Abstract
For the synthesis of optically active compounds, chiral catalysts have attracted much attention because large quantities of optically active molecules can be prepared from a small amount of a chiral source. However, many chiral catalysts are often unstable in air (oxygen) and/or in the presence of water. This is especially the case in chiral Lewis acid catalysis, because most Lewis acids are air- and moisture-sensitive. Therefore, many catalysts are prepared in situ in an appropriate solvent just before use, and they cannot be stored for extended periods. We have developed air-stable, storable, and highly efficient chiral zirconium Lewis acids. The catalysts promoted asymmetric Mannich-type, aza Diels–Alder, aldol, and hetero Diels–Alder reactions efficiently with high enantioselectivities. A key to stabilizing the catalysts is an appropriate combination of chiral zirconium Lewis acids with molecular sieves, and the zirconium–molecular sieves-combined catalysts can be stored for extended periods in air at room temperature without loss of activity. Moreover, it has been demonstrated that the catalysts can be recovered and reused.
Enzymatic reactions that attain perfect yields and selectivities under mild conditions are an ideal goal for organic chemists who are developing new reactions. Instead of enzymes, chemists often like to use “artificial catalysts” to reach the goal. The catalysts have advantages over enzymes and antibodies such as a wide scope of substrates applied and large-scale preparation of target products, etc. For the synthesis of optically active compounds, chiral catalysts have attracted much attention because large quantities of optically active molecules can be prepared from a small amount of a chiral source (1, 2). Although several excellent chiral catalysts have been developed in oxidation and reduction, carbon–carbon bond-forming reactions, and other transformations, they are often unstable in air and/or in the presence of water. This is especially the case in chiral Lewis acid catalysis, because most Lewis acids are air- and moisture-sensitive (3). Therefore, many catalysts are prepared in situ in an appropriate solvent just before use, and they cannot be stored for extended periods (some chiral Lewis acid catalysts are stable; see ref. 4).
To address this issue, we focused on molecular sieves (MS), which are a kind of zeolite and often used as dehydration agents in organic synthesis. MS contain Lewis acidic and Lewis basic sites in their structure. We assumed that the Lewis basic sites might interact with chiral Lewis acids under equilibrium conditions, leading to stabilization of the chiral Lewis acids.
In this article, we describe air-stable, storable, and highly selective chiral zirconium Lewis acid catalysts for asymmetric carbon–carbon bond-forming reactions such as Mannich-type, aza Diels–Alder, aldol, and hetero Diels–Alder reactions. The catalyst is prepared by using MS and can be stored for >3 months in air at room temperature without loss of activity (for preliminary communications see refs. 5 and 6).
Experimental Procedures
General. 1H and 13C NMR spectra were recorded on a JEOL JNM-LA300, JNM-LA400, or JNM-LA500 spectrometer in CDCl3. Tetramethylsilane served as internal standard (δ = 0) for 1H NMR, and CDCl3 was used an internal standard (δ = 77.0) for 13C NMR. When dichloromethane-d2 was used, it served as internal standard (δ = 5.32) for 1H NMR, and δ = 53.8 for 13C NMR. HPLC was carried out by using the following apparatuses: liquid chromatograph Shimadzu LC-10AT (liquid chromatograph), Shimadzu SPD-10A (UV detector), and Shimadzu C-R6A or C-R8A (chromatopac). Fluorescence x-ray analysis was measured with Shimadzu Rayny EDX-800. Column chromatography was performed on Silica gel 60 (Merck), and preparative TLC was carried out by using Wakogel B-5F.
MS 3A, 4A, and 5A (powder) were purchased from Aldrich and dried [200°C, 0.2 mmHg (1 mmHg = 133 Pa), 8 h] before use. Zr(OtBu)4 was purchased from Trichemical Laboratory Co. (Yamanashi, Japan). N-methylimidazole (NMI) was purchased from Tokyo Chemical Industry (Tokyo). Aldimines of aromatic aldehydes and heteroaromatic aldehydes were prepared from the corresponding aldehydes and 2-aminophenol by usual methods. The crude aldimines were recrystallized from ethanol to give the pure materials. Ketene silyl acetals were prepared according to the literature (7, 8). (R)-6,6′-bis(pentafluoroethyl)2-1,1′-binaphthene-2,2′-diol (BINOL), (R)-3,3′-(m-CF3C6H4)2BINOL, (R)-3,3′-Br2BINOL, and (R)-3,3′-I2BINOL were prepared according to the literature (9–11). Dichloromethane (CH2Cl2) was distilled from P2O5, then from CaH2, and dried over MS 4A. Toluene and benzene were dried over CaCl2, distilled, and stored over MS 4A. Tetrahydrofuran (THF) was freshly distilled from Na-benzophenone ketyl before use. All other solvents and chemical compounds were purified based on standard procedures. All reactions were carried out under an argon atmosphere in dried glassware.
Preparation of (R)-6-C2F5-ZrMS. To a suspension of powdered MS 5A (240 mg) in benzene (0.5 ml) were added (R)-6,6′-bis(pentafluoroethyl)-BINOL [(R)-6,6′-C2F5BINOL, 35.5 mg, 0.08 mmol] in benzene (0.5 ml), NMI (13.1 mg, 0.16 mmol) in benzene (0.5 ml), and Zr(OtBu)4 (15.3 mg, 0.04 mmol) in benzene (0.5 ml) at room temperature. The mixture was stirred and heated at 80°C for 2 h. After removal of the solvent under reduced pressure (0.2 mmHg) at 50°C for 1 h, the Zr–(R)-6,6′-(C2F5)2BINOL MS-combined catalyst [(R)-6-C2F5-ZrMS] was formed. The (R)-6-C2F5-ZrMS was handled in air and stored at ambient temperature.
Preparation of (R)-3-(m-CF3C6H4)-ZrMS. To a suspension of powdered MS 3A (240 mg) in benzene (0.5 ml) were added (R)-3,3′-bis(m-trif luoromethylphenyl)-BINOL [(R)-3,3′-(m-CF3C6H4)2BINOL, 68.9 mg, 0.12 mmol] in benzene (1.0 ml), NMI (13.1 mg, 0.16 mmol) in benzene (0.5 ml), and Zr(OtBu)4 (15.3 mg, 0.04 mmol) in benzene (0.5 ml) at room temperature. The mixture was stirred and heated at 80°C for 2 h. After removal of the solvent under reduced pressure (0.2 mmHg) at 50°C for 1 h, the (R)-3-(m-CF3C6H4)-ZrMS catalyst was formed.
Preparation of (R)-3-Br-ZrMS. To a solution of Zr(OtBu)4 (15.3 mg, 0.04 mmol) in THF (0.5 ml) was added (R)-3,3′-dibromo-BINOL (3,3′-Br2BINOL, 17.8 mg, 0.04 mmol) in THF (1.0 ml) at room temperature, and the mixture was stirred for 30 min at the same temperature. H2O (1.4 mg, 0.08 mmol) then was added, and after the whole was stirred for an additional 30 min, MS 3A (240 mg) was added to the mixture. After stirring for 5 min, the solvent was removed under reduced pressure (0.2 mmHg) at room temperature for 1 h to form the (R)-3-Br-ZrMS catalyst.
Preparation of (R)-3-I-ZrMS. To a suspension of (R)-3,3′-diiodo-BINOL [(R)-3,3′-I2BINOL, 645 mg, 1.2 mmol] in toluene (9 ml) was added zirconium propoxide propanol complex [Zr(OPr)4-PrOH, 1.0 mmol] in toluene (11 ml) at room temperature, and the whole was stirred for 3 h at the same temperature. MS 5A (2.5g), which contained 10% (wt/wt) H2O, then was added, and the mixture was stirred for 5 min. After removal of the solvents under reduced pressure (0.2 mmHg) at room temperature for 1 h, the (R)-3-I-ZrMS catalyst was formed.
A Typical Experimental Procedure for the Enantioselective Mannich-Type Reactions Using (R)-6-C2F5-ZrMS. A typical experimental procedure is described for the reaction of aldimine 1a with ketene silyl acetal 2a. To a suspension of the (R)-6-C2F5-ZrMS catalyst (0.02 mmol) in CH2Cl2 (0.3 ml) was added NMI (0.04 mmol) in CH2Cl2 (0.2 ml) at room temperature. The mixture was stirred for 0.5 h at the same temperature and then cooled to –45°C. Dichloromethane solutions (1.0 ml) of 1a (0.2 mmol) and 2a (0.24 mmol) were added successively. The mixture was stirred for 18 h, and saturated aqueous NaHCO3 then was added to quench the reaction. The aqueous layer was extracted with CH2Cl2, and the crude adduct was treated with THF/1 M HCl (10:1) at 0°C for 30 min. After usual work-up, the crude product was purified by preparative TLC on silica gel to give the desired Mannich adduct. The optical purity was determined by HPLC analysis using a chiral column.
A Typical Experimental Procedure for the Enantioselective Mukaiyama Aldol Reactions Using (R)-3-I-ZrMS. A typical experimental procedure is described for the reaction of benzaldehyde (5a) with 2f. To a suspension of the (R)-3-I-ZrMS catalyst (74.5 mg, 5 mol %) in toluene (0.9 ml) was added PrOH (19.2 mg, 0.32 mmol) in toluene (0.3 ml) at room temperature, and the mixture was stirred for 1 h at the same temperature. After being cooled to 0°C, 5a (42.5 mg, 0.4 mmol) in toluene (0.4 ml) and 2f (107 mg, 0.48 mmol) in toluene (0.4 ml) were added successively, and the whole was stirred for 18 h at the same temperature. The reaction was quenched with saturated aqueous NaHCO3, and CH2Cl2 was added. The organic layer was separated, and the aqueous layer was extracted with CH2Cl2. After usual work-up, the crude mixture was purified by preparative TLC on silica gel to afford the desired aldol adduct. The diastereomer ratio was determined by 1H NMR analysis, and the optical purity was determined by HPLC analysis using a chiral column after acetylation.
Results and Discussion
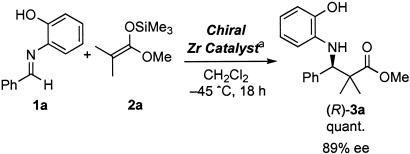
Mannich-Type Reactions. Asymmetric Mannich-type reactions of imines with enolate components are one of the most important carbon–carbon bond-forming reactions in organic synthesis (12). The reactions open useful ways to chiral β-amino ketones or esters (Mannich bases), which are versatile chiral building blocks for the synthesis of many nitrogen-containing, biologically important compounds including β-amino acids, β-lactams, etc. Compared with asymmetric Mannich-type reactions using stoichiometric amounts of chiral sources (13–16), little is known concerning catalytic asymmetric versions. In 1997, we reported an example of truly catalytic enantioselective Mannich-type reactions of imines with silicon enolates by using a zirconium catalyst prepared from zirconium (I V) tert-butoxide [Zr(OtBu)4], 2 eq of (R)-6,6′-dibromo-BINOL [(R)-6,6′-Br2BINOL], and NMI [ref. 17; for recent examples of catalytic asymmetric Mannich-type reactions, see refs. 5 (and references therein) and 18]. After that, several modifications have been made to improve catalytic activity of the chiral Zr catalysts (9).
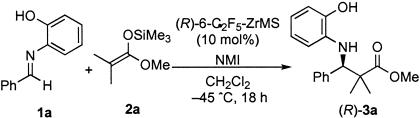
In this work, we first investigated stabilization of a zirconium catalyst prepared from Zr(OtBu)4, (R)-6,6′-bis(pentafluoroethyl)-BINOL [(R)-6,6′-(C2F5)2BINOL], and NMI, which was effective for an asymmetric Mannich-type reaction (Scheme 1 and Fig. 1) (9). We assumed that a Lewis acidic site of the chiral zirconium catalyst might interact with Lewis basic sites of MS under equilibrium conditions. The zirconium–MS-combined catalyst (ZrMS) was prepared by mixing a Zr catalyst and powdered, well dried MS and removing the solvent and drying. {A detailed procedure to form the ZrMS catalyst of Zr–(R)-6,6′-(C2F5)2BINOL complex [(R)-6-C2F5-ZrMS] is shown in Experimental Procedures.} As a model, the Mannich-type reaction of imine 1a prepared from benzaldehyde and 2-aminophenol with ketene silyl acetal 2a derived from methyl isobutyrate was selected, and the reaction was performed in the presence of 10 mol % of the (R)-6-C2F5-ZrMS catalyst. When the catalyst prepared in the absence of MS 5A was used, the desired Mannich-type adduct was obtained quantitatively with 82% enantiomeric excess (ee) (Table 1, entry 1). It is noted that the enantioselectivity was slightly decreased compared with that using the catalyst prepared in situ (Scheme 1). Because this catalyst was stable for heating but was unstable when treated in air and moisture, some additional treatments after preparation might cause a slight decrease of the selectivity. On the other hand, when the (R)-6-C2F5-ZrMS catalyst was used, the reaction proceeded in high yield, albeit with almost no selectivity (Table 1, entry 2). However, addition of NMI to the (R)-6-C2F5-ZrMS catalyst increased the enantioselectivity significantly (Table 1, entries 2–4), and the best results were obtained when 20 mol % of NMI was used (Table 1, entry 3). The yield and enantioselectivity were comparable with those obtained by using the catalyst prepared in situ (Scheme 1). In addition, the (R)-6-C2F5-ZrMS catalyst was found to be remarkably stable to air and moisture; it was stable at least 13 weeks in air at room temperature (Table 2). It should be noted that a significant decrease of the yield and selectivity was observed when a zirconium catalyst, which was prepared from Zr(OtBu)4, (R)-6,6′-(C2F5)2BINOL, and NMI in situ, was used after 1 day of storage in air at room temperature, presumably because of decomposition of the catalyst.
Scheme 1.
Asymmetric Mannich-type reaction using Zr–6,6′-(C2F5)2BINOL. *, The Zr catalyst was prepared from Zr(OtBu)4 (10 mol %), (R)-6,6′-(C2F6)2BINOL (20 mol %), and NMI (20 mol %).
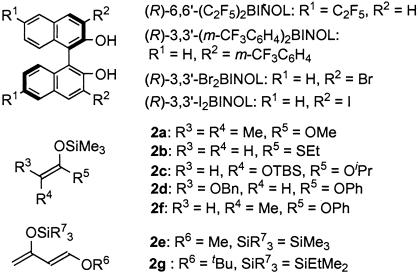
Fig. 1.
BINOLs, silicon enolates, and Danishefsky's dienes.
Table 1.
Quant., quantitative.
The catalyst used was prepared without MS 5A.
ee of (S)-3a.
Table 2. 6-C2F5-ZrMS as a storable chiral Lewis acid.
| Storage time, week | 0 | 2 | 5 | 13 |
|---|---|---|---|---|
| Yield, % | Quant. | Quant. | 98 | 99 |
| ee, % | 90 | 90 | 90 | 90 |
The results of the reactions of 1a with 2a using (R)-6-C2F5-ZrMS (10 mol %) and NMI (20 mol %). (R)-3a was obtained in all cases. Quant., quantitative.
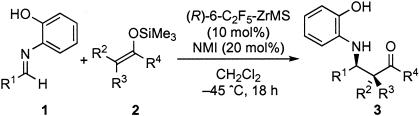
Other substrates (imines and silicon enolates) then were tested by using the (R)-6-C2F5-ZrMS catalyst, and the results are shown in Table 3. Imine 1b and 1c derived from other aromatic aldehydes reacted with the ketene silyl acetal smoothly to afford the desired Mannich-type adducts in high yields with high enantioselectivities (Table 3, entries 1–3). Not only ketene silyl acetal 2a derived from methyl isobutyrate but also the ketene silyl acetals from S-ethyl ethanethioate (2b) or α-oxygenated esters (2c, 2d) worked well, and the reactions proceeded with high enantioselectivities. In the reactions of 2c and 2d, whereas the syn-adduct was obtained by using α-tert-butyldimethylsilyloxy enolate 2c, the anti-adduct was produced by using α-BnO enolate 2d, both in high diastereo- and enantioselectivities (19). These yields and selectivities were almost comparable with those obtained by using a chiral zirconium catalyst prepared in situ in dichloromethane under an argon atmosphere (Table 3, in parentheses).
Table 3.
| Entry | R1 | Si enolate | Yield, %* | ee, %* |
|---|---|---|---|---|
| 1 | Ph (1a) | 2a | Quant. (Quant.) | 90 (89) |
| 2 | 1-Naph (1b) | 2a | 79 (Quant.) | 90 (89) |
| 3 | p-ClC6H4 (1c) | 2a | 96 (Quant.) | 85 (88) |
| 4 | Ph (1a) | 2b | 89 (99) | 90 (89) |
| 5 | 1-Naph (1b) | 2b | 93 (Quant.) | 90 (88) |
| 6 | p-ClC6H4 (1c) | 2b | 91 (98) | 94 (94) |
| 7 | 2-Furyl (1d) | 2b | 92 (96) | 89 (89) |
| 8† | i-C4H9 (1e) | 2b | 60 (61) | 92 (92) |
| 9‡ | Ph (1a) | 2c§ | 92 (80) 97/3 (92/8)** | 96 (90)∥ |
| 10 | Ph (1a) | 2d¶ | 70 (74) 8/92 (5/95)** | 89 (93)∥ |
Quant., quantitative.
Yields and ee values of the Mannich-type reactions using the chiral zirconium catalyst prepared in situ from Zr(OtBu)4, (R)-6,6′-(C2F5)2BINOL, and NMI in dichloromethane are shown in parentheses.
The imine was prepared from isovaleraldehyde and 2-amino-m-cresol in situ.
1,2-Dimethylimidazole was used instead of NMI, and toluene was used as a solvent at -78°C.
Entgegen/Zusammen (E/Z) = >99/<1.
Entgegen/Zusammen (E/Z) = <1/>99.
Major diastereomere.
syn/anti.
For asymmetric Mannich-type reactions, some other excellent chiral zirconium catalysts with (R)-3,3′-(m-CF3C6H4)2BINOL and (R)-3,3′-Br2BINOL were developed (10, 20, 21). However, although these catalysts showed high activity when prepared in situ just before use, they are unstable in air and moisture and cannot be treated in air and cannot be stored for extended periods. We then decided to prepare ZrMS based on these catalysts to confirm generality of the idea, that is, stabilization of Lewis acid catalysts combining with MS.
Indeed, a complex prepared from Zr(OtBu)4, (R)-3,3′-(m-CF3C6H4)2BINOL, and NMI (10) and a complex prepared from Zr(OtBu)4, (R)-3,3′-Br2BINOL, and water (21) were tested. These two catalyst systems already have been reported to be effective in asymmetric Mannich-type reactions, and both catalyst systems were reported to require MS 3A in the reaction systems to realize high enantioselectivity. Therefore, ZrMS formations were conducted by using MS 3A. First, the (R)-3-m-CF3C6H4-ZrMS catalyst, which was prepared according to a similar procedure (see Experimental Procedures), was applied to asymmetric Mannich-type reactions (Table 4) (10, 20). In the presence of the (R)-3-m-CF3C6H4-ZrMS catalyst (10 mol %) and NMI (20 mol %), the reactions of the aromatic imines with 2a or 2b proceeded smoothly to afford the desired adducts in high yields with high enantioselectivities (Table 4). It is noted that the ZrMS catalyst can be stored for at least 17 days without loss of activity.
Table 4. Asymmetric Mannich-type reactions using 3-m-CF3C6H4-ZrMS.
| Entry | Imine | Si enolate | Yield, % | ee, %* |
|---|---|---|---|---|
| 1 | 1a | 2a | 80 (84)† | 93 (92)† |
| 2 | 1b | 2a | 77 | 88 |
| 3 | 1c | 2a | 74 | 93 |
| 4 | 1a | 2b | 76 | 89 |
| 5 | 1b | 2b | 60 | 78 |
| 6‡ | 1c | 2b | 73 | 83 |
(R)-3-m-CF3C6H4-ZrMS (10 mol %) and NMI (30 mol %) were used, and the Mannich-type reactions were performed in toluene at 0°C for 18 h.
In all cases, the absolute configuration of the products was opposite to that obtained by using (R)-6-C2F5-ZrMS.
After 17 days.
At 50°C.
Next we investigated stabilization of a chiral zirconium catalyst prepared from Zr(OtBu)4, (R)-3,3′-Br2BINOL, and water (21). It was reported that the catalyst system required not NMI but propanol (PrOH) as an additive in asymmetric Mannich-type reactions. The corresponding (R)-3-Br-ZrMS catalyst was prepared by a procedure shown in Experimental Procedures. The Mannich-type reactions using this catalyst system (10 mol %) and PrOH (30 mol %) then were conducted, and the results are summarized in Table 5. Imines 1a–1c reacted with 2a and 2b smoothly in THF, and the desired Mannich-type adducts were obtained in high yields with high enantioselectivity. The (R)-3-Br-ZrMS catalyst also was found to be stable for at least 1 month, and no loss of activity was observed when using the stored catalyst.
Table 5. Asymmetric Mannich-type reactions using 3-Br-ZrMS.
| Entry | Imine | Si enolate | Yield, % | ee, %* |
|---|---|---|---|---|
| 1 | 1a | 2a | 93 (92)† | 95 (95)† |
| 2 | 1b | 2a | 98 | 94 |
| 3 | 1c | 2a | Quant. | 91 |
| 4 | 1a | 2b | 92 | 77 |
| 5 | 1b | 2b | 67 | 84 |
| 6 | 1c | 2b | 78 | 65 |
(R)-3-Br-ZrMS (10 mol %) and PrOH (30 mol %) were used, and the Mannich-type reactions were performed in THF at -45°C for 18 h. Quant., quantitative.
In all cases, the absolute configuration of the products was the same as that obtained by using (R)-6-C2F5-ZrMS.
After 31 days.
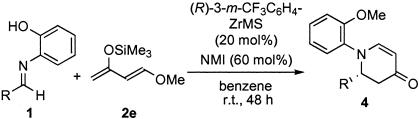
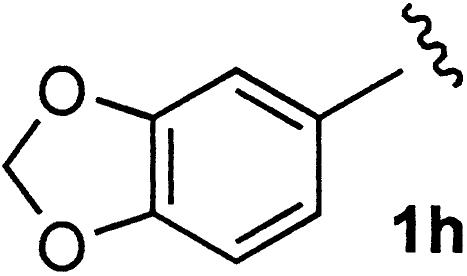
aza Diels–Alder Reactions. The (R)-3-m-CF3C6H4-ZrMS catalyst also worked well in asymmetric aza Diels–Alder reactions, which are one of the most effective methods for the preparation of optically active 2,3-dihydro-4-pyridone structure (refs. 10, 20, and 22; for other examples see ref. 23). It already has been reported that chiral zirconium complexes prepared from Zr(OtBu)4, 3,3′-diaryl-BINOL, and NMI were effective for aza Diels–Alder reactions of imines with Danishefsky's dienes. In the presence of the (R)-3-m-CF3C6H4-ZrMS catalyst and NMI, imines reacted with Danishefsky's diene 2e to afford the desired pyridone derivatives in good yields with high enantioselectivities (Table 6). It is noted that higher selectivity was obtained by using the ZrMS compared with that of previous methods.
Table 6.
| Entry | Imine; R | Yield, % | ee, % |
|---|---|---|---|
| 1 | 1a | 72 | 88 |
| 2 | 1b | 53 | 96 |
| 3 | o-MeC6H4(1f) | 72 | 97 |
| 4 | 2-Naph(1g) | 47 | 95 |
| 5 | 1h | 64 | 97 |
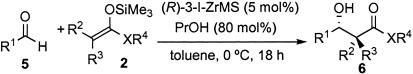
Aldol Reactions. Catalytic asymmetric aldol reactions are powerful tools for the preparation of optically active β-hydroxy carbonyl compounds and are often used successfully in total synthesis of optically active natural products (for recent reviews see refs. 1, 2, and 24–26). Recently we developed highly anti-selective asymmetric aldol reactions of silicon enolates with aldehydes by using a chiral zirconium catalyst that was prepared from Zr(OtBu)4, (R)-3,3′-I2BINOL, primary propanol (PrOH), and a small amount of H2O in situ (27). Although this catalyst has attained a high level of enantioselectivity with a wide scope of substrates, the catalyst was sensitive to water and even moisture in air. We then investigated generation of an air-stable and storable catalyst for practical and highly stereoselective asymmetric aldol reactions.
In this study, we also found that a combination of a chiral zirconium catalyst and MS was important to realize storage of the catalyst for extended periods without loss of activity. The zirconium catalyst with MS [(R)-3-I-ZrMS] was initially prepared according to the following procedure. Zr(OtBu)4, 3,3′-I2BINOL, PrOH, and H2O were combined in toluene at room temperature for 3 h, and then MS (2.5g/mmol) was added, and the mixture was stirred for 5 min. After removal of the solvents under reduced pressure at room temperature for 1 h, the (R)-3-I-ZrMS catalyst was formed.
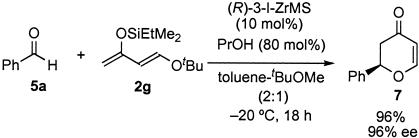
In the first trial, we used well dried MS 5A as a support to prepare the (R)-3-I-ZrMS catalyst. The aldol reaction of benzaldehyde (5a) with ketene silyl acetal (2f) was carried out by using 5 mol % of the (R)-3-I-ZrMS catalyst in toluene at 0°C in the presence of PrOH (80 mol %). The reaction proceeded smoothly to afford the desired product in 74% yield with high diastereo- and enantioselectivities (syn/anti = 9/91, 85% ee). The selectivities were improved by adding H2O (10 mol %) (96% yield, syn/anti = 9/91, 93% ee). We then used the (R)-3-I-ZrMS catalyst prepared from wet MS 5A, which contained 10% (wt/wt) H2O, and it was found that additional H2O was not needed in any catalyst-preparation steps to obtain high yield and selectivities (quantitative yield, syn/anti = 5/95, 99% ee). We also investigated the effect of MS 3A and MS 4A; however, a slight decrease of the enantioselectivity was observed (MS 3A: syn/anti = 7/93, 91% ee; MS 4A: syn/anti = 7/93, 96% ee). The result obtained by using (R)-3-I-ZrMS was almost comparable with that of the reaction using the zirconium catalyst prepared in situ. It is noteworthy that this (R)-3-I-ZrMS catalyst was remarkably stable in air and moisture and that the catalyst could be stored at least for 13 weeks at room temperature without loss of reactivity and selectivity (Table 7). (R)-3-I-ZrMS catalyse was also prepared from zirconium propoxide propanol complex [Zr(OnPr)·PrOH].
Table 7. 3-I-ZrMS as a storable chiral Lewis acid.
| Storage time, week | 0 | 2 | 6 | 13 |
|---|---|---|---|---|
| Yield, % | Quant. | Quant. | Quant. | Quant. |
| syn/anti | 5/95 | 5/95 | 5/95 | 5/95 |
| ee, % (anti) | 99 | 99 | 99 | 99 |
The results of the reaction of 5a with 2f using (R)-3-I-ZrMS (10 mol %) and PrOH (80 mol %). Quant., quantitative.
The (R)-3-I-ZrMS catalyst was applied successfully to asymmetric aldol reactions of various substrates (aldehydes and silicon enolates), and the results are summarized in Table 8. In the reactions of benzaldehyde (5a) with other silicon enolates (2a and 2b), the (R)-3-I-ZrMS catalyst worked well, and excellent yields and enantioselectivities were obtained (Table 8, entries 1–3). In the reactions of aromatic aldehydes (5b and 5c) with the ketene silyl acetal 2f, anti-aldol adducts were obtained with high diastereo- and enantioselectivities (Table 8, entries 4–6). In the reactions using cinnamaldehyde (5d) and 3-phenylpropionaldehyde (5e), a slight decrease of the yield and selectivity was observed (Table 8, entries 7 and 8). It is noted that a high level of stereocontrol was achieved in the reactions of various aldehydes and that anti-aldol adducts were obtained with high diastereo- and enantioselectivities.
Table 8.
| Entry | 1; R1 | Si enolate | Yield, % | syn/anti | ee, % (anti) |
|---|---|---|---|---|---|
| 1 | Ph (5a) | 2a | 92 | — | 94 |
| 2 | Ph (5a) | 2b | Quant. | — | 92 |
| 3* | Ph (5a) | 2b | 97 | — | 94 |
| 4 | Ph (5a) | 2f | Quant. | 5/95 | 99 |
| 5 | p-MeOC6H4 (5b) | 2f | 80 | 5/95 | 94 |
| 6 | p-ClC6H4 (5c) | 2f | Quant. | 8/92 | 95 |
| 7 | PhCH=CH (5d) | 2f | 94 | 16/84 | 98 |
| 8 | PhCH2CH2 (5e) | 2f | 65 | 15/85 | 87 |
Quant., quantitative.
Catalyst (10 mol %) was used.
Hetero Diels–Alder Reaction. It was found also that the hetero Diels–Alder reaction of benzaldehyde (5a) with Danishefsky's diene 2g proceeded smoothly in the presence of the (R)-3-I-ZrMS catalyst to afford the desired product in high yield with high enantioselectivity (Scheme 2) (ref. 28; for a review see ref. 29). High levels of the yield and selectivity also were attained by using (R)-3-I-ZrMS.
Scheme 2.
Asymmetric hetero Diels–Alder reaction using 3-I-ZrMS. The obtained product was treated with trifluoroacetic acid.
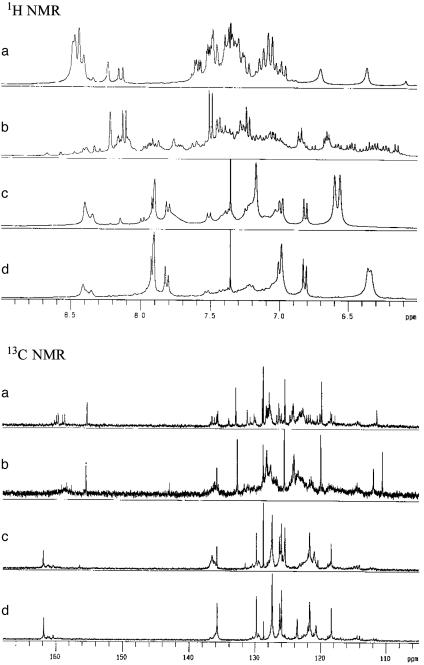
Structure of ZrMS. It is remarkable that simple treatment with powdered MS enhanced the stability of the chiral Lewis acid significantly without loss of activity. We then performed NMR experiments to clarify the structure of the ZrMS catalysts. First, the (R)-6-C2F5-ZrMS catalyst was treated with CD2Cl2 at room temperature for 30 min, and after filtration, 1H and 13C NMR spectra were measured (Fig. 2a). The spectra were complicated, and an oligomeric structure of Zr was assumed, because a similar oligomeric structure was formed by the combination of Zr(OtBu)4 and (R)-6,6′-C2F5BINOL (Fig. 2b). This oligomeric catalyst was known to give low selectivity in the Mannich-type reaction. On the other hand, rather simple spectra were recorded when ZrMS was combined with NMI in CD2Cl2 at room temperature for 30 min (Fig. 2c; after filtration). It should be noted that the spectra were similar to those of the chiral zirconium catalyst prepared from Zr(OtBu)4, (R)-6,6′-C2F5BINOL, and NMI, which was an efficient catalyst for the Mannich-type reactions of imines with silicon enolates (Fig. 2d). These results indicate that additional NMI plays an important role to regenerate the real catalyst.
Fig. 2.
NMR experiment. (a) 6-C2F5-ZrMS in CD2Cl2. (b) Zr(OtBu)4 and 2.0 eq of 6,6′-(C2F5)2BINOL in CD2Cl2. (c) 6-C2F5-ZrMS and 2.0 eq of NMI in CD2Cl2. (d) Zr(OtBu)4, 2.0 eq of 6,6′-(C2F5)2BINOL, and NMI, 2.0 eq of CD2Cl2.
Recovery and Reuse of the ZrMS Catalysts. Recovery and reuse of a chiral catalyst is a current interest in the chemistry of catalytic asymmetric synthesis. Insoluble, solid-supported catalysts often can be recovered easily and used several times. Interaction between the zirconium catalysts and MS would make easy recovery of the catalysts possible. Therefore, recovery and reuse of the ZrMS catalysts were investigated. In the presence of the (R)-6-C2F5-ZrMS catalyst (10 mol %), imine 1a was treated with ketene silyl acetal 2a in CH2Cl2 at –45°C. After 18 h, the reaction vessel was warmed to room temperature, and MS 5A was added. After all volatile solvents and materials were removed under reduced pressure (3 h), hexane was added and decantation was conducted three times. Although the desired product was isolated from the hexane solution (89% yield, 92% ee), the residue was pumped up under reduced pressure for 3 h and was used for the second run. Almost the same levels of yields and selectivities were obtained (second run: 94% yield, 91% ee; third run: 99% yield, 90% ee), although a small amount of the Zr (≈5%) was leached in each run according to this procedure.
Conclusions and Perspective
We have developed air-stable, storable, and highly selective chiral Lewis acid catalysts (the ZrMS catalysts) that were used successfully in asymmetric Mannich-type, aza Diels–Alder, aldol, and hetero Diels–Alder reactions. Procedures for the preparation of the ZrMS catalysts are very simple, and handling of the ZrMS catalysts is very easy. It has been revealed that treatment with powdered MS is a key to create these remarkable Lewis acids. The catalyst was stable for more than several weeks in air at room temperature without loss of activity. It is expected that other Lewis acid catalysts may also be stabilized to air and moisture based on a similar concept.
The use of chiral Lewis acids in air and moisture is really remarkable, because most chiral Lewis acids decomposed rapidly under the conditions. However, the ZrMS catalysts still cannot be used in water, because decomposition of the catalysts occurred in the presence of a large amount of water. From synthetic and environmental points of view, the next goal may be asymmetric catalysis in water, and for this purpose, development of chiral catalysts that work well in water is desired. It is interesting to mention that enzymes work efficiently in the presence of a large amount of water but that even they catalyze few carbon–carbon bond-forming reactions compared with other transformations.
Acknowledgments
This work was partially supported by Core Research for Evolutional Science and Technology, the Solution Oriented Research for Science and Technology program, Exploratory Research for Advanced Technology, Japan Science and Technology Agency, and a grant-in-aid for Scientific Research from the Japan Society of the Promotion of Science.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MS, molecular sieves; NMI, N-methylimidazole; BINOL, 1,1′-binaphthene-2,2′-diol; THF, tetrahydrofuran; ee, enantiomeric excess.
References
- 1.Jacobsen, E. N., Pfaltz, A. & Yamamoto, H., eds. (1999) Comprehensive Asymmetric Catalysis (Springer, Berlin), Vol. 1–3.
- 2.Ojima, I., ed. (2000) Catalytic Asymmetric Synthesis (Wiley–VCH, New York), 2nd Ed.
- 3.Yamamoto H., ed. (2000) Lewis Acid in Organic Synthesis (Wiley–VCH, 2000), Vols. 1 and 2.
- 4.Kim, Y. S., Matsunaga, S., Das, J., Sekine, A., Ohshima, T. & Shibasaki, M. (2000) J. Am. Chem. Soc. 122, 6506–6507. [Google Scholar]
- 5.Ueno, M., Ishitani, H. & Kobayashi, S. (2002) Org. Lett. 4, 3395–3397. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi, S., Saito, S., Ueno, M. & Yamashita, Y. (2003) Chem. Commun., 2016–2017. [DOI] [PubMed]
- 7.Kobayashi, S., Manabe, K., Ishitani, H. & Matsuo, J. (2002) in Science of Synthesis, Houben-Weyl Methods of Molecular Transformations, eds. Bellus, D., Ley, S. V., Noyori, R., Regitz, M., Schaumann, E., Shinkai, I., Thomas, E. J. & Trost, B. M. (Thieme, Stuttgart), Vol. 4, pp. 317–369. [Google Scholar]
- 8.Ishihara, K., Maruyama, T., Mouri, M., Gao, Q., Furuta, K. & Yamamoto, H. (1993) Bull. Chem. Soc. Jpn. 66, 3483–3491. [Google Scholar]
- 9.Ishitani, H., Ueno M. & Kobayashi, S. (2000) J. Am. Chem. Soc. 122, 8180–8186. [Google Scholar]
- 10.Kobayashi, S., Kusakabe, K. & Ishitani, H. (2000) Org. Lett. 2, 1225–1227. [DOI] [PubMed] [Google Scholar]
- 11.Cox, P. J., Wang, W. & Snieckus, V. (1992) Tetrahedron Lett. 33, 2253–2256. [Google Scholar]
- 12.Kleinman, E. F. (1991) in Comprehensive Organic Syhthesis, ed. Trost, B. M. (Pergamon, Oxford), Vol. 2, pp. 893–951. [Google Scholar]
- 13.Corey, E. J., Decicco, C. P. & Newbold, R. C. (1991) Tetrahedron Lett. 39, 5287–5290. [Google Scholar]
- 14.Ishihara, K., Miyata, M., Hattori, K., Tada, T. & Yamamoto, H. (1994) J. Am. Chem. Soc. 116, 10520–10524. [Google Scholar]
- 15.Enders, D., Ward, D., Adam, J. & Raabe, G. (1996) Angew. Chem. Int. Ed. Engl. 35, 981–984. [Google Scholar]
- 16.Risch, N. & Esser, A. (1992) Liebigs Ann. Chem., 233–237.
- 17.Ishitani, H., Ueno M. & Kobayashi, S. (1997) J. Am. Chem. Soc. 119, 7153–7154. [Google Scholar]
- 18.Taggi, A. E., Hafez, A. M. & Lectka, T. (2003) Acc. Chem. Res. 36, 10–19. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi, S., Ishitani, H. & Ueno, M. (1998) J. Am. Chem. Soc. 120, 431–432. [Google Scholar]
- 20.Kobayashi, S., Kusakabe, K., Komiyama, S. & Ishitani, H. (1999) J. Org. Chem. 64, 4220–4221. [Google Scholar]
- 21.Kobayashi, S., Ishitani, H., Yamashita, Y., Ueno, M. & Shimizu, H. (2001) Tetrahedron 57, 864–866. [Google Scholar]
- 22.Kobayashi, S., Komiyama, S. & Ishitani, H. (1999) Angew. Chem. Int. Ed. Engl. 37, 979–984. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, S. (2002) in Cycloaddition Reactions in Organic Synthesis, eds. Kobayashi, S. & Jørgensen K. A. (Wiley–VCH, Weinheim, Germany), pp. 187–207.
- 24.List, B. (2002) Tetrahedron 58, 5573–5590. [Google Scholar]
- 25.Alcaide, B. & Almendros, P. (2002) Eur. J. Org. Chem. 1595–1601.
- 26.Palomo, C., Oiarbide, M. & Garcia, J. M. (2002) Chem. Eur. J. 8, 36–44. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita, Y., Ishitani, H., Shimizu, H. & Kobayashi, S. (2002) J. Am. Chem. Soc. 124, 3292–3302. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita, Y., Saito, S., Ishitani, H. & Kobayashi, S. (2003) J. Am. Chem. Soc. 125, 3793–3798. [DOI] [PubMed] [Google Scholar]
- 29.Jørgensen, K. A. (2000) Angew. Chem. Int. Ed. Engl. 39, 3558–3588.11091406 [Google Scholar]