Abstract
Background:
Renal transplantation has been associated with a significantly increased risk of developing cancers during long-term follow-up, but for bladder cancer, this risk is less clear. We therefore performed a meta-analysis to determine whether bladder cancer risk in renal transplant recipients was increased.
Methods:
Eligible studies were identified through searches of PubMed and other public resources. Random-effects meta-analyses were used to pool overall estimates for standardised incidence ratios (SIRs). Heterogeneity test, sensitivity analysis, and assessment of publishing bias were also performed.
Results:
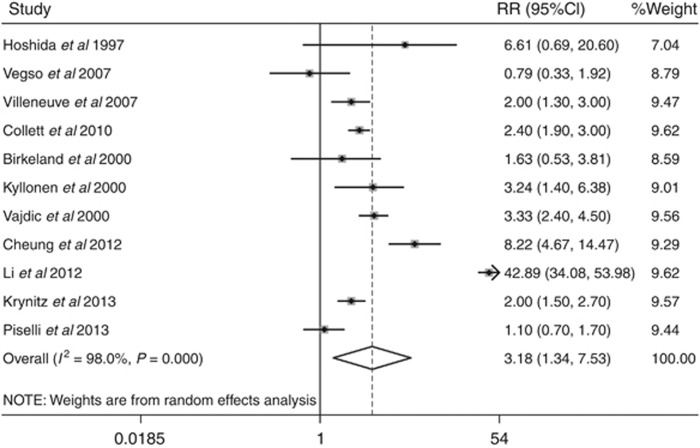
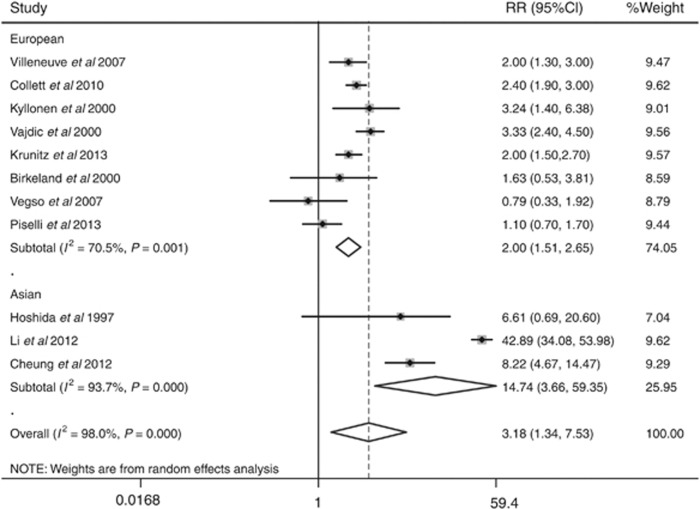
We identified a 3.18-fold higher SIR (95% confidence intervals (CI): 1.34–7.53, P=0.008) of bladder cancer in patients following renal transplantation compared with the general population, based on data from 79 988 patients with a total follow-up of 308 458 patient-years. When stratified by ethnicity, the SIRs for bladder cancer were 2.00 (95% CI: 1.51–2.65, P=0.001) and 14.74 (95% CI: 3.66–59.35, P<0.001) between European and Asian renal transplant recipients, respectively.
Conclusions:
Our study demonstrated that the risk of developing bladder cancer in transplant populations was increased. Such association suggests that physicians should be more vigilant in checking for bladder cancer in transplantation recipient population.
Keywords: renal transplantation, bladder cancer, standardised incidence ratio, surveillance
Renal transplantation is the treatment offered for patients with end-stage renal failure. The outcomes of renal transplantation have improved considerably over the past decades, especially with the introduction of highly active immunosuppressive drugs that dramatically decrease the incidence of acute graft rejection and improve graft and patient survival rates (O'Grady et al, 2002; Knight et al, 2009). However, treatment of renal transplant recipients (RTRs) with immunosuppressive agents was considered to lead to malignancy by supporting oncogenesis caused by certain viruses or by impairing immune surveillance resulting in faster tumour growth (Rama and Grinyo, 2010). As a matter of fact, malignancy is the third leading cause of death among RTRs after transplation, following cardiovascular disease and infection (Briggs, 2001). Compared with the general population, an overall two- to seven-fold elevated risk of malignancies was documented among RTRs (Kyllonen et al, 2000; Adami et al, 2003; Vajdic et al, 2006; Villeneuve et al, 2007; Krynitz et al, 2013). The most frequent malignancies are skin cancers and lymphomas, followed by Kaposi's sarcoma, lip, cervical, perineal, renal, hepatocellular carcinomas, and other sarcomas (Penn, 2000; Kauffman et al, 2006). Increased incidence of other malignancies, such as thyroid and lung in transplant recipients, has also been reported (Grulich et al, 2007; Karamchandani et al, 2010).
Several studies have showed increased risk of bladder cancer after renal transplantation (Hoshida et al, 1997; Cheung et al, 2012; Li et al, 2012). However, not all studies have shown a similar association (Serraino et al, 2005; Vegso et al, 2007). In this study, we performed a meta-analysis to determine whether the overall SIR of bladder cancer is increased in RTRs compared with the general population, which might be helpful in determining whether conclusive recommendations for bladder cancer screening in RTRs are needed.
Materials and methods
Identification of eligible studies
A comprehensive literature search was performed via public database PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), Embase (http://www.embase.com), and ISI Wed of Knowledge (http://isiknowledge.com), with the last update in September 2013. The search was restricted to studies published in English. The key search terms used were ‘renal', ‘kidney', ‘transplantation', ‘bladder', and ‘cancer'. The search results were restricted to the presence of these keywords in the title or abstract of the articles. A manual search of the references from eligible studies was performed afterwards to check for additional potentially relevant studies for inclusion.
Inclusion and exclusion criteria
Eligible studies were selected according to the following inclusion criteria: (a) studies should be population-based cohort studies of RTRs; (b) studies matched the transplant population to a standardised population to calculate a standardised incidence ratio (SIR); (c) Sufficient information on SIR, relative risk (RR), or observed cases of bladder cancer in RTRs had to be provided in the studies. The following exclusion criteria were used: (a) other organ transplantation studies; (b) case series and case reports; and (c) studies that collected data on incident cancer through cancer registries in the developed countries. Those studies that merely accepted bladder cancer or other cancer diagnoses without confirming that these were notified to a cancer registry were excluded.
Data extraction
Two investigators (LY and PC) independently extracted the data and reached consensus on all items. All retrieved data were organised into a data extraction tables. Parameters extracted from the studies included: the first author, publication year, geographic origin, data source, number of patients, number of renal transplant cases, number of all cancers, length of follow-up time, mean follow-up time (years), patient-years (years), mean age at transplantation (years), mean age at diagnosis of malignancy (years), median time to development of any type of cancer (months), number of expected cases of bladder cancer, number of observed cases of bladder cancers, the SIRs of commonly known cancers and bladder cancer, and mean time to development of bladder cancer (months), if available.
Statistical analysis
In this meta-analysis, the unadjusted RR with 95% confidence intervals (CIs) was estimated. Because of possible heterogeneity between studies, a random-effects model was used to pool effects for RR (DerSimonian and Kacker, 2007). Between-study heterogeneity was calculated using the chi-squared-based Q-statistic (significance level at P<0.1) and by estimating I2, which was documented for the percentage of the observed between-study variability due to heterogeneity rather than chance, with ranges from 0% to 100% (I2=0–25%, no heterogeneity; I2=25–50%, moderate heterogeneity; I2=50–75%, large heterogeneity; I2=75–100%, extreme heterogeneity). In addition, one-way sensitivity analyses were performed after the sequential removal of each study, and the new pooled results reflected the influence of that deleted study to the overall RR. The Begg's funnel plot and Egger's test were performed to statistically analyse the publication bias (Egger et al, 1997). All statistical analyses were performed using the STATA 11.0 software (STATA Corp, College Station, TX, USA).
Results
Study characteristics
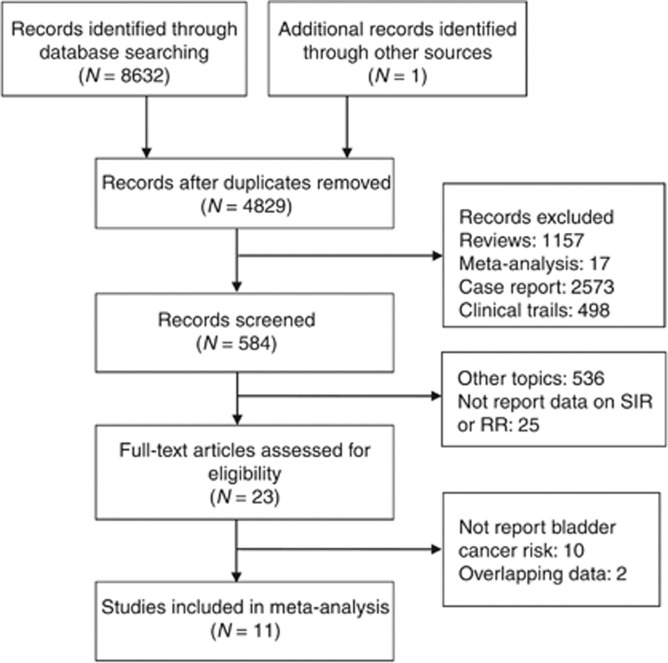
A total of 11 studies met our inclusion criteria (Hoshida et al, 1997; Birkeland et al, 2000; Kyllonen et al, 2000; Vajdic et al, 2006; Vegso et al, 2007; Villeneuve et al, 2007; Collett et al, 2010; Cheung et al, 2012; Li et al, 2012; Krynitz et al, 2013; Piselli et al, 2013), comprising a total of 81 394 renal transplants performed on 79 988 patients. A diagram schematising the selection process of identified studies is presented in Figure 1. The studies and their details are listed in Table 1. All the studies were retrospective, and the largest study had 25 104 renal transplant patients. These studies were based on patients in several countries, including one in Denmark (Birkeland et al, 2000), two in China (Cheung et al, 2012; Li et al, 2012), one in the UK (Collett et al, 2010), one in Japan (Hoshida et al, 1997), one in Sweden (Krynitz et al, 2013), one in Finland (Kyllonen et al, 2000), one in Italy (Piselli et al, 2013), one in Australia and New Zealand (Vajdic et al, 2006), one in Hungary (Vegso et al, 2007), and one in Canada (Villeneuve et al, 2007). All were multicentre-based studies except two (Kyllonen et al, 2000; Vegso et al, 2007).
Figure 1.
Flowchart of article selection.
Table 1. Summary of studies included in the analysis.
| Study | Multicentre | Type of transplant | Geographic origin | Data source | Number of pantients | Number of renal transplant cases |
|---|---|---|---|---|---|---|
|
Birkeland et al, 2000 |
Yes |
Renal |
Denmark |
Danish registry |
1821 |
1821 |
|
Cheung et al, 2012 |
Yes |
Renal |
China |
Hong Kong Renal Registry |
4674 |
4895 |
|
Collett et al, 2010 |
Yes |
Multiorgan |
UK |
UK Transplant Registry |
25 104 |
25 104 |
|
Hoshida et al, 1997 |
Yes |
Renal |
Japan |
Multicentre, Japan |
1744 |
1744 |
|
Krynitz et al, 2013 |
Yes |
Multiorgan |
Sweden |
Swedish National Patient Register |
7952 |
7952 |
|
Kyllonen et al, 2000 |
No |
Renal |
Finland |
Finland Transplant Registry |
2890 |
3440 |
|
Li et al, 2012 |
Yes |
Renal |
China |
Taiwan National Health Insurance Research Database (NHIRD) |
4716 |
4716 |
|
Piselli et al, 2013 |
Yes |
Renal |
Italy |
Italian KT centre |
7217 |
7299 |
|
Vajdic et al, 2006 |
Yes |
Renal |
Australia and New Zealand |
Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) |
10 180 |
10 180 |
|
Vegso et al, 2007 |
No |
Renal |
Hungary |
Budapest transplantation center |
2535 |
2852 |
|
Villeneuve et al, 2007 |
Yes |
Renal |
Canada |
Canadian Organ Replacement Registry |
11 155 |
11 391 |
| Total | 79 988 | 81 394 |
Table 2 showed the demographic details of the included studies. As noted, the 79 988 RTRs included in this analysis were followed-up for a total of 308 458 person-years, with a mean follow-up duration of 7.6 years (range: 4.8–9.8 years). The mean age at transplantation and diagnosis of malignancy were 43.7 (range: 38.9–53.1) and 46.8 (range: 40.0–53.1) years old, respectively. During the period of observation, the overall diagnosed cancers and haematological malignancies was 10 902.
Table 2. Demographic details of the included studies.
| Study | Number of all cancers | Length of follow-up time | Mean follow-up time (years) | Patient-years (years) | Mean age at transplantation (years) | Mean age at diagnosis of malignancy (years) | Number of expected cases of bladder cancer | Number of observed cases of bladder cancers | Mean time to development of bladder cancer (months) |
|---|---|---|---|---|---|---|---|---|---|
|
Birkeland et al, 2000 |
209 |
NR–1995 |
7.5 |
13 734 |
38.9 |
|
3.06 |
5 |
|
|
Cheung et al, 2012 |
299 |
1972–2011 |
8.2 |
40 246 |
43.7 |
53.1 |
1.46 |
12 |
57.6 |
|
Collett et al, 2010 |
4422 |
1980–2007 |
|
|
|
|
31.90 |
76 |
|
|
Hoshida et al, 1997 |
46 |
1970–1995 |
7.4 |
12 982 |
|
40.0 |
0.30 |
2 |
63.0 |
|
Krynitz et al, 2013 |
2774 |
1970–2008 |
9.7 |
77 288 |
|
|
21.00 |
42 |
|
|
Kyllonen et al, 2000 |
230 |
1964–1997 |
7.2 |
20 817 |
41.5 |
47.2 |
2.47 |
8 |
|
|
Li et al, 2012 |
320 |
1997–2008 |
4.8 |
22 556 |
44.1 |
|
1.68 |
72 |
|
|
Piselli et al, 2013 |
395 |
1997–2009 |
5.5 |
39 598 |
|
|
18.10 |
20 |
|
|
Vajdic et al, 2006 |
1236 |
1982–2003 |
8.5 |
|
41.0 |
|
12.61 |
42 |
105.6 |
|
Vegso et al, 2007 |
193 |
1973–2007 |
9.8 |
|
53.1 |
|
3.80 |
3 |
|
|
Villeneuve et al, 2007 |
778 |
1981–1998 |
7.3 |
81 237 |
|
|
12.10 |
24 |
|
| Total | 10902 | 7.6 | 308 458 | 43.7 | 46.8 | 108.48 | 306 | 75.4 |
In this meta-analysis, 306 cases of bladder cancer were identified, compared with 108.48 expected cases.
Evidence synthesis
Meta-analysis for the SIR for bladder cancer suggested a significantly increased risk (SIR=3.18, 95% CI: 1.34–7.53; P=0.008). Figure 2 shows the forest plot for individual and overall RR measures. Table 3 shows the cancer-specific SIRs. Cancers caused by viral infections, including liver cancer (hepatitis C and B viruses), non-Hodgkin lymphoma and Hodgkin lymphoma (both due to Epstein Barr virus), lip, and non-melanoma skin cancer (both possibly due to human papillomavirus virus (HPV)), were found to be significantly increased among RTRs. Thyroid and kidney cancers also had an increased risk of 5.29 and 8.61 times more than the general population, respectively, whereas common epithelial cancers such as breast, ovary, prostate, and pancreas cancers occurred almost at the same rate as the general population.
Figure 2.
Forest plot of overall bladder cancer risk, showing the relative ratio (RR) and 95% confidence intervals (CI).The squares and horizontal lines correspond to the study-specific RR and 95% CI. The area of the squares reflects the study-specific weight.
Table 3. Cancer-specific standardised incidence ratios (SIRs).
| Cancer site | Birkeland et al, 2000 | Cheung et al, 2012 | Collett et al, 2010 | Hoshida et al, 1997 | Krynitz et al, 2013 | Kyllonen et al, 2000 | Li et al, 2012 | Piselli et al, 2013 | Vajdic et al, 2006 | Vegso et al, 2007 | Villeneuve et al, 2007 | Overall SIR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lip |
13.02 (10.75–15.63) |
|
65.6 (49.9–84.6) |
|
46 (35–59) |
22.95 (12.55–38.51) |
|
9.4 (3.1–22.0) |
47 (41.76–52.91) |
|
31.3 (23.5–40.8) |
29.45 (17.85–48.59) |
| Stomach |
|
2.85 (1.62–5.02) |
2.0 (1.4–2.6) |
1.40 (0.45–2.68) |
1.8 (1.0–2.9) |
|
1.84 (0.83–4.09) |
1.4 (0.8–2.3) |
|
|
2.1 (1.2–3.4) |
1.93 (1.60–2.34) |
| Colon |
|
1.75 (1.22–2.52) |
1.8 (1.6–2.1) |
|
2.3 (1.8–2.9) |
3.94 (2.10–6.74) |
2.04 (1.13–3.54) |
|
1.71 (1.38–2.09) |
0.34 |
1.4 (1.0–1.8) |
1.89 (1.61–2.22) |
| Liver |
|
2.53 (1.63–3.91) |
2.4 (1.5–3.8) |
1.36 (0.24–3.36) |
2.7 (1.6–4.1) |
|
5.07 (3.89–6.42) |
0.4 (0.1–1.1) |
3.19 (1.53–5.8) |
3.25 |
1.8 (0.6–4.3) |
2.63 (1.91–3.62) |
| Pancreas |
|
1.57 (0.51–4.87) |
1.5 (1.2–2.1) |
|
2.2 (1.3–3.5) |
|
|
0.9 (0.3–2.0) |
|
|
1.1 (0.4–2.2) |
1.55 (1.24–1.93) |
| Lunga |
|
1.68 (1.17–2.42) |
1.4 (1.2–1.6) |
|
1.7 (1.3–2.2) |
|
4.81 (2.73–8.48) |
1.1 (0.8–1.6) |
|
0.65 |
2.1 (1.7–2.5) |
1.70 (1.30–2.21) |
| Melanoma |
1·35 (0·28–3·93) |
9.09 (2.27–36.34) |
|
|
|
|
5.40 (0.76–38.18) |
1.8 (0.9–3.3) |
|
|
|
2.33 (1.39–3.92) |
| Non-melanoma skin |
10.68 (8.84–12.79) |
|
16.6 (15.9–17.3) |
|
54 (52–56) |
39.10 (29.20–51.27) |
2.30 (0.86–6.13) |
|
|
2.58 |
|
11.93 (4.84–29.45) |
| Breast |
1.45 (0.72–2.60) |
1.66 (1–2.75) |
1.0 (0.8–1.2) |
1.53 (0.18–5.27) |
1.2 (0.9–1.5) |
1.20 (0.64–2.05) |
|
0.8 (0.5–1.2) |
1.03 (0.78–1.34) |
0.86 |
1.3 (1.0–1.7) |
1.12 (1.00–1.24) |
| Ovary |
|
3.29 (1.37–7.9) |
1.4 (0.9–2.0) |
|
1.9 (1.1–3.1) |
|
1.14 (0.64–1.89) |
1.1 (0.2–3.1) |
1.15 (0.46–2.38) |
|
1.5 (0.6–3.0) |
1.50 (1.19–1.89) |
| Prostate |
|
0.88 (0.39–1.95) |
1.1 (0.9–1.4) |
|
1.1 (0.9–1.3) |
|
|
1.7 (1.2–2.3) |
0.95 (0.68–1.29) |
0.65 |
0.9 (0.6–1.3) |
1.11 (0.99–1.25) |
| Kidney |
4.08 (1.50–8.88) |
12.5 (8.51–18.36) |
7.9 (6.7–9.3) |
79.96 (39.98–114.95) |
6.2 (4.8–7.9) |
7.97 (5.00–12.07) |
|
4.9 (3.4–6.8) |
7.3 (5.69–9.22) |
6.77 |
7.3 (5.7–9.2) |
8.61 (6.73–11.02) |
| Bladder |
1.63 (0.53–3.81) |
8.22 (4.67–14.47) |
2.4 (1.9–3.0) |
6.61 (0.69–20.60) |
2.0 (1.5–2.7) |
3.24 (1.40–6.38 ) |
42.89 (34.08–53.98) |
1.1 (0.7–1.7) |
3.33 (2.4–4.5) |
0.79 |
2.0 (1.3–3.0) |
3.18 (1.34–7.53) |
| Thyroid |
0·91 (0·02–5·09) |
4.35 (2.41–7.85) |
7.0 (4.8–9.8) |
12.43 (2.38–33.70) |
4.1 (2.1–7.2) |
8.09 (4.04–14.47) |
2.41 (1.08–5.34) |
1.9 (0.9–3.6) |
6.9 (4.69–9.8) |
8.95 |
5.0 (3.1–7.4) |
5.29 (3.87–7.24) |
| Hodgkin's lymphoma |
8.0 (1.65–23.38) |
|
7.4 (5.3–10.2) |
|
|
|
|
2.3 (0.5–6.8) |
3.74 (1.51–7.71) |
|
3.6 (1.7–6.9) |
5.92 (4.53–7.73) |
| Non-Hodgkin lymphoma |
5.48 (2.37–10.80) |
|
12.5 (11.2–13.8) |
|
|
|
|
|
9.86 (8.37–11.54) |
1.32 |
8.8 (7.4–10.5) |
6.59 (4.33–10.04) |
| All cancers | 3.59 (3.12–4.11) | 2.94 (2.62–3.29) | 4.5 | 2.78 (1.8–3.28) | 6.5 (6.3–6.8) | 3.33 (2.92–3.79) | 3.75 (3.36–4.18) | 1.7 (1.6–1.9) | 3.4 (3.22–3.59) | 1.33 | 2.5 (2.3–7.1) | 3.19 (2.22–4.60) |
Includes trachea, bronchus, and lung.
Sensitivity analysis indicates that the omission of any of the studies led to changes in estimates between 2.36 (95% CI: 1.69–3.30) and 3.64 (95% CI: 1.48–8.94) (Table 4). The changes were not significant.
Table 4. Sensitivity analysis.
| Study omitted | RR | 95% confidence intervals | Pheterogeneity | I2 | Pbias | |
|---|---|---|---|---|---|---|
|
Birkeland et al, 2000 |
3.39 |
1.37 |
8.39 |
<0.001 |
98.2 |
0.422 |
|
Cheung et al, 2012 |
2.89 |
1.14 |
7.30 |
<0.001 |
98.2 |
0.344 |
|
Collett et al, 2010 |
3.28 |
1.20 |
8.94 |
<0.001 |
98.1 |
0.204 |
|
Hoshida et al, 1997 |
3.01 |
1.23 |
7.38 |
<0.001 |
98.2 |
0.310 |
|
Krynitz et al, 2013 |
3.34 |
1.28 |
8.72 |
<0.001 |
98.1 |
0.342 |
|
Kyllonen et al, 2000 |
3.18 |
1.27 |
7.95 |
<0.001 |
98.2 |
0.394 |
|
Li et al, 2012 |
2.36 |
1.69 |
3.30 |
<0.001 |
80.0 |
0.969 |
|
Piselli et al, 2013 |
3.55 |
1.44 |
8.79 |
<0.001 |
98.1 |
0.461 |
|
Vajdic et al, 2006 |
3.17 |
1.19 |
8.44 |
<0.001 |
98.2 |
0.393 |
|
Villeneuve et al, 2007 |
3.34 |
1.31 |
8.51 |
<0.001 |
98.2 |
0.433 |
| Vegso et al, 2007 |
3.64 |
1.48 |
8.94 |
<0.001 |
98.2 |
0.483 |
| Combined | 3.18 | 1.34 | 7.53 | <0.001 | 98.0 | 0.373 |
Abbreviation: RR=relative risk.
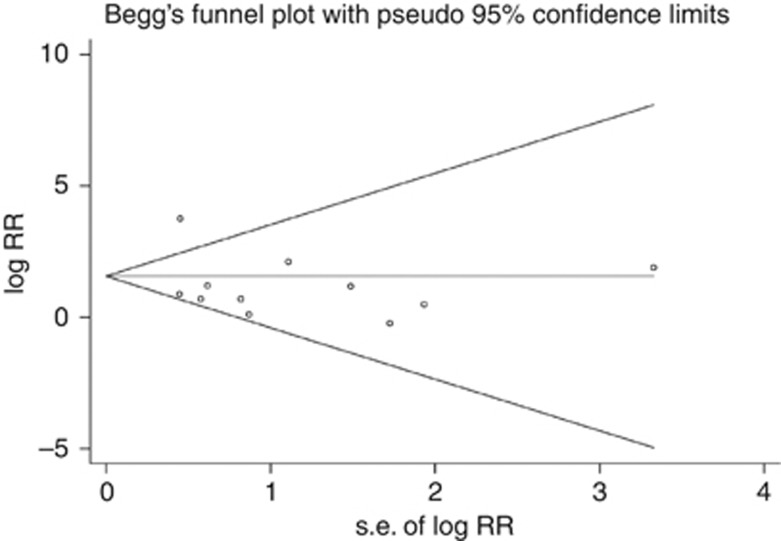
Significant heterogeneity existed (I2=98.0%, Pheterogeneity<0.001) in the pooled analysis. When stratified by ethnicity, the SIRs for bladder cancer were 2.00 (95% CI: 1.51–2.65, P=0.001, I2=70.50%, Pheterogeneity=0.001) for European RTRs and 14.74 (95% CI: 3.66–59.35, P<0.001, I2=93.70%, Pheterogeneity<0.001) for Asian RTRs (Figure 3). With the limited information available, we could not detect any sources contributing to the substantial heterogeneity. Furthermore, no evidence of publication bias was observed by Egger's test (P=0.373) or Begg's test (P=0.276, Figure 4).
Figure 3.
Forest plot of bladder cancer risk stratified by ethnicity.
Figure 4.
Begg's funnel plot to detect publication bias in overall populations.Each circle represents a separate study for the indicated association.
Discussion
The aim of this meta-analysis was to determine whether the risk of bladder cancer among RTRs is increased such that separate surveillance recommendations would be warranted. Our results showed a significantly increased risk of developing bladder cancer in transplant populations compared with the general population (SIR=3.18, 95% CI: 1.34–7.53, P=0.008). When stratified by ethnicity, the SIRs for bladder cancer were 2.00 (95% CI: 1.51–2.65, P=0.001) among European and much higher (14.74) among Asian RTRs (95% CI: 3.66–59.35, P<0.001), suggesting the presence of ethnicity-based differences.
Most bladder cancers in RTRs are transitional cell carcinoma (TCC). However, in RTRs, some studies reported an invasive and fatal nature of bladder cancers (Buzzeo et al, 1997), a factor that should impact on intensive surveillance during the post-transplant period. The exact reason for the increased risk of bladder cancer in RTRs still remains unknown. Several possible factors have been proposed. Both viral and nonviral factors are involved in the development of bladder cancer after renal transplantation. The long-term use of immunosuppressive therapy for the graft is another possible factor promoting bladder cancer, as reported for other malignancies. The underlying possible mechanisms include direct cellular damage by immunosuppressants (e.g., cyclophosphamide) and impaired ability to repair damage to cellular DNA or destroy damaged cells due to the immunocompromised state (Buzzeo et al, 1997). In this meta-analysis, the incidence of bladder cancer after renal transplantation was much higher in the Asian (specifically Chinese) populations than in European populations. This difference may be due to differences in genetic background and environmental factors. Aristolochic acid in Chinese herbs and chronic arsenic poisoning from underground water intake may both contribute to a high incidence of bladder cancers in transplant patients (Wu et al, 2004). Studies in patients from the Chinese mainland showed a RR of 5.85 for developing TCC after kidney transplantation when exposed to Chinese herbs. Furthermore, infection with high-risk HPV (especially HPV16; Li et al, 2011) and various gene polymorphisms (Yuan et al, 2012; Xie et al, 2013) have been postulated to have a role in bladder carcinogenesis.
Some limitation may exist in this study. First, the amount of heterogeneity between studies is high, which may be due to the following reasons: (i) no detail information on the confounders were available that allow us to perform an adjustment for these potential confounders, including cigarette smoking, obesity, aristolochic acid in Chinese herbs, diabetic drug rosiglitazone or pioglitazone, and so on (Wu et al, 2004; Holick et al, 2007; Hsiao et al, 2013; Wyszynski et al, 2014); (ii) although all the studies used the general population as the reference population, the criteria used for matching might be differently applied; and (iii) a lack of case–control studies that examined bladder cancer risk following renal transplant to better control of confounding bias. Second, the RTRs were not screened for bladder cancer before transplantation, and the length of time from transplantation to the diagnosis of bladder cancer was very short (from 4.8 to 8.8 years) in our results. Therefore, we could not exclude the presence of possible pre-existing tumours in these recipients. Finally, the pooled increased risk estimate for bladder cancer after transplantation was generated from 11 publications. A larger, multiple-centre prospective study may still be necessary to evaluate the risks of bladder cancer development in RTRs.
In conclusion, in this meta-analysis, we showed an increased risk of developing bladder cancer in transplant populations. Such an association suggests that physicians should be more vigilant in checking for bladder cancer in the transplantation population.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81172694, No. 81070367, and No. 81270537), the practice innovation training program projects for the Jiangsu College students (2012JSSPTTP1018), the Jiangsu Province's Qinglan project (JX2161015124), and the project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Adami J, Gabel H, Lindelof B, Ekstrom K, Rydh B, Glimelius B, Ekbom A, Adami HO, Granath F. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221–1227. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkeland SA, Lokkegaard H, Storm HH. Cancer risk in patients on dialysis and after renal transplantation. Lancet. 2000;355:1886–1887. doi: 10.1016/s0140-6736(00)02298-4. [DOI] [PubMed] [Google Scholar]
- Briggs JD. Causes of death after renal transplantation. Nephrol Dial Transplant. 2001;16:1545–1549. doi: 10.1093/ndt/16.8.1545. [DOI] [PubMed] [Google Scholar]
- Buzzeo BD, Heisey DM, Messing EM. Bladder cancer in renal transplant recipients. Urology. 1997;50:525–528. doi: 10.1016/S0090-4295(97)00305-1. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Lam MF, Chu KH, Chow KM, Tsang KY, Yuen SK, Wong PN, Chan SK, Leung KT, Chan CK, Ho YW, Chau KF. Malignancies after kidney transplantation: Hong Kong Renal Registry. Am J Transplant. 2012;12:3039–3046. doi: 10.1111/j.1600-6143.2012.04209.x. [DOI] [PubMed] [Google Scholar]
- Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10:1889–1896. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- Dersimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grulich AE, Van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- Holick CN, Giovannucci EL, Stampfer MJ, Michaud DS. Prospective study of body mass index, height, physical activity and incidence of bladder cancer in US men and women. Int J Cancer. 2007;120:140–146. doi: 10.1002/ijc.22142. [DOI] [PubMed] [Google Scholar]
- Hoshida Y, Tsukuma H, Yasunaga Y, Xu N, Fujita MQ, Satoh T, Ichikawa Y, Kurihara K, Imanishi M, Matsuno T, Aozasa K. Cancer risk after renal transplantation in Japan. Int J Cancer. 1997;71:517–520. doi: 10.1002/(sici)1097-0215(19970516)71:4<517::aid-ijc3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Hsiao FY, Hsieh PH, Huang WF, Tsai YW, Gau CS. Risk of bladder cancer in diabetic patients treated with rosiglitazone or pioglitazone: a nested case-control study. Drug Saf. 2013;36:643–649. doi: 10.1007/s40264-013-0080-4. [DOI] [PubMed] [Google Scholar]
- Karamchandani D, Arias-Amaya R, Donaldson N, Gilbert J, Schulte KM. Thyroid cancer and renal transplantation: a meta-analysis. Endocr Relat Cancer. 2010;17:159–167. doi: 10.1677/ERC-09-0191. [DOI] [PubMed] [Google Scholar]
- Kauffman HM, Cherikh WS, Mcbride MA, Cheng Y, Hanto DW. Post-transplant de novo malignancies in renal transplant recipients: the past and present. Transpl Int. 2006;19:607–620. doi: 10.1111/j.1432-2277.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation. 2009;87:785–794. doi: 10.1097/TP.0b013e3181952623. [DOI] [PubMed] [Google Scholar]
- Krynitz B, Edgren G, Lindelof B, Baecklund E, Brattstrom C, Wilczek H, Smedby KE. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008—a Swedish population-based study. Int J Cancer. 2013;132:1429–1438. doi: 10.1002/ijc.27765. [DOI] [PubMed] [Google Scholar]
- Kyllonen L, Salmela K, Pukkala E. Cancer incidence in a kidney-transplanted population. Transpl Int. 2000;13 (Suppl 1:S394–S398. doi: 10.1007/s001470050369. [DOI] [PubMed] [Google Scholar]
- Li N, Yang L, Zhang Y, Zhao P, Zheng T, Dai M. Human papillomavirus infection and bladder cancer risk: a meta-analysis. J Infect Dis. 2011;204:217–223. doi: 10.1093/infdis/jir248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WH, Chen YJ, Tseng WC, Lin MW, Chen TJ, Chu SY, Hwang CY, Chen CC, Lee DD, Chang YT, Wang WJ, Liu HN. Malignancies after renal transplantation in Taiwan: a nationwide population-based study. Nephrol Dial Transplant. 2012;27:833–839. doi: 10.1093/ndt/gfr277. [DOI] [PubMed] [Google Scholar]
- O'grady JG, Burroughs A, Hardy P, Elbourne D, Truesdale A. Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomised controlled trial. Lancet. 2002;360:1119–1125. doi: 10.1016/s0140-6736(02)11196-2. [DOI] [PubMed] [Google Scholar]
- Penn I. Cancers in renal transplant recipients. Adv Ren Replace Ther. 2000;7:147–156. doi: 10.1053/rr.2000.5269. [DOI] [PubMed] [Google Scholar]
- Piselli P, Serraino D, Segoloni GP, Sandrini S, Piredda GB, Scolari MP, Rigotti P, Busnach G, Messa P, Donati D, Schena FP, Maresca MC, Tisone G, Veroux M, Sparacino V, Pisani F, Citterio F. Risk of de novo cancers after transplantation: results from a cohort of 7217 kidney transplant recipients, Italy 1997-2009. Eur J Cancer. 2013;49:336–344. doi: 10.1016/j.ejca.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Rama I, Grinyo JM. Malignancy after renal transplantation: the role of immunosuppression. Nat Rev Nephrol. 2010;6:511–519. doi: 10.1038/nrneph.2010.102. [DOI] [PubMed] [Google Scholar]
- Serraino D, Piselli P, Angeletti C, Minetti E, Pozzetto A, Civati G, Bellelli S, Farchi F, Citterio F, Rezza G, Franceschi S, Busnach G. Risk of Kaposi's sarcoma and of other cancers in Italian renal transplant patients. Br J Cancer. 2005;92:572–575. doi: 10.1038/sj.bjc.6602346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajdic CM, Mcdonald SP, Mccredie MR, Van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- Vegso G, Toth M, Hidvegi M, Toronyi E, Langer RM, Dinya E, Toth A, Perner F, Jaray J. Malignancies after renal transplantation during 33 years at a single center. Pathol Oncol Res. 2007;13:63–69. doi: 10.1007/BF02893443. [DOI] [PubMed] [Google Scholar]
- Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7:941–948. doi: 10.1111/j.1600-6143.2007.01736.x. [DOI] [PubMed] [Google Scholar]
- Wu MJ, Lian JD, Yang CR, Cheng CH, Chen CH, Lee WC, Shu KH, Tang MJ. High cumulative incidence of urinary tract transitional cell carcinoma after kidney transplantation in Taiwan. Am J Kidney Dis. 2004;43:1091–1097. doi: 10.1053/j.ajkd.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Wyszynski A, Tanyos SA, Rees JR, Marsit CJ, Kelsey KT, Schned AR, Pendleton EM, Celaya MO, Zens MS, Karagas MR, Andrew AS. Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer. 2014;120:408–414. doi: 10.1002/cncr.28394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Gong Y, Dai J, Wu X, Gu J.2013Genetic variations in base excision repair pathway and risk of bladder cancer: A case-control study in the United States Mol Carcinoge-pub ahead of print 4 September 2013; doi: 10.1002/mc.22073 [DOI] [PubMed]
- Yuan Q, Wang M, Zhang Z, Zhang W. Macrophage migration inhibitory factor gene -173G>C polymorphism and risk of bladder cancer in southeast China: a case-control analysis. Mol Biol Rep. 2012;39:3109–3115. doi: 10.1007/s11033-011-1075-9. [DOI] [PubMed] [Google Scholar]