Abstract
The development of new catalytic asymmetric reactions can lead to exciting new strategies for organic synthesis. This article describes the synthetic utility of the combined C—H activation/Cope rearrangement, achieved by dirhodium tetraprolinate-catalyzed reaction of vinyldiazoacetates with compounds containing allylic C—H bonds. The transformation is highly diastereoselective and enantioselective. The product distribution, however, is highly substrate dependent, the major side products being either direct C—H activation or cyclopropanation.
The field of chiral catalysis has experienced explosive growth over the last two decades (1, 2). By now, many of the classic reactions of organic synthesis can be achieved in a highly asymmetric manner. One of the new frontiers for asymmetric catalysis is the discovery of new catalytic asymmetric methods that do not have an established achiral counterpart (3). For some time we have been exploring the chemistry of donor/acceptor-substituted rhodium carbenoids (2) as a possible source of these new catalytic asymmetric transformations (Eq. 1) (4). Because of the presence of the donor group (typically vinyl or aryl), 2 are much more stabilized than the conventional carbenoids, which contain only electron acceptor groups (typically ester, keto, phosphonate, sulfonate, cyano or nitro) (5, 6). Consequently, 2 undergo a range of transformations
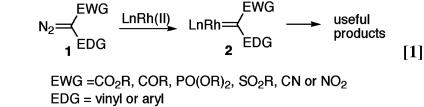 |
with unprecedented regioselectivity and stereoselectivity (4).
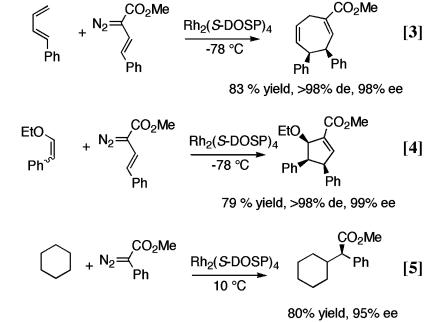
Our studies into the chemistry of donor/acceptor-substituted carbenoids have already led to a number of exciting methods for catalytic asymmetric synthesis. The dirhodium tetraprolinate complex Rh2(S-DOSP)4 [S-DOSP is S-(N-dodecylbenzenesulfonyl)prolinate; see Eq. 2] is an exceptional chiral catalyst for these carbenoids, resulting in generally high levels of asymmetric induction (7). These methods include cyclopropanation of alkenes (Eq. 2) (8), [3 + 4] cycloaddition between vinylcarbenoids and dienes (Eq. 3) (9), [3 + 2] cycloaddition between vinylcarbenoids and vinyl ethers (Eq. 4) (10), and intermolecular C—H activation (Eq. 5)
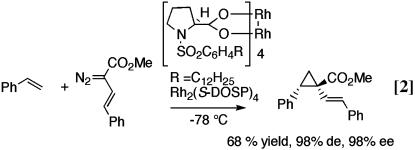 |
(11). A common feature of all of these transformations is the opportunity for the stereoselective generation of multiple stereocenters in a single step.
Figure 1.
In these equations, de indicates diastereomeric excess and ee indicates enantiomeric excess.
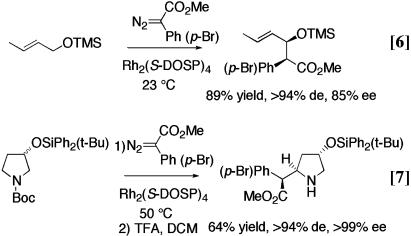
Intermolecular C—H activation is a very exciting reaction because it represents an additional strategic reaction for organic synthesis. Complementary carbenoid versions of many of the classic synthetic reactions of organic chemistry have been achieved. β-Hydroxy esters, usually derived from an aldol reaction, are stereoselectively formed by C—H activation α to oxygen (Eq. 6) (12).
 |
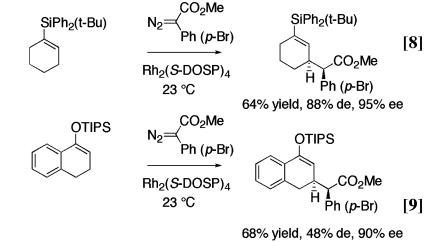 |
Similarly, β-amino esters, classically prepared by a Mannich reaction, are obtained by C—H activation α to nitrogen (Eq. 7) (13). Allylic C—H activation can lead to γ,δ-unsaturated esters, the typical products of a Claisen rearrangement (Eq. 8) (14), or O-silylated 1,5-dicarbonyl compounds, the typical products of a Michael addition (Eq. 9) (15). As can be seen in the illustrative examples of Eqs. 6–9, the C—H activation can be highly stereoselective and a range of functionality is compatible with this chemistry.
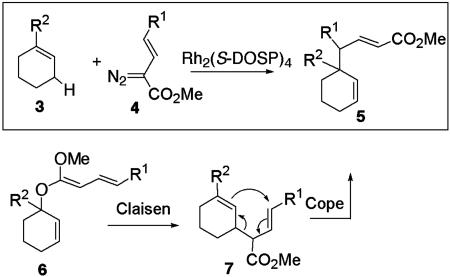
The chemistry of vinyldiazoacetates has been rich and varied because, in addition to the highly stereoselective reactions, many unprecedented carbenoid transformations have been discovered involving the reaction at the vinylogous position to the carbenoid (10, 16–18). In this article, we describe a further example of the vinylogous reactivity in the Rh2(S-DOSP)4-catalyzed reaction between vinyldiazoacetates 4 and cycloalkenes 3, a combined C—H activation/Cope rearrangement (Scheme 1). This leads to the highly stereoselective formation of products 5 containing two new stereocenters, including a quaternary carbon atom at the ring junction (Scheme 1) (19). Conceptually, the reaction can be considered as complementary to a tandem Claisen rearrangement/Cope rearrangement (6 to 7 to 5) (20).
Scheme 1.
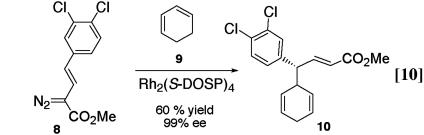
We have previously communicated examples of the reaction of vinylcarbenoids with allylic C—H bonds. An impressive example is the reaction of the vinyldiazoacetate 8 with 1,3-cyclohexadiene (9) to form 10 in 99% ee (Eq. 10) (21). This reaction was used as the key step in a formal asymmetric synthesis of the antidepressant (+)-sertraline (21). Because of the unusual bond migration that occurred, the reaction was considered to be a combined C—H activation/Cope rearrangement (21). In this article, we describe the extension of this reaction to more elaborate substrates and show that two stereogenic centers can be formed in a highly diastereoselective fashion.
 |
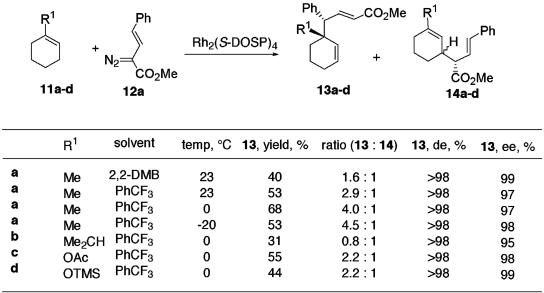
To explore the possibility of controlling the diastereoselectivity of the reaction of vinylcarbenoids with allylic C—H bonds, the reaction of the phenylvinyldiazoacetate 12a with methylcyclohexene (11a) was examined (Scheme 2). The Rh2(S-DOSP)4-catalyzed reaction between 12a and 11a in 2,2-dimethylbutane (2,2-DMB) as solvent resulted in the formation of two types of products. The major product was the unsaturated ester 13a, which is analogous to the product formed in the reaction of 1,3-cyclohexadiene shown in Eq. 10 (21). Remarkably, 13a is formed in >98% de and 99% ee. This transformation is potentially a very powerful method for the construction of functionalized cycloalkenes containing a quaternary carbon center. The minor product 14a was the direct C—H activation product, which was formed as a mixture of two diastereomers. The enantioselectivity in the formation of the major diastereomer of 14a was also found to be very high (97% ee). The formation of only 13a and 14a demonstrates the regioselectivity of the vinylcarbenoid chemistry because methylcyclohexene (11a) has three allylic sites but reaction at only one of the sites is involved in the formation of 13a and 14a. The ratio of 13a/14a can be modified in favor of the combined C—H activation/Cope rearrangement product 13a by using trifluorotoluene as solvent instead of 2,2-DMB and by lowering the reaction temperature from 23°C to –20°C. The reaction can be extended to a range of 1-substituted cyclohexenes 11b–d. In each case a mixture of the combined C—H activation/Cope rearrangement product 13, and the direct C—H activation product 14 is formed. The ratio of the two products depends on the structure of the substrate, but in all cases, 13 is formed as a single diastereomer in >95% ee.
Scheme 2.
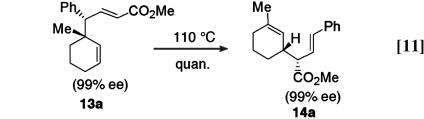
The most obvious mechanism for the formation of 13 would be by a Cope rearrangement of the direct C—H activation product 14. This mechanistic interpretation, however, was ruled out in our earlier studies with cyclohexadiene (21) and can also be ruled out here because there is no clear thermodynamic driving force for the conversion of 14 to 13. Under the reaction conditions both 13a and 14a are stable and indeed, on heating at 110°C in toluene it is clear that the C—H activation product is thermodynamically the most stable because 13a undergoes a quantitative rearrangement to 14a (Eq. 11). In this case the product is produced in 99% ee and as a single diastereomer. The (E) alkene configuration in 14a is indicative of a Cope rearrangement occurring through a chair transition state (22), and the drawn relative stereochemistry in 14a is the predicted stereochemistry that would be formed from such a chair transition state (22). The rearrangement of 13a to 14a is convincing proof that the formation of 13a is not caused by a tandem C—H activation/Cope rearrangement but some form of combined reaction. Even though these results indicate that it would be possible to prepare 14a in a one-pot process by heating the crude reaction mixture of 13a and 14a, the isolation of 13a followed by its thermal rearrangement is a better process because under these conditions 14a is formed as a single diastereomer.
 |
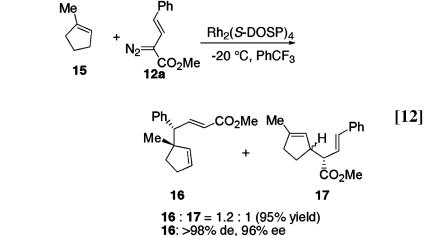
1-Methylcyclopentene 15 is also a suitable substrate for this chemistry (Eq. 12). Rh2(S-DOSP)4-catalyzed reaction of 15 with the vinyldiazoacetate 12a generates a 1.2:1 mixture of 16 and 17, in a combined yield of 95%. The combined C—H activation/Cope rearrangement product 16 is formed in >98% de, 96% ee, and 51% isolated yield.
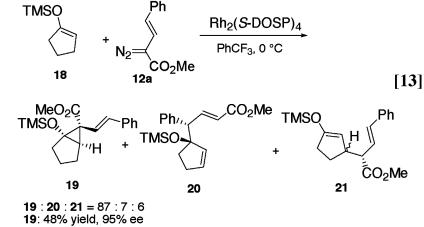
Even though the combined C—H activation/Cope rearrangement product is formed in a highly stereoselective manner, controlling potential side reactions in this chemistry is still quite challenging. The competing direct C—H activation is usually a fairly significant side reaction. In general, the donor/acceptor-substituted carbenoids do not cyclopropanate trisubstituted alkenes but there are some exceptions (14). For example, extension of the chemistry to siloxycyclopentene 18 as substrate was not successful because in this case, cyclopropane 19 was the dominant product rather than allylic C—H functionalization products 20 and 21 (Eq. 13) The relative configuration of the cyclopropane 19 was determined by nuclear Overhauser effect analysis, and the absolute configuration was assigned on the basis of the predictive model developed for these cyclopropanations (8).
 |
 |
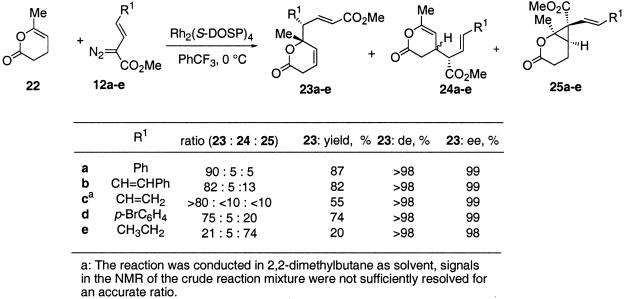
An exceptional substrate for the combined C—H activation/Cope rearrangement is the dihydropyranone 22. Rh2(S-DOSP)4-catalyzed reaction of various vinyldiazoacetates resulted in high yielding reactions with a strong preference for the formation of the C—H activation/Cope rearrangement product 23 over the direct C—H activation product 24 (Scheme 3). In each case the product was formed in >98% de and ≥98% ee. The relative and absolute stereochemistry of 23d was determined from its x-ray crystallographic data. The stereochemistry of all of the other C—H activation/Cope rearrangement products are tentatively assigned by analogy to the stereochemistry for formation of 23d. The subtleties of this chemistry are clearly seen in the reaction of the alkylvinyldiazoacetate 12e with 22. In this case, the combined C—H activation/Cope rearrangement product 23e is a minor product while cyclopropanation to form 25e becomes the dominant reaction pathway. The relative configuration of the cyclopropane 25e was determined by nuclear Overhauser effect analysis, and the absolute configuration was assigned on the basis of the predictive model developed for these cyclopropanations (8).
Scheme 3.
The combined C—H activation/Cope rearrangement is an exceptional reaction, displaying spectacular levels of asymmetric induction. The mechanism is clearly not a C—H activation followed by a Cope rearrangement. Both the C—H activation and the Cope rearrangement need to occur during the same concerted process. This would require a very definite trajectory of approach of the trapping agent to the vinylcarbenoid and may explain why the reaction is so enantioselective. Some possibilities on how the combined C—H activation/Cope rearrangement products are formed would be by an intercepted C—H activation process or by means of an ene reaction where the vinylcarbenoid reacts as a 2π system (21). At this stage, however, the mechanistic understanding of this reaction is not well developed, and further studies are needed.
In summary, these studies describe a very unusual transformation between vinylcarbenoids and compounds containing allylic C—H bonds. The reactions are incredibly stereoselective and lead to useful chirons for application in synthesis. The major challenge is to develop further the factors that control the selective formation of the C—H activation/Cope rearrangement products because currently, the product distribution is very sensitive to the nature of the vinylcarbenoid and the trapping substrate.
Supplementary Material
Acknowledgments
We thank Dr. Pingda Ren for some preliminary studies and Andrey Yu. Kovalevsky for the x-ray crystallographic structural determination. Financial support of this work by the National Science Foundation (CHE-0350536) is gratefully acknowledged.
Appendix: Experimental Material
All of the solvents are degassed with argon before use. The carbenoid reactions were run at 0.5-mmol scale unless otherwise noted. A typical procedure is the following: To a solution of 1-methyl-1-cyclohexene (11a) (0.5 mmol) and Rh2(S-DOSP)4 (19 mg, 0.01 mmol) in trifluorotoluene (2 ml) was added a solution of methyl (E)-phenylvinyldiazoacetate (12a) (203 mg, 1.0 mmol) in trifluorotoluene (5 ml) at 0°C over a 45-min period via syringe pump. The solvent was then evaporated, and the residue was purified by flash chromatography on silica gel (20:1 pentane/ether eluent) to provide 13a (92 mg, 68% yield) and 14a (23 mg, 17% yield).
13a. Rf 0.56 (5:1 pentane/ether); [α]D25 –6.4° (c 4.00, CHCl3); Fourier transform IR (film) 3,023, 2,933, 2,868, 1,724, 1,649, 1,452, 1,434, 1,269, 1,247, 1,189, 1,166 cm–1; 1H NMR (500 MHz, CDCl3) δ 7.38 (dd, J = 15.6, 9.2 Hz, 1H), 7.30–7.25 (m, 2H), 7.24–7.22 (m, 1H), 7.21–7.17 (m, 2H), 5.78 (dd, J = 15.6, 0.9 Hz, 1H), 5.69 (dt, J = 10.1, 3.9 Hz, 1H), 5.36 (d, J = 10.1 Hz, 1H), 3.70 (s, 3H), 3.31 (d, J = 9.2 Hz, 1H), 1.98–1.85 (m, 2H), 1.64–1.52 (m, 3H), 1.42–1.36 (m, 1H), 0.98 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 166.9, 149.2, 139.8, 134.4, 129.6, 128.0, 127.2, 126.6, 122.4, 59.0, 51.4, 38.5, 33.1, 25.5, 24.9, 18.7; LC-MS (electrospray ionization) m/z (relative intensity): 249 (100), 271 (M++H, 58), 293 (M++Na, 23); HPLC analysis: 97% ee (Chiralcel OD-H, 5% i-PrOH in hexane, 0.8 ml/min, λ = 254 nm, tR = 6.2 min, major; tR = 11.7 min, minor); Analysis. Calculated for C17H22O2: C, 79.96; H, 8.20. Found: C, 79.89; H, 8.37.
14a. (mixture of two diastereomers, ratio 4:1) as a colorless oil: Rf 0.65 (5:1 pentane/ether); Fourier transform IR (film) 2,928, 2,858, 1,734, 1,448, 1,434, 1,157 cm–1; minor: 1H NMR (500 MHz, CDCl3) δ 7.39 (d, J = 7.5 Hz, 2H), 7.32 (t, J = 7.5 Hz, 2H), 7.24 (t, J = 7.5 Hz, 1H), 6.46 (d, J = 15.8 Hz, 1H), 6.21 (dd, J = 15.8, 9.8 Hz, 1H), 5.34 (s, 1H), 3.70 (s, 3H), 2.97 (t, J = 9.8 Hz, 1H), 2.56 (br m, 1H), 1.96–1.83 (m, 2H), 1.80–1.67 (m, 2H), 1.64 (s, 3H), 1.56–1.47 (m, 1H), 1.27–1.20 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 174.0, 136.8, 136.2, 133.4, 128.5, 127.5, 126.5, 126.4, 121.7, 55.6, 51.7, 38.3, 29.9, 27.2, 23.9, 21.7; major: 1H NMR (500 MHz, CDCl3) δ 7.37 (d, J = 7.5 Hz, 2H), 7.30 (t, J = 7.5 Hz, 2H), 7.22 (t, J = 7.5 Hz, 1H), 6.46 (d, J = 15.8 Hz, 1H), 6.17 (dd, J = 15.8, 9.8 Hz, 1H), 5.20 (s, 1H), 3.72 (s, 3H), 2.97 (t, J = 9.8 Hz, 1H), 2.56 (br m, 1H), 1.96–1.83 (m, 2H), 1.75–1.67 (m, 2H), 1.65 (s, 3H), 1.56–1.47 (m, 1H), 1.27–1.20 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 174.0, 136.7, 136.4, 133.0, 128.5, 127.5, 126.7, 126.3, 122.4, 55.8, 51.7, 38.0, 30.0, 26.2, 24.0, 21.1; GC-MS (electron impact) m/z (relative intensity): minor, 95 (100), 115 (50), 176 (55), 211 (8); major, 95 (100), 115 (40), 176 (55), 211 (5), 270 (M+, 1); HPLC analysis: major, 95% ee (R,R-Whelk-01 OD-H, 1% i-PrOH in hexane, 0.8 ml/min, λ = 254 nm, tR = 13.1 min, major; tR = 14.9 min, minor); Analysis. Calculated for C17H22O2 (mixture of diastereomers): C, 79.96; H, 8.20. Found: C, 80.01; H, 8.37.
Other Compounds and X-Ray Structure for 23d. Synthesis and characterization of other compounds and x-ray structure data for 23d are published as supporting information on the PNAS web site.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: S-DOSP, S-(N-dodecylbenzenesulfonyl)prolinate; de, diastereomeric excess; ee, enantiomeric excess.
References
- 1.Jacobsen, E. N., Pfaltz, A. & Yamamoto, H., eds. (1999) Comprehensive Asymmetric Catalysis (Springer, Berlin), Vols. I–III.
- 2.Ojima, I. (2000) Catalytic Asymmetric Synthesis (Wiley, New York), 2nd Ed.
- 3.Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. eds. (2003) Comprehensive Asymmetric Catalysis (Springer, Berlin), Suppl. 1.
- 4.Davies, H. M. L. (1998) Curr. Org. Chem. 2, 463–488. [Google Scholar]
- 5.Davies, H. M. L. & Antoulinakis, E. G. (2001) Org. Reactions 57, 1–326. [Google Scholar]
- 6.Doyle, M. P., McKervey, M. A. & Ye, T. (1998) Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides (Wiley, New York).
- 7.Davies, H. M. L. (1999) Eur. J. Org. Chem. 2459–2469.
- 8.Davies, H. M. L., Bruzinski, P., Hutcheson, D. K., Kong, N. & Fall, M. J. (1996) J. Am. Chem. Soc. 118, 6897–6907. [Google Scholar]
- 9.Davies, H. M. L. (1999) Adv. Cycloaddition 5, 119–164. [Google Scholar]
- 10.Davies, H. M. L., Xiang, B., Kong, N. & Stafford, D. G. (2001) J. Am. Chem. Soc. 123, 7461–7462. [DOI] [PubMed] [Google Scholar]
- 11.Davies, H. M. L., Hansen, T. & Churchill, M. R. (2000) J. Am. Chem. Soc. 122, 3063–3070. [Google Scholar]
- 12.Davies, H. M. L., Beckwith, R. E. J., Antoulinakis, E. G. & Jin, Q. (2003) J. Org. Chem. 68, 6126–6132. [DOI] [PubMed] [Google Scholar]
- 13.Davies, H. M. L., Venkataramani, C., Hansen, T. & Hopper, D. W. (2003) J. Am. Chem. Soc. 125, 6462–6468. [DOI] [PubMed] [Google Scholar]
- 14.Davies, H. M. L., Ren, P. & Jin, Q. (2001) Org. Lett. 3, 3587–3590. [DOI] [PubMed] [Google Scholar]
- 15.Davies, H. M. L. & Ren, P. (2001) J. Am. Chem. Soc. 123, 2070–2071. [DOI] [PubMed] [Google Scholar]
- 16.Davies, H. M. L., Saikali, E., Clark, T. J. & Chee, E. H. (1990) Tetrahedron Lett. 31, 6299–6302. [Google Scholar]
- 17.Davies, H. M. L. & Hu, B. (1992) Tetrahedron Lett. 33, 453–456. [Google Scholar]
- 18.Davies, H. M. L., Hu, B., Saikali, E. & Bruzinski, P. R. (1994) J. Org. Chem. 59, 4535–4541. [Google Scholar]
- 19.Corey, E. J. & Perez, A. G. (1998) Angew. Chem. Int. Ed. Engl. 37, 388–401. [DOI] [PubMed] [Google Scholar]
- 20.Frauenrath, H. (1996) in Houben-Weyl E 21: Stereoselective Synthesis, eds. Helmchen, G., Hoffmann, R. W., Mulzer, J. & Schaumann, E. (Thieme, Stuttgart), Vol. 6, pp. 3711–3719. [Google Scholar]
- 21.Davies, H. M. L., Stafford, D. G. & Hansen, T. (1999) Org. Lett. 1, 233–236. [DOI] [PubMed] [Google Scholar]
- 22.Evans, D. A. & Nelson, J. V. (1980) J. Am. Chem. Soc. 102, 774–782. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.