Abstract
Background:
Retreatment with ipilimumab has been shown to re-establish disease control in some patients with disease progression. Here, we report the efficacy and safety of retreatment with ipilimumab 3 mg kg−1 among patients participating in an expanded access programme in Italy.
Methods:
Patients who achieved disease control during induction therapy were retreated with ipilimumab upon progression (3 mg kg−1 every 3 weeks for up to four doses), providing they had not experienced toxicity that precluded further dosing. Tumour assessments were conducted after retreatment, and patients were monitored throughout for adverse events.
Results:
Of 855 patients treated with ipilimumab, 51 were retreated upon disease progression. Of these, 28 (55%) regained disease control upon retreatment and 42% were alive 2 years after the first induction dose of ipilimumab; median overall survival was 21 months. Eleven patients (22%) had a treatment-related adverse event of any grade during retreatment. These were generally mild-to-moderate and resolved within a median of 4 days. No new types of toxicity were reported.
Conclusions:
For patients who meet predefined criteria, retreatment with ipilimumab is generally well tolerated and can translate into clinical benefit. This strategy should be compared with other therapeutic options in randomised controlled trials.
Keywords: melanoma, ipilimumab, expanded access, retreatment, treatment outcome, safety, disease control
The annual mortality rate for patients with metastatic melanoma is increasing faster than for most other types of cancer (Lens and Dawes, 2004; Ferlay et al, 2010). Until recently, patients with unresectable stage III or stage IV (advanced) melanoma had limited treatment options and a poor prognosis, with less than one-third of patients surviving up to 1 year (Tarhini and Agostara, 2006; Korn et al, 2008; Agarwala, 2009). The monoclonal antibody ipilimumab was the first agent to provide a significant survival benefit compared with control in randomised phase III trials of patients with metastatic melanoma (Hodi et al, 2010; Robert et al, 2011), and was subsequently approved for use in Europe and the United States in 2011. Ipilimumab has consistently demonstrated a long-term survival benefit with durable antitumour responses and disease stabilisation in phase II and III clinical trials of patients with advanced melanoma, with some patients still alive for >5 years after starting treatment (Weber et al, 2009; Hodi et al, 2010; O'Day et al, 2010; Wolchok et al, 2010; Hamid et al, 2011; Robert et al, 2011; Prieto et al, 2012; Lebbé et al, 2013; Maio et al, 2013). Associated adverse events (AEs) are generally inflammatory in nature, are mostly mild or moderate in severity and can generally be managed by implementing established treatment algorithms (Chin et al, 2008; Weber et al, 2009; Hodi et al, 2010; Hoos et al, 2010; O'Day et al, 2010; Wolchok et al, 2010; Hamid et al, 2011; Robert et al, 2011; Weber et al, 2012; YERVOY Summary of Product Characteristics, 2013).
Unlike chemotherapies, which kill tumour cells by direct cytotoxicity, ipilimumab augments T-cell proliferation and infiltration into tumours by blocking cytotoxic T-lymphocyte-associated antigen-4, a negative regulator of T-cell activation (Korman et al, 2006; Peggs et al, 2006; Schneider et al, 2006; Fong and Small, 2008; Robert and Ghiringhelli, 2009). Although responses to ipilimumab can be long lasting, even in the absence of continued treatment (Prieto et al, 2012), patients whose tumours are not completely eliminated may require further activation of the immune system in order to prevent tumour growth. In addition, persistent activation of the immune system, for example, through the continued administration of immunotherapy, may change the tumour phenotype, potentially reducing the effectiveness of treatment over time (Reiman et al, 2007). Several strategies to potentiate inefficient immune responses or overcome tolerance have been considered, including the use of agents that target multiple tumour antigens, or treatment with a variety of different drugs to reduce selective pressure for tumour resistance. An alternative approach could be to reinitiate immunotherapy to reactivate the primed immune system to recognise and respond to any remaining tumour cells (Robert et al, 2013).
Available data suggest that retreatment with ipilimumab upon disease progression may be a valid approach to overcome immune tolerance among eligible patients (Table 1). On the basis of predefined criteria, patients enrolled in an expanded access programme (EAP), which was initiated to provide ipilimumab to patients who were not eligible to receive the drug within clinical trials, were eligible for retreatment with ipilimumab 3 mg kg−1. The EAP provided a valuable opportunity to evaluate the safety and efficacy of retreatment with ipilimumab in patients with advanced melanoma outside of a clinical trial setting (Pigozzo et al, 2012).
Table 1. Retreatment with ipilimumab in previous clinical trials.
|
MDX010-20 (phase III) |
CA184-025 (rollover phase II study) |
||||
|---|---|---|---|---|---|
| |
Treatmenta |
Ipilimumab dose in parent study |
|||
| Ipilimumab plus gp100 (n=29) | Ipilimumab (n=9) | 0.3 mg kg−1 (n=24) | 3 mg kg−1 (n=34) | 10 mg kg−1 (n=53) | |
| Retreatment dose of ipilimumab, mg kg−1 |
3 |
10 |
|||
| Median number of doses, n |
4 |
4 |
2 |
4 |
4 |
| BORR, n/n (%) |
3/23 (13) |
3/8 (38) |
28/122 (23)b |
||
| DCR, n (%) |
15/23 (65) |
6/8 (75) |
59/122 (48)b |
||
| irAEs, n (%) | 15 (52) | 7 (78) | 18 (75) | 23 (68) | 30 (57) |
Abbreviations: BORR=best overall response rate; DCR=disease control rate; irAE=immune-related adverse event.
Treatment received during the induction phase and retreatment; patients received ipilimumab at 3 mg kg−1.
Includes three patients who were previously treated with other doses or regimens of ipilimumab and eight patients who were retreated with ipilimumab 3 mg kg−1.
Patients and methods
Patients
Patients with life-threatening unresectable stage III or stage IV melanoma were eligible to be included in the EAP if they had failed to respond or were intolerant to at least one systemic therapy and if no alternative treatment option was available. All patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2, and an interval of at least 28 days since completion of treatment with chemotherapy, biochemotherapy, surgery, radiation, or immunotherapy was recommended. Patients who progressed following either stable disease (SD) of ⩾ 3 months duration or an initial objective response (partial (PR) or complete response (CR)) were eligible to receive retreatment with ipilimumab providing they met predefined safety criteria (no unacceptable toxicity requiring discontinuation of ipilimumab during the induction phase excepting reversible autoimmune hepatitis, medically manageable endocrinopathy or reversible dermatological toxicity). The protocol for the EAP was approved by a local independent ethics committee and all participating patients provided signed informed consent before enrolment.
Study design
During the induction phase, ipilimumab 3 mg kg−1 was administered intravenously over 90 min every 3 weeks for four doses. Tumour assessments were conducted at baseline and after completion of induction therapy (Week 12), and classified according to immune-related response criteria (Wolchok et al, 2009). Clinical response was defined as an immune-related complete response (irCR) (disappearance of all index lesions), immune-related partial response (irPR) (⩾50% decrease from baseline in the sum of the product of diameters of defined index lesions), immune-related progressive disease (⩾25% increase from the smallest recorded sum of the product of diameters of defined index lesions), or an immune-related SD (irSD) (criteria not met for CR, PR or progressive disease). Immune-related disease control (irDC) was defined as irSD lasting ⩾3 months, irPR, or irCR, and the irDC rate (irDCR) was the percentage of patients achieving irDC. Each retreatment cycle consisted of four doses of ipilimumab 3 mg kg−1 given in an identical schedule to that used during the induction phase. Tumour assessments were carried out 12 weeks after initiation of retreatment. AEs, including irAEs, were monitored continuously and graded using Common Terminology Criteria for Adverse Events, version 3.0.
Objective
The aim of this analysis was to evaluate the efficacy and safety of retreatment with ipilimumab 3 mg kg−1 in patients with advanced melanoma outside of a clinical trial setting.
Statistical analysis
Patient and disease characteristics were analysed using descriptive statistics. Discrete variables were expressed as relative frequencies (percentages) and continuous variables as median and range. With a total of 51 patients, the largest s.e. in the response rate would be 7% (50% response rate), corresponding to a 95% confidence interval (CI) width of ∼±14%. Overall survival (OS) was estimated using the Kaplan–Meier analysis.
Results
Patient characteristics
Of the 855 patients participating in the Italian EAP, 126 patients had disease progression following a response to ipilimumab induction therapy but did not receive retreatment and 51 patients (6%) were retreated with ipilimumab 3 mg kg−1. Of these retreated patients, 31 patients had irSD lasting ⩾3 months as their best response to induction therapy, and 20 patients had an irPR with induction therapy. Patient characteristics at the beginning of retreatment are provided in Table 2. Among the 51 patients who started a first retreatment cycle, 37 received all four doses and 2 patients began a second retreatment cycle (Table 3). Reasons for not completing all four retreatment doses comprised death (n=6), dose omission (for surgery or other reasons not including toxicity; n=4), disease progression (n=3), and toxicity (grade 3 diarrhoea; n=1). The median time between first induction dose and first retreatment dose was 36 weeks (range: 24–66 weeks).
Table 2. Patient characteristics before retreatment (N=51).
| Characteristic | |
|---|---|
| Median age, years (range) |
61 (19–85) |
|
Gender, n (%) | |
| Male | 24 (47) |
| Female |
27 (53) |
|
ECOG performance status, n (%) | |
| 0 | 36 (71) |
| 1 | 15 (29) |
| Time from diagnosis, months (range) |
50 (4–199) |
|
Number of prior therapies (excluding ipilimumab), n (%) | |
| 1 | 22 (43) |
| 2 | 20 (39) |
| ⩾3 |
9 (18) |
|
Types of previous therapy | |
| Dacarbazine | 26 (51) |
| Fotemustine | 18 (35) |
| Platinum-based chemotherapy | 27 (53) |
| BRAF inhibitor | 1 (2) |
| Temozolomide | 10 (20) |
| Patients with brain metastases, n (%) | 3 (6) |
| Patients with liver metastases, n (%) | 16 (31) |
Abbreviation: ECOG=Eastern Cooperative Oncology Group.
Table 3. Overview of retreatment.
| Ipilimumab 3 mg kg−1 | Patients, n (%) |
|---|---|
| Started first retreatment cycle |
51 |
|
Number of doses received | |
| One dose | 4 (8) |
| Two doses | 2 (4) |
| Three doses | 8 (16) |
| Four doses |
37 (72) |
| Started second retreatment cycle | 2 (4) |
Efficacy
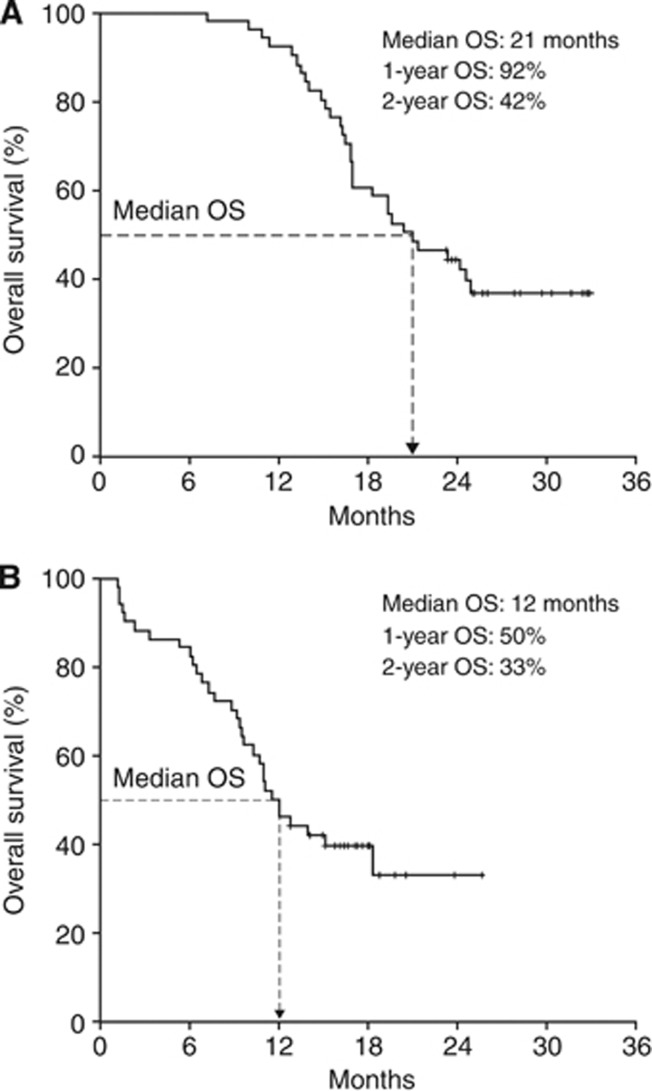
Among the 51 patients who received retreatment with ipilimumab, two patients achieved an irCR and four an irPR with ipilimumab retreatment for an immune-related best overall response rate of 12% (3–20%). This included one patient whose best response to induction therapy had been irSD. An additional 22 patients had irSD in response to retreatment for an irDCR of 55% (41–69%). Best response to induction therapy for the 22 patients with irSD on retreatment had been an irPR in 8 patients and irSD in 14 patients (Table 4). With a median follow-up of 20 months (range: 7–33 months), median OS from the beginning of induction therapy was 21 months (95% CI: 16–26 months; Figure 1A) for patients who were retreated with ipilimumab and 13 months (95% CI: 11–15 months; P<0.0001) for those who were not retreated (median follow-up 13 months; range: 3–29 months). The 1- and 2-year OS rates for patients given retreatment were 92% and 42%, respectively. Median OS from the start of retreatment was 12 months (95% CI: 10–14 months; Figure 1B), and the 1- and 2-year OS rates were 50 and 33%, respectively, for retreated patients.
Table 4. Tumour response among retreated patients (N=51).
|
Patients,
n
(%) |
||||
|---|---|---|---|---|
| |
Response after retreatment according to irRC |
|||
| Response after induction according to irRC | irCR | irPR | irSD | irPD |
| irCR | 0 | 0 | 0 | 0 |
| irPR | 1 | 4 | 8 | 7 |
| irSD | 1 | 0 | 14 | 16 |
Abbreviations: irCR=immune-related complete response; irPD=immune-related progressive disease; irPR=immune-related partial response; irRC=immune-related response criteria; irSD=immune-related stable disease.
Figure 1.
OS from beginning of induction therapy (A) and from start of retreatment (B). Abbreviation: OS=overall survival.
Safety
Of the 51 patients retreated with ipilimumab 3 mg kg−1, drug-related toxicity had been reported in 20 patients (39%) during the induction phase, including 2 patients with a grade 3 AE (diarrhoea and thrombocytopenia) that had not precluded further dosing. Upon retreatment, 14 patients (27%) reported an AE of any grade, which were considered drug-related in 11 patients (22% Table 5). Of these 14 patients, 7 had experienced treatment-related AEs and 3 had experienced AEs that were unrelated to ipilimumab during the induction period. Ten patients (20%) had grade 1 or 2 AEs upon retreatment, which were considered to be treatment related in 8 patients (16%). Grade 3 or 4 AEs were reported upon retreatment in 5 patients (10%) and were considered to be treatment related in 3 patients (6%) (Table 5).
Table 5. Treatment-related AEs experienced upon retreatment (N=51).
| |
Patients,
n
(%) |
|
|---|---|---|
| Treatment-related AE | Any grade | Grade 3/4 |
| Total | 11 (22) | 3 (6) |
| Diarrhoea | 2 (4) | 1 (2) |
| Pruritus | 4 (8) | 0 |
| Liver toxicity | 1 (2) | 0 |
| Fatigue | 2 (4) | 0 |
| Hypothyroidism | 1 (2) | 0 |
| Hypokalaemia | 1 (2) | 1 (2) |
| Bone marrow aplasia | 1 (2) | 1 (2) |
Abbreviation: AE=adverse event.
The most common treatment-related AEs of any grade experienced upon retreatment were pruritus, diarrhoea, and fatigue. For the three patients who had Grade 3 or 4 treatment-related AEs, these included hypokalaemia, which was controlled, enabling the patient to undergo a second retreatment cycle; diarrhoea, which was observed after the third cycle of retreatment causing the patient to discontinue treatment with ipilimumab. The third patient had pancytopenia 4 months after completing the fourth dose of ipilimumab retreatment, and a bone marrow biopsy performed 1 month later showed a hyperplasia of immature myeloid series. The patient's symptoms improved with steroids, erythropoietin and blood transfusions and she was able to receive dacarbazine for disease progression.
Grade 3 AEs considered unrelated to treatment were an acute abdomen after the first cycle of retreatment, which resolved in 10 days, and one case of pain (not specified) that occurred after the first cycle of retreatment; these patients were both able to continue ipilimumab retreatment. Treatment-related AEs were experienced at a similar frequency during retreatment compared with induction, and no new types of toxicity were observed. Treatment-related AEs were generally reversible with treatment as per protocol-specific guidelines, with a median time to resolution of 4 days (range: 1–21).
Discussion
The reported efficacy and safety profile of ipilimumab retreatment in this EAP is consistent with previously observed outcomes (Table 1) (Margolin et al, 2013; Neyns et al, 2013; Robert et al, 2013). For example, among 38 patients retreated with ipilimumab with or without gp100 in a phase III trial, 65–75% re-established DC and 61% survived for >2 years from initial randomisation at study entry (Hodi et al, 2010; Robert et al, 2013). Similarly, of 122 patients who achieved DC in one of several completed phase II trials and subsequently progressed, 48% regained DC after retreatment with ipilimumab (Neyns et al, 2013). In the US EAP, median OS from the first ipilimumab dose was 21.1 months for the 108 patients who were retreated with ipilimumab 3 mg kg−1 upon disease progression compared with 7.6 months for all 2155 patients who were treated in the EAP (Margolin et al, 2013).
Compared with DCRs of 48–75% among retreated patients in clinical trials (Neyns et al, 2013; Robert et al, 2013), approximately half the patients retreated with ipilimumab in this analysis regained DC. Interestingly, the DCR was higher among retreated patients in our analysis than had previously been reported following initial induction therapy with ipilimumab 3 mg kg−1 in clinical trials (Hodi et al, 2010; Wolchok et al, 2010; Hamid et al, 2011; Hersh et al, 2011). However, this may be because the patients included in this analysis were those who had previously benefited from induction therapy with ipilimumab, suggesting that they had tumours or immune systems that were more responsive to ipilimumab (Robert et al, 2013).
The response of some patients to ipilimumab is improved upon retreatment compared with induction, possibly because of the time it can take to mount an immune response against the tumour. For example, in the phase III trial MDX010-20, 3 out of 21 patients whose best response to induction therapy was SD had a PR following retreatment with ipilimumab plus gp100 or ipilimumab alone (Robert et al, 2013). In addition, in this analysis of EAP data, 1 patient with irSD as their best response to induction therapy went on to have an irPR with retreatment.
The precise mechanism by which retreatment with ipilimumab induces renewed or even deeper antitumour activity is unclear. In patients who progress after an initial response to ipilimumab, it is possible that new lesions develop with a novel antigen repertoire that is not recognised by the existing T-cell population, thereby allowing their escape and continued proliferation (Reiman et al, 2007). Retreatment with ipilimumab may result in the expansion of T-cell clones specific for the new antigen repertoire, thus reactivating the antitumour immune response. Alternatively, ipilimumab may amplify immune adaptation through shifting T-cell responses, a mechanism for overcoming immune tolerance that has been observed in a long-term survivor of metastatic melanoma in the absence of immunotherapy (Yamshchikov et al, 2005). In addition, it has been shown that the effectiveness of continuous immunotherapy may be counteracted by large numbers of immunosuppressive cells in the tumour microenvironment (Stewart et al, 2004), or the induction of functionally corrupt memory T cells (Klebanoff et al, 2006). Following completion of ipilimumab induction therapy, the balance between immune effector and regulatory cells, together with the function of the immune state, may be ‘reset' and potentially more conducive to subsequent treatment.
In the current analysis, ipilimumab retreatment was generally well tolerated with most patients receiving the full four doses of retreatment. In line with other reports (Margolin et al, 2013; Neyns et al, 2013; Robert et al, 2013), the frequencies of treatment-related AEs observed during retreatment were similar to those observed during induction, and no new types of toxicities were reported. Within the EAP, retreatment-related AEs resolved quickly and effectively with treatment as per protocol-specific guidelines, suggesting that retreatment with ipilimumab is safe outside of a clinical trial setting. Indeed, established management algorithms appear to be applicable to AEs that develop during ipilimumab retreatment as well as those that emerge during induction therapy.
As we develop a greater understanding of the mechanisms of tumour immunoediting and immune escape, it is important to identify how immune-based approaches can be designed to limit or overcome these processes. In addition to retreatment upon disease progression, in some trials patients with an objective tumour response or SD after the induction period have received an additional dose of ipilimumab every 12 weeks as ‘maintenance therapy' for as long as tolerated, until progressive disease. A notable proportion of patients who received maintenance therapy in a phase III trial had long-term survival of >5 years, indicating that this approach is also feasible and warrants further evaluation (Maio et al, 2013).
This EAP has demonstrated that retreatment with ipilimumab can be effective in patients who progress after achieving initial clinical benefit with ipilimumab treatment. A retreatment response rate of 12% and irDCR of 55% is encouraging, suggesting that retreatment with ipilimumab can translate into further clinical benefit. However, the small sample size means that further prospective data are needed to confirm the safety and efficacy of retreatment protocols. Further evaluation of this strategy is required in randomised controlled clinical trials to help define the potential benefit for patients.
Acknowledgments
This work was supported in part by the Associazione Italiana per la Ricerca sul Cancro, the Italian Ministry of Health, via the Ricerca Finalizzata 2010. The EAP was sponsored by Bristol-Myers Squibb (BMS). Editorial and writing assistance was provided by StemScientific, funded by BMS. Statistical support was provided by Clinical Research Services, funded by BMS. We would like to thank the patients and investigators who participated in the European EAP.
VC-S has received travel expenses for medical meetings and conferences and honoraria for advisory boards and consultancy from Bristol-Myers Squibb, GlaxoSmithKline, Merck Sharp & Dohme and Roche-Genentech. PAA has served in a consultancy/advisory role for Bristol-Myers Squibb, Merck Sharp & Dohme, Roche-Genentech, GlaxoSmithKline, Amgen and Celgene; he has also received research funding from Bristol-Myers Squibb, and honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Roche-Genentech and GlaxoSmithKline. MM has had an advisory role and received funding for communication programmes from Bristol-Myers Squibb, Roche-Genentech and Merck Sharp & Dohme and has received research funding from Bristol-Myers Squibb. PM has served in an advisory role for Bristol-Myers Squibb, GlaxoSmithKline, Novartis and Roche-Genentech. AT received honoraria and travel support from Bristol-Myers Squibb for advisory board participation. PQ has received honoraria from and served as a consultant and in an advisory role for Bristol-Myers Squibb, GlaxoSmithKline and Roche-Genentech. MDV has served as a consultant or in an advisory role for Merck Sharp & Dohme/Schering-Plough and GlaxoSmithKline; he has received research funding from Celgene, Novartis and Roche. The remaining authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Agarwala SS. Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther. 2009;9:587–595. doi: 10.1586/era.09.25. [DOI] [PubMed] [Google Scholar]
- Chin K, Ibrahim R, Berman D, Yellin M, Lowy I, Lin R, Hoos A.2008Treatment guidelines for the management of immune-related adverse events in patients treated with ipilimumab, an anti-CTLA4 therapy Ann Oncol 19viii244abstract 787P. [Google Scholar]
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, Guida M, Hyams DM, Gomez H, Bastholt L, Chasalow SD, Berman D. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh EM, O'Day SJ, Powderly J, Khan KD, Pavlick AC, Cranmer LD, Samlowski WE, Nichol GM, Yellin MJ, Weber JS. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs. 2011;29:489–498. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoos A, Ibrahim R, Korman A, Abdallah K, Berman D, Shahabi V, Chin K, Canetta R, Humphrey R. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–546. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA, Parulekar W, Markovic SN, Saxman S, Kirkwood JM. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- Lebbé C, Weber JS, Maio M, Neyns B, Harmankaya K, Hamid O, O'Day S, Chin KM, Opatt McDowell D, Cykowski L, McHenry B, Wolchok JD. Long-term survival in patients with metastatic melanoma who received ipilimumab in four phase II trials. J Clin Oncol. 2013;31:abstract 9053. [Google Scholar]
- Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150:179–185. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- Maio M, Bondarenko I, Robert C, Thomas L, Garbe C, Testori A, Lu H, Chin K, Wolchok J. Survival analysis with 5 years of follow-up in a phase III study of ipilimumab and dacarbazine in metastatic melanoma. Eur J Cancer. 2013;49:abstract 3704. [Google Scholar]
- Margolin KA, Hamid O, Weber JS, Pavlick AC, Hodi FS, Amin A, Bennett K, Michener T, Minor DR. Ipilimumab retreatment following induction therapy: the expanded access program (EAP) experience. J Clin Oncol. 2013;31:abstract 9041. [Google Scholar]
- Neyns B, Weber JS, Lebbé C, Maio M, Harmankaya K, Hamid O, O'Day S, Chin KM, Opatt McDowell D, Cykowski L, McHenry B, Wolchok JD. Ipilimumab (Ipi) retreatment at 10 mg/kg in patients with metastatic melanoma previously treated in phase II trials. J Clin Oncol. 2013;31:abstract 9059. [Google Scholar]
- O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, Queirolo P, Lundgren L, Mikhailov S, Roman L, Verschraegen C, Humphrey R, Ibrahim R, de Pril V, Hoos A, Wolchok JD. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Pigozzo J, Ascierto PA, Calabrò L, De Galitiis F, Ferrucci PF, Queirolo P, Bernengo MG, Aglietta M, Mandalà M, Del Vecchio M. Efficacy and safety of ipilimumab reinduction therapy in patients with pretreated advanced melanoma participating in an expanded access programme in Italy. Ann Oncol. 2012;23:abstract 1130P. [Google Scholar]
- Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman JM, Kmieciak M, Manjili MH, Knutson KL. Tumor immunoediting and immunosculpting pathways to cancer progression. Semin Cancer Biol. 2007;17:275–287. doi: 10.1016/j.semcancer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Ghiringhelli F. What is the role of cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Oncologist. 2009;14:848–861. doi: 10.1634/theoncologist.2009-0028. [DOI] [PubMed] [Google Scholar]
- Robert C, Schadendorf D, Messina M, Hodi FS, O'Day S. Efficacy and safety of retreatment with ipilimumab in patients with pretreated advanced melanoma who progressed after initially achieving disease control. Clin Cancer Res. 2013;19:2232–2239. doi: 10.1158/1078-0432.CCR-12-3080. [DOI] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Jr., Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- Stewart TJ, Fernando GJ, Frazer IH, Leggatt GR. Tumour susceptibility to innate and adaptive immunotherapy changes during tumour maturation. Immunol Cell Biol. 2004;82:455–461. doi: 10.1111/j.0818-9641.2004.01273.x. [DOI] [PubMed] [Google Scholar]
- Tarhini AA, Agostara B. Cutaneous melanoma: available therapy for metastatic disease. Dermatol Ther. 2006;19:19–25. doi: 10.1111/j.1529-8019.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D, Siegel J, O'Day SJ. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr., Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O'Day SJ, Lebbe C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- Yamshchikov GV, Mullins DW, Chang CC, Ogino T, Thompson L, Presley J, Galavotti H, Aquila W, Deacon D, Ross W, Patterson JW, Engelhard VH, Ferrone S, Slingluff CL., Jr Sequential immune escape and shifting of T-cell responses in a long-term survivor of melanoma. J Immunol. 2005;174:6863–6871. doi: 10.4049/jimmunol.174.11.6863. [DOI] [PubMed] [Google Scholar]
- YERVOY Summary of Product Characteristics 2013. Available at http://www.medicines.org.uk/emc/medicine/24779 2013.