σ– reveals a linear Hammett correlation with a ρ value of 0.30.
Table 1.
| Entry | X-Group | Product | (kobs)·103 (M·s-1) |
|---|---|---|---|
| 1 | CH3OC- | 13 | 3.3 |
| 2 | CF3 | 14 | 2.8 |
| 3 | H | 15 | 1.9 |
| 4 | OMe | 16 | 1.7 |
| 5 | Me | 17 | 1.6 |
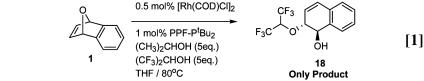
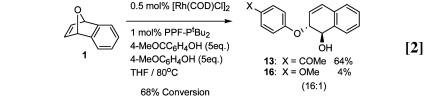
To further probe the relative importance of acidity and nucleophilicity in these reactions, competition experiments were performed. These experiments were carried out with pairs of alcohol and phenol nucleophiles. In one experiment, 5 eq of isopropanol and hexafluoroisopropanol (HFIP) were reacted with 1, and the reaction progress was monitored by periodic aliquot removal and crude 1H NMR analysis. In all cases, the exclusive product was 18 arising from HFIP addition. Even at 100% conversion, no isopropanol incorporation could be detected (Eq. 1). In another experiment, 5 eq of 4-hydroxyacetophenone and 4-hydroxyanisole were used. At 68% conversion, a 16:1 ratio of 13 to 16 was obtained, indicating that the more acidic phenol reacted preferentially (Eq. 2).
 |
 |
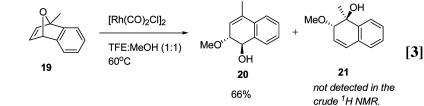
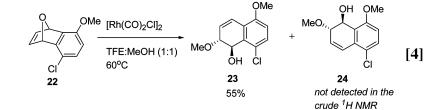
Diastereoselective Ring-Opening Reactions. Two different substitution locations were probed to determine whether a directing effect was present. In the first case, the effect of bridgehead substitution was investigated by reaction of methyl-substituted 19. In MeOH:TFE (1:1) and catalytic [Rh(CO)2Cl]2, only regioisomer 20 is produced arising from C–O-bond cleavage at the more highly substituted bridgehead carbon (Eq. 3). More remote substituent effects were also examined. By choosing aryl substituents with differing electronic properties, the steric influence can be minimized while creating an electronic bias that could direct the ring opening event. When 22 was reacted with catalytic [Rh(CO)2Cl]2 in TFE/MeOH (1:1) at 60°C, only regioisomer 23 is produced, indicating that a remote donating substituent affects the ring-opening step (Eq. 4).
 |
 |
Influence of Remote Substitution on the Oxabicyclic Alkene on Reaction Outcome
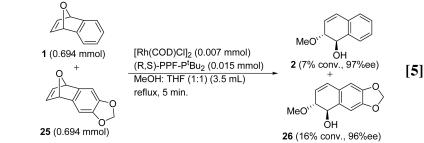
Competition Experiments. To probe the importance of cationic stabilization, competition experiments were conducted with three oxabicyclic substrates. In one reaction, equimolar quantities of 1 and the methylenedioxy-substituted 25 were reacted with methanol. After 5 min, 16% conversion of 25 and 7% conversion of 1 had occurred (Eq. 5). In contrast, when equimolar amounts of 1 and difluoro-substituted 27 were reacted, unsubstituted 1 reacted more rapidly. After 5 min, 10% conversion
 |
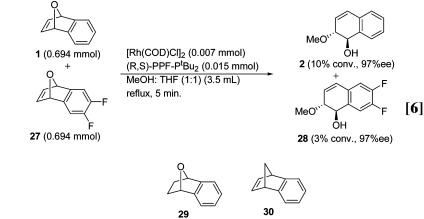 |
of 1 and 3% conversion of 27 occurred (Eq. 6). These results indicate that the presence of electron-donating groups on the aromatic ring of the oxabenzonorbornadiene activates the system toward reaction with the rhodium catalyst and that electron-withdrawing groups deactivate the system.
Substrates lacking various elements of 1 were reacted under the standard reaction conditions to determine the essential requirements for a successful reaction. For example, substrate 29 lacking the olefinic functionality does not react with methanol under the standard conditions. Analogously, 30 lacking the bridging ether functionality is also inert. Both the alkene and the oxygen group are necessary for reaction to occur.
Product Stability to the Rhodium Catalyst. Because the products of these ring opening reactions contain allylic ether/phenolic moieties that are known to react with palladium complexes (19–21), it might be anticipated that the rhodium catalyst will interact with the ring opened products and induce ionization. To test for reversibility in the nucleophile addition step, crossover experiments were conducted. For example, reaction of racemic 2 under conditions used for phenol addition for 1 h gave compete recovery of the starting material 2 with no phenol incorporation detectable in the crude 1H NMR (Eq. 7). Analogously, subjecting 15 to the conditions used for methanol addition gave only 15 in the crude 1H NMR and in >90% recovered yield (Eq. 8). These results support the notion that the nucleophilic addition step in these reactions is irreversible.
 |
 |
In another experiment, treatment of enantioenriched 2 (96% ee) with an achiral catalyst generated from [Rh(COD)Cl]2 and dppf in refluxing methanol:THF (1:1) for 1 h gave complete recovery of 2 with no erosion of ee (Eq. 9). Similarly, racemic 2 and 15 were subjected to the standard reaction conditions in the presence of a chiral catalyst generated from [Rh(COD)Cl]2 and PPF-PtBu2. In both reactions, the starting material was recovered unchanged in >90% yield as racemates (Eqs. 10 and 11). These results indicate that the high enantioselectivity obtained in these reactions is kinetic in origin, and that, if insertion of the rhodium catalyst into the allylic alcohol/phenol moiety does occur, ring closure of the oxabenzonorbornadiene to regenerate the oxabicyclic alkene does not occur.
 |
 |
 |
Discussion
Analysis of previously reported allylic functionalizations provides little analogy with the present reactions. These rhodium-catalyzed ARO reactions of oxabicyclic alkenes proceed with overall inversion of stereochemistry. Inversion of stereochemistry has previously been documented with palladium (0) catalysts and hard nucleophiles (such as organometallics) where the nucleophile is delivered from the metal (19–21). A similar mode of reactions is very unlikely here because hard nucleophiles such as arylboronic acids react with the same catalyst and substrate to give retention of stereochemistry (58). A net SN2′ displacement of the bridgehead leaving group is observed that is also different from what is obtained with rhodium catalysts and allylic carbonates where nucleophilic attack occurs at the same carbon from which the leaving group departs (11–17). Nucleophilic attack at the carbon bearing the leaving group has also been documented with Ru (48) and Fe (44) catalysts. On the other hand, Ni (27–33), Pd (19–21), and Pt (34, 35) all typically produce a mixture of regioisomeric products, whereas Ir (37–43), Mo (22–26), and W (50) favor attack at the more highly substituted allylic terminus.
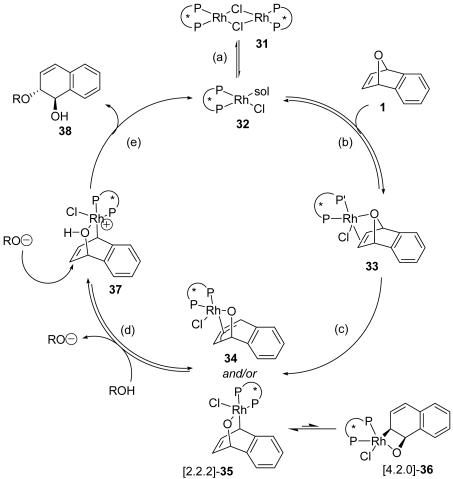
Based on our mechanistic and ligand studies, we propose the mechanism outlined in Scheme 6. When [Rh(COD)Cl]2 is used as the rhodium source, dimeric complex 31 is cleaved by solvation, substrate binding, or reaction with the nucleophile to give monomeric complex 32. Reversible exo coordination of the substrate is followed by oxidative insertion with retention into a bridgehead C–O bond to give the π-allyl or enyl rhodium alkoxide complexes 34 or 35. It is likely that the formation of these rhodium(III) alkoxide complexes will be irreversible because of the release of the ring strain present in the oxabicyclic alkene substrate. We propose that the oxidative cleavage of the C–O bond is the enantiodiscriminating step in the catalytic cycle.
Scheme 6.
a, Cleavage of the dimeric rhodium complex; b, exo coordination of the oxabicyclic alkene; c, oxidative insertion into the bridgehead C–O bond with retention; d, protonation of the rhodium alkoxide by the nucleophile; e, nucleophilic attack with inversion and product liberation.
Regardless of the precise mode of binding to the allyl moiety, we propose that the rhodium metal will be situated closer to the benzylic carbon atom because of the directing influence of the alkoxide ligand. The two enyl complexes 35 and 36 illustrate possible rhodium complexes. Because there will be a significant degree of ring strain associated with the [4.2.0] structure 36, this enyl complex should be disfavored compared to the more stable [2.2.2] structure 35. A similar argument can also be applied to the π-allyl rhodium complex 34. By shifting the rhodium metal on the π-allyl moiety to the distal position relative to the alkoxide, ring strain will be minimized.
Once 34 or 35 has been formed, the rhodium alkoxide complex could be protonated by the alcohol prenucleophile to generate cationic rhodium complex 37 and an alkoxide or phenoxide. This proton transfer has two effects. The organorhodium species is made more electrophilic as a result of the positive charge, and the nucleophile is rendered more nucleophilic by becoming deprotonated. It is noteworthy that reaction of an alkoxide or phenoxide does not give ring-opened product.
The positioning of the rhodium metal on the π-allyl moiety will influence the regioselectivity of nucleophilic attack. Nucleophilic attack with inversion is proposed to occur adjacent to the alkoxy group in an SN2′ fashion relative to the rhodium metal. The product is subsequently liberated and the rhodium monomer is regenerated, which will either reform the dimer (if another rhodium monomer is encountered) or continue the catalytic cycle.
Experimental evidence points to the active catalyst being a rhodium monomer. First, the monomeric complex [Rh(CO)Cl(PPF-PtBu2)] is an active catalyst for these reactions giving similar yields and enantioselectivity to the use of [Rh(COD)Cl]2/2 PPF-PtBu2. In addition, the NLE observed with a catalyst generated from [Rh(COD)Cl]2/2 PPF-PtBu2 can be rationalized on the basis of the formation of diastereomeric hetero- and homochiral dimeric complexes (59) that undergo bridge cleavage at different rates.
Although the NLE results obtained with [Rh(COD)Cl]2, on their own, could be interpreted as possible evidence for the presence of diastereomeric complexes along the reaction pathway (60), the linear relationship between auxiliary and product ee with [Rh(CO)2Cl]2 points to another explanation. Reaction of [Rh(CO)2Cl]2 with a variety of bidentate ligands results in the extrusion of one CO ligand per rhodium atom. The remaining carbonyl ligand fills the square planar four coordinate geometry of the d8 rhodium(I) complex producing the monomeric [Rh(CO)Cl(PPF-PtBu2)]. Importantly, the enantiomeric excess of the monomeric rhodium complex corresponds directly to the ee of the chiral ligand. The experimentally observed linear relationship between ligand and product ee supports the notion that the catalyst is monomeric in structure and that diastereomeric complexes are not implicated.
Exo coordination of the rhodium catalyst to the oxabicyclic substrate is a reasonable assumption. On the basis of steric arguments, coordination should be favored at the more accessible exo face. Indeed, exo coordination has been proposed in both nickel-catalyzed reductive ARO and palladium-catalyzed alkylative ARO reactions of the same substrates (1). In our subsequent work with rhodium-catalyzed arylative and alkenylative ARO reactions (58), for which there is a larger body of work on which to base the mechanism (61), exo coordination of the aryl rhodium(I) complex to the olefin is again invoked to explain the stereochemical outcome.
A key step in the working model is oxidative insertion of the rhodium complex into the bridgehead C–O bond with retention of configuration; a pathway that distinguishes it from the majority of known/proposed mechanisms for other allylic functionalizations, including previous reports with rhodium catalysts. Of the metals that are known to catalyze allylic substitutions, only molybdenum has been shown to oxidatively add with retention of configuration (62, 63). Precoordination of the Lewis basic leaving group to the Lewis acidic molybdenum metal before alkene binding is believed to be responsible for this outcome (64, 65). The putative predisposition of the oxabicyclic core to bind to the rhodium metal on its exo face through the alkene and the oxygen atom may predispose the complex to this type of reactivity. Precoordination of the metal to the alkene and the leaving group has been used to rationalize the stereochemical dichotomy between palladium and molybdenum catalyzed reactions (64–70).
The results obtained in the regio-selective ring opening reactions also point to the ionization of the C–O bond as a key step in the catalytic cycle. For example, oxabicycle 19 reacts to give hydronaphthalene 20 exclusively (Eq. 3). If oxidative insertion were occurring, ionization of the tertiary C–O bond should be preferred because the tertiary cation will be more stable. In the regio-selective ring opening of 22, where no steric influence in the ring opening step should be present, the selective formation of 23 can be rationalized by the resonance stabilization of positive charge at the bridgehead by the methoxy substituent.
We propose that a proton transfer occurs between the phenol nucleophile and the rhodium(III) alkoxide intermediate 34 to give a phenoxide and a cationic rhodium(III) complex 35. The competition and kinetics experiments with different phenol nucleophiles lend experimental support to this notion. In kinetics experiments, more acidic phenols gave faster reactions than more basic ones; this influence was found to follow a linear Hammett relationship with a positive ρ value of 0.303. A positive ρ value corresponds with an accumulation of negative charge on the phenol in the transition state of the rate-determining step, most likely the result of a deprotonation. Because the reaction is run under neutral conditions, the base is likely be a species associated with the rhodium catalyst. Rhodium alkoxide and hydroxide complexes have been demonstrated to be sufficiently basic to deprotonate acetylacetone (ref. 71 and references therein). More acidic species, such as HCl, can also protonate rhodium alkoxide/hydroxide complexes (72). We propose that it is the rhodium alkoxide 34/35 that is serving as the base in these reactions. This deprotonation would have two effects. First, the creation of a cationic rhodium(III) species 37 makes the π-allyl (or enyl) moiety more electrophilic, and secondly, the deprotonation of the nucleophile renders it more nucleophilic.
The proposal that nucleophilic attack occurs in an SN2′ fashion with inversion is consistent with the well documented mechanism of palladium π-allyl chemistry (73–76) and the proposed mechanism of rhodium-catalyzed reactions of allylic carbonates put forward by Evans (12). The proposal that the alkoxide moiety directs the nucleophilic attack by shifting the position of the metal on the allyl fragment has precedent in the chemistry of palladium where neighboring groups have been found to exert this type of directing influence (77, 78).
Conclusion
A better understanding of the important properties of the PPF-PtBu2 ligand that contribute to the high reactivities and enantioselectivities obtained in the rhodium-catalyzed asymmetric ring opening (ARO) reaction of oxabicyclic alkenes has been achieved. These results lay down guidelines that may be used in the search for more reactive and selective catalysts. To maximize reactivity, both phosphines should be electron-rich. For maximal enantioselectivity, the two phosphines should be of different size with the larger phosphine located at the benzylic position. Finally, the optimal diastereomers contain (R) central and (S) planar chirality [or the enantiomeric (S,R) pair]. Catalyst and substrate studies were also conducted to gain mechanistic insight into the rhodium-catalyzed ARO reactions. Based on these results a mechanistic working model has been proposed that rationalizes all experimental observations.
Supplementary Material
Acknowledgments
We thank Solvias AG for generously providing us with the Josiphos ligands used in these studies. We thank the Natural Sciences and Engineering Research Council, the Ontario Research and Development Challenge Fund, AstraZeneca, and the University of Toronto for financial support of our research. K.F. thanks the Natural Sciences and Engineering Research Council for a doctoral scholarship.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: ee, enantiomeric excess.
References
- 1.Lautens, M., Fagnou, K. & Hiebert, S. (2002) Acc. Chem. Res. 36, 48–58. [DOI] [PubMed] [Google Scholar]
- 2.Chiu, P. & Lautens, M. (1997) Top. Curr. Chem. 190, 1–85. [Google Scholar]
- 3.Lautens, M., Fagnou, K. & Zunic, V. (2002) Org. Lett. 4, 3465–3468. [DOI] [PubMed] [Google Scholar]
- 4.Lautens, M. & Fagnou, K. (2001) J. Am. Chem. Soc. 123, 7170–7171. [DOI] [PubMed] [Google Scholar]
- 5.Lautens, M., Fagnou, K. & Taylor, M. (2000) Org. Lett. 2, 1677–1679. [DOI] [PubMed] [Google Scholar]
- 6.Lautens, M., Fagnou, K. & Rovis, T. (2000) J. Am. Chem. Soc. 122, 5650–5651. [Google Scholar]
- 7.Lautens, M. & Fagnou, K. (2001) Tetrahedron 57, 5067–5072. [Google Scholar]
- 8.Lautens, M., Fagnou, K., Taylor, M. & Rovis, T. (2001) J. Organomet. Chem. 624, 259–270. [Google Scholar]
- 9.Tsuji, J., Minami, I. & Shimizu, I. (1984) Tetrahedron Lett. 25, 5157–5160. [Google Scholar]
- 10.Minami, I., Shimizu, I. & Tsuji, J. (1985) J. Organomet. Chem. 296, 269–280. [Google Scholar]
- 11.Evans, P. A. & Nelson, J. D. (1998) Tetrahedron Lett. 39, 1725–1728. [Google Scholar]
- 12.Evans, P. A. & Nelson, J. D. (1998) J. Am. Chem. Soc. 120, 5581–5582. [Google Scholar]
- 13.Evans, P. A., Robinson, J. E. & Nelson, J. D. (1999) J. Am. Chem. Soc. 121, 6761–6762. [Google Scholar]
- 14.Evans, P. A., Robinson, J. E. & Moffett, K. K. (2001) Org. Lett. 3, 3269–3271. [DOI] [PubMed] [Google Scholar]
- 15.Evans, P. A. & Leahy, D. K. (2000) J. Am. Chem. Soc. 122, 5012–5013. [Google Scholar]
- 16.Evans, P. A. & Kennedy, L. J. (2001) J. Am. Chem. Soc. 123, 1234–1235. [DOI] [PubMed] [Google Scholar]
- 17.Evans, P. A. & Robinson, J. E. (2001) J. Am. Chem. Soc. 123, 4609–4610. [DOI] [PubMed] [Google Scholar]
- 18.Fagnou, K. & Lautens, M. (2000) Org. Lett. 2, 2319–2321. [DOI] [PubMed] [Google Scholar]
- 19.Trost, B. M. & Van Vranken, D. L. (1996) Chem. Rev. 96, 395–422. [DOI] [PubMed] [Google Scholar]
- 20.Johannsen, M. & Jorgensen, K. A. (1998) Chem. Rev. 98, 1689–1708. [DOI] [PubMed] [Google Scholar]
- 21.Tsuji, J. (1995) Palladium Reagents and Catalysis (Wiley, Toronto).
- 22.Trost, B. M. & Lautens, M. (1987) J. Am. Chem. Soc. 109, 1469–1478. [Google Scholar]
- 23.Trost, B. M. & Hachiya, I. (1998) J. Am. Chem. Soc. 120, 1104–1105. [Google Scholar]
- 24.Belda, O., Kaiser, N.-F., Bremberg, U., Larhed, M., Hallberg, A. & Moberg, C. (2000) J. Org. Chem. 65, 5868–5870. [DOI] [PubMed] [Google Scholar]
- 25.Malkov, A. V., Spoor, P., Vinader, V. & Koèovský, P. (2001) Tetrahedron Lett. 42, 509–512. [Google Scholar]
- 26.Trost, B. M., Hildebrand, S. & Dogra, K. (1999) J. Am. Chem. Soc. 121, 10416–10417. [Google Scholar]
- 27.Nomura, N. & RajanBabu, T. V. (1997) Tetrahedron Lett. 38, 1713–1716. [Google Scholar]
- 28.Consiglio, G., Piccolo, O., Roncetti, L. & Morandi, F. (1986) Tetrahedron 42, 2043–2053. [Google Scholar]
- 29.Furukawa, J., Kiji, J., Yamamoto, K. & Tojo, T. (1973) Tetrahedron 29, 3149–3151. [Google Scholar]
- 30.Yamamoto, T., Ishizu, J. & Yamamoto, A. (1981) J. Am. Chem. Soc. 103, 6863–6869. [Google Scholar]
- 31.Cuvigny, T. & Julia, M. (1983) J. Organomet. Chem. 250, C21–C24. [Google Scholar]
- 32.Cuvigny, T. & Julia, M. (1986) J. Organomet. Chem. 317, 383–390. [Google Scholar]
- 33.Alvarez, E., Cuvigny, T. & Julia, M. (1988) J. Organomet. Chem. 339, 199–212. [Google Scholar]
- 34.Blacker, A. J., Clark, M. L., Loft, M. S., Mahon, M. F., Humphries, M. E. & Williams, J. M. J. (2000) Chem. Eur. J. 6, 353–368. [DOI] [PubMed] [Google Scholar]
- 35.Blacker, A. J., Clark, M. L., Loft, M. S. & Williams, J. M. J. (1999) Chem. Commun. 913–914.
- 36.Bhatia, B., Reddy, M. M. & Iqbal, J. (1993) Tetrahedron Lett. 34, 6301–6304. [Google Scholar]
- 37.Takeuchi, R. & Kashio, M. (1997) Angew. Chem. Int. Ed. Engl. 36, 263–265. [Google Scholar]
- 38.Takeuchi, R. & Kashio, M. (1998) J. Am. Chem. Soc. 120, 8647–8655. [Google Scholar]
- 39.Takeuchi, R. & Shiga, N. (1999) Org. Lett. 1, 265–268. [Google Scholar]
- 40.Takeuchi, R., Ue, N., Tanabe, K., Yamashita, K. & Shiga, N. (2001) J. Am. Chem. Soc. 123, 9525–9534. [DOI] [PubMed] [Google Scholar]
- 41.Bartels, B. & Helmchen, G. (1999) Chem. Commun. 741–743.
- 42.Takeuchi, R. & Tanabe, K. (2000) Angew. Chem. Int. Ed. Engl. 39, 1975–1978. [DOI] [PubMed] [Google Scholar]
- 43.Janssen, J. P. & Helmchen, G. (1997) Tetrahedron Lett. 38, 8025–8026. [Google Scholar]
- 44.Xu, Y. & Zhou, B. (1987) J. Org. Chem. 52, 974–977. [Google Scholar]
- 45.Kondo, T., Ono, H., Satake, N., Mitsudo, T-a. & Watanabe, Y. (1995) Organometallics 14, 1945–1953. [Google Scholar]
- 46.Morisaki, Y., Kondo, T. & Mitsudo, T.-a. (1999) Organometallics 18, 4742–4746. [Google Scholar]
- 47.Konda, T., Morisaki, Y., Uenoyama, S.-y., Wada, K. & Mitsuda, T.-a. (1999) J. Am. Chem. Soc. 121, 8657–8658. [Google Scholar]
- 48.Trost, B. M., Fraisse, P. & Ball, Z. T. (2002) Angew. Chem. Int. Ed. Engl. 41, 1059–1062. [DOI] [PubMed] [Google Scholar]
- 49.Matsushuma, Y., Onitsuka, K., Kondo, T., Mitsudo, T.-a. & Takahashi, S. (2001) J. Am. Chem. Soc. 123, 10405–10406. [DOI] [PubMed] [Google Scholar]
- 50.Lloyd-Jones, G. C. & Pfaltz, A. (1995) Angew. Chem. Int. Ed. Engl. 34, 462–464. [Google Scholar]
- 51.Pastor, S. D. & Togni, A. (1989) J. Am. Chem. Soc. 111, 2333–2334. [Google Scholar]
- 52.Pastor, S. D. & Togni, A. (1990) J. Org. Chem. 55, 1649–1664. [Google Scholar]
- 53.Schnyder, A., Togni, A. & Wiesli, U. (1997) Organomet. 16, 255–260. [Google Scholar]
- 54.Girard, C. & Kagan, H. B. (1998) Angew. Chem. Int. Ed. Engl. 37, 2922–2959. [DOI] [PubMed] [Google Scholar]
- 55.Avalos, M., Babiano, R., Cintas, P., Jimenez, J. L. & Palacios, J. A. (1997) Tetrahedron Asymmetry 8, 2997–3017. [Google Scholar]
- 56.Bolm, C. (1996) in Advanced Asymmetric Synthesis, ed. Stephenson, G. R. (Blackie, Glasgow, Scotland), pp. 9–26.
- 57.Blackmond, D. C. (2000) Acc. Chem. Res. 33, 402–411. [DOI] [PubMed] [Google Scholar]
- 58.Lautens, M., Dockendorff, C., Fagnou, K. & Malicki, A. (2002) Org. Lett. 4, 1311–1314. [DOI] [PubMed] [Google Scholar]
- 59.Dorta, R., Egli, P., Zarcher, F. & Togni, A. (1997) J. Am. Chem. Soc. 119, 10857–10858. [Google Scholar]
- 60.Hansen, K. B., Leighton, J. L. & Jacobsen, E. N. (1996) J. Am. Chem. Soc. 118, 10924–10925. [Google Scholar]
- 61.Fagnou, K. & Lautens, M. (2003) Chem. Rev. 103, 169–196. [DOI] [PubMed] [Google Scholar]
- 62.Faller, J. W. & Linebarrier, D. (1988) Organometallics 7, 1670–1672. [Google Scholar]
- 63.Rubio, A., Liebeskind, L. S. & Bombrun, A. (1993) J. Am. Chem. Soc. 115, 891–901. [Google Scholar]
- 64.Dvorak, D., Stary, L. & Koèovský, P. J. (1995) J. Am. Chem. Soc. 117, 6130–6131. [Google Scholar]
- 65.Ward, Y. D., Villaneuva, L. A., Allred, G. D. & Liebeskind, L. S. (1996) J. Am. Chem. Soc. 118, 897–898. [Google Scholar]
- 66.Starý, I. & Koèovský, P. (1989) J. Am. Chem. Soc. 111, 4981–4982. [Google Scholar]
- 67.Starý, I., Zajìèek, J. & Koèovský, P. (1992) Tetrahedron 48, 7229–7240. [Google Scholar]
- 68.Kurosawa, H., Ogoshi, S., Kawasaki, Y., Murai, S., Miyoshi, M. & Ikead, I. (1990) J. Am. Chem. Soc. 112, 2813–2814. [Google Scholar]
- 69.Kurosawa, H., Kajimaru, H., Ogoshi, S., Yoneda, H., Miki, K., Murai, S. & Ikead, I. (1992) J. Am. Chem. Soc. 114, 8417–8424. [Google Scholar]
- 70.Farthing, C. N. & Koèovský, P. (1998) J. Am. Chem. Soc. 120, 6661–6672. [Google Scholar]
- 71.Hayashi, T., Takahashi, M., Takaya, Y. & Ogasawara, M. (2002) J. Am. Chem. Soc. 124, 5052–5058. [DOI] [PubMed] [Google Scholar]
- 72.Sakuma, S. & Miyaura, N. (2001) J. Org. Chem. 66, 8944–8946. [DOI] [PubMed] [Google Scholar]
- 73.Albinati, A., Pregosin, P. S. & Wick, K. (1996) Organometallics 15, 2419–2421. [Google Scholar]
- 74.Togni, A., Burckhardt, U., Gramlich, V., Pregosin, P. S. & Salzmann, R. (1996) J. Am. Chem. Soc. 118, 1031–1037. [Google Scholar]
- 75.Sprinz, J., Kiefer, M., Helmchen, G., Reggelin, M., Huttner, G., Walter, O. & Zsolnai, L. (1994) Tetrahedron Lett. 35, 1523–1526. [Google Scholar]
- 76.Evans, D. A., Campos, K. R., Tedrow, J. S., Michael, F. E. & Gagne, M. R. (2000) J. Am. Chem. Soc. 122, 7905–7920. [Google Scholar]
- 77.Krafft, M. E., Wilson, A. M., Fu, Z., Preoctor, M. J. & Dasse, O. A. (1998) J. Org. Chem. 63, 1748–1749. [Google Scholar]
- 78.Krafft, M. E., Sugiura, M. & Abboud, K. A. (2001) J. Am. Chem. Soc. 123, 9174–9175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.