Abstract
Background:
The putative role of personality in cancer risk has been controversial, and the evidence remains inconclusive.
Methods:
We pooled data from six prospective cohort studies (British Household Panel Survey; Health and Retirement Study; Household, Income, and Labour Dynamics in Australia; Midlife in the United Survey; Wisconsin Longitudinal Study Graduate; and Sibling samples) for an individual-participant meta-analysis to examine whether personality traits of the Five Factor Model (extraversion, neuroticism, agreeableness, conscientiousness, and openness to experience) were associated with the incidence of cancer and cancer mortality in 42 843 cancer-free men and women at baseline (mean age 52.2 years, 55.6% women).
Results:
During an average follow-up of 5.4 years, there were 2156 incident cancer cases. In random-effects meta-analysis adjusted for age, sex, and race/ethnicity, none of the personality traits were associated with the incidence of all cancers or any of the six site-specific cancers included in the analysis (lung, colon, breast, prostate, skin, and leukaemia/lymphoma). In the three cohorts with cause-specific mortality data (421 cancer deaths among 21 835 participants), none of the personality traits were associated with cancer mortality.
Conclusions:
These data suggest that personality is not associated with increased risk of incident cancer or cancer-related mortality.
Keywords: personality, cancer, big five, meta-analysis, psychosomatic medicine
Personality refers to individual differences in cognitive styles, behavioural dispositions, and emotional responsiveness. The role of personality in predisposing to cancer has been a topic of long-running controversy. Early psychosomatic theories suggested that high extraversion and low neuroticism would increase cancer risk (Kissen and Eysenck, 1962). Anti-emotional and overly rational thinking (Butow et al, 2000; Lemogne et al, 2013), and the suppression of negative emotions (White et al, 2007) have also been hypothesised to increase cancer risk. Plausible mechanisms mediating these associations include accumulated stress responses (Eysenck, 1994) disrupting the immune and endocrine systems (Antoni and Lutgendorf, 2007) and increased chronic inflammation (Hänsel et al, 2010). Personality may also influence cancer risk indirectly via adoption of poor health behaviours, such as smoking and not participating in cancer screenings (Bogg and Roberts, 2004; Arai et al, 2009).
Recent research has found only limited evidence to support personality as a risk factor for cancer (Schapiro et al, 2001; Lillberg et al, 2002; Nakaya et al, 2003, 2010; Hansen et al, 2005; Bleiker et al, 2008; Ranchor et al, 2010). However, most of these studies have been severely limited in the assessment of personality, so the lack of associations may reflect incomplete measurement of the relevant psychological traits. The majority of studies have focused only on extraversion and neuroticism (Schapiro et al, 2001; Lillberg et al, 2002; Nakaya et al, 2003, 2010; Hansen et al, 2005) but these two traits cover only part of personality variation. Thus, these earlier personality measures have left potentially important personality differences unmeasured. In modern personality psychology, the Five Factor Model is one of the most widely accepted comprehensive frameworks delineating the main personality dimensions (John et al, 2008). In addition to extraversion and neuroticism, the model includes personality traits conscientiousness, agreeableness, and openness to experience. Low conscientiousness, that is, lack of persistence, self-discipline, and industriousness, in particular, has been associated with many health outcomes (Martin et al, 2007; Jokela et al, 2013a). With some notable exceptions (Hansen et al, 2005; Nakaya et al, 2010), many previous studies of personality and cancer have also been limited with respect to sample size and follow-up time.
The aim of the present study was to assess whether personality traits of the Five Factor Model are associated with cancer risk. To overcome some of the limitations of previous studies, we pooled data from six prospective cohort studies from the United States, United Kingdom, and Australia for an individual-participant meta-analysis. Personality was assessed with the Five Factor Model in all of the six studies. We examined the overall risk of incident cancer, cancer mortality, and six common site-specific cancers separately. On the basis of earlier studies on cancer and other chronic illnesses, we hypothesised that extraversion and neuroticism are not associated with cancer while higher conscientiousness may be related to lower cancer risk. We had no specific hypotheses for agreeableness and openness to experience.
Materials and methods
Participants
We searched the data collections of the Inter-University Consortium for Political and Social Research (ICPSR; http://www.icpsr.umich.edu/icpsrweb/ICPSR/) and the UK Data Service (http://ukdataservice.ac.uk/) to identify eligible large-scale cohort studies for which data were publicly available. We included all available prospective studies that were sufficiently large (n>1000) and had data on participant's cancer status and personality assessed at baseline using at least the 15-item questionnaire based on the Five Factor Model. Six cohort studies with individual-level data were identified: the British Household and Panel Survey (BHPS); the Household, Income, and Labour Dynamics in Australia (HILDA); the US Health and Retirement Study (HRS); the Midlife in the United States (MIDUS); and the Wisconsin Longitudinal Study graduate (WLSG) and sibling (WLSS) samples. All studies were approved by local ethics committees. Only individuals with no cancer at baseline were included, excluding 171 participants in BHPS, 394 in HILDA, 2181 in HRS, 449 in MIDUS, 142 in WLSG, and 273 in WLSS, leaving a total of 42 843 participants for analysis of cancer incidence and 21 835 participants for analysis of cancer mortality (Table 1).
Table 1. Descriptive statistics of the six cohort studies.
| BHPS | HILDA | HRS | MIDUS | WLSG | WLSS | |
|---|---|---|---|---|---|---|
| Agea |
45.6 (18.1) |
44.6 (15.9) |
66.1 (10.2) |
46.1 (12.3) |
54.1 (0.5) |
52.3 (7.0) |
| Age range |
16–98 |
17–92 |
25–104 |
20–75 |
53–56 |
33–75 |
| Sex (% female) |
54.7 (7012) |
53.6 (3393) |
60.2 (6493) |
53.0 (2290) |
54.2 (3090) |
53.1 (1530) |
| Race (% non-White) |
13.1 (1685) |
14.0 (884) |
22.9 (2469) |
8.7 (374) |
— |
— |
| Follow-up (years)a |
2.9 (0.4) |
4.0 (0.1) |
3.2 (1.0) |
9.0 (0.5) |
11.2 (0.4) |
11.2 (0.5) |
| Incident cancer cases |
1.9 (239) |
4.6 (290) |
4.7 (510) |
8.1 (350) |
9.4 (538) |
7.9 (229) |
| Cancer deaths |
— |
— |
159 |
— |
175 |
87 |
| Participants (n) | 12 820 | 6333 | 10 787 | 4319 | 5703 | 2881 |
Abbreviations: BHPS=British Household Panel Survey, HILDA=Household, Income, and Labour Dynamics in Australia, HRS=Health and Retirement Study, MIDUS=Midlife in the United States, WLSG=Wisconsin Longitudinal Study, Graduate sample, WLSS=Wisconsin Longitudinal Study, Sibling sample.
Values are means (and standard deviations).
Values percentages (and numbers of participants).
Measures
Personality was assessed using standardised questionnaire instruments, described in more detail in the Supplementary Material. Briefly, the Five Factor Model of personality used in the present study is widely recognised as the most comprehensive model of the major dimensions of personality (John et al, 2008). Extraversion reflects characteristics such as social assertiveness, sociability, and sensitivity to positive emotions; neuroticism is associated with low emotional stability, sensitivity to negative emotions, and anxiety proneness; agreeableness measures cooperativeness, altruism, and trust towards other people; conscientiousness is expressed as self-control, orderliness, and adherence to social norms; and openness to experience correlates with curiosity, broad-ranging interests, and open-mindedness. Data on diagnosed cancers were based on self-reported data, as described in more detail in Supplementary Material. Cancer mortality was recorded using ICD-9 or ICD-10 codes in the WLSG and WLSS cohorts, and with a group of cancer-related causes of death in HRS. Six site-specific cancers (lung, breast, colorectal, prostate, skin, and leukaemia/lymphoma) were included in the analysis on the basis of data availability on these cancer types across the different samples. Smoking, obesity (body mass index ⩾30, based on self-reported data on height and weight at baseline), alcohol consumption, physical activity, and education were included as additional covariates in all samples (except for alcohol consumption and physical activity in the BHPS), as these variables have been associated with some specific cancer types. In order not to lose participants due to missing data in the covariates, missing data imputation with regression was applied to all the five covariates with age, sex, and race/ethnicity as predictors. The results of the adjusted models were substantially the same without the data imputation (data not shown).
Statistical analysis
Personality traits were standardised as z-scores within each sample (mean=0, s.d.=1). Associations with cancer incidence were tested using logistic regression analysis, adjusted for sex, age, race/ethnicity (0=white, 1=non-white), and follow-up time between baseline and follow-up in months. Associations with cancer mortality were examined using Cox's proportional hazards model, adjusted for age, sex, and race/ethnicity. The five personality traits were entered simultaneously in the model to estimate their independent effects. Meta-analysis was performed using the two-stage approach in which the logistic and proportional hazard models were first fitted separately in each cohort, and the cohort-specific results were then pooled together using random-effect meta-analysis (Stewart et al, 2012). We used STATA 12.1 statistical software (StataCorp, College Station, TX, USA) to analyse the data.
Results
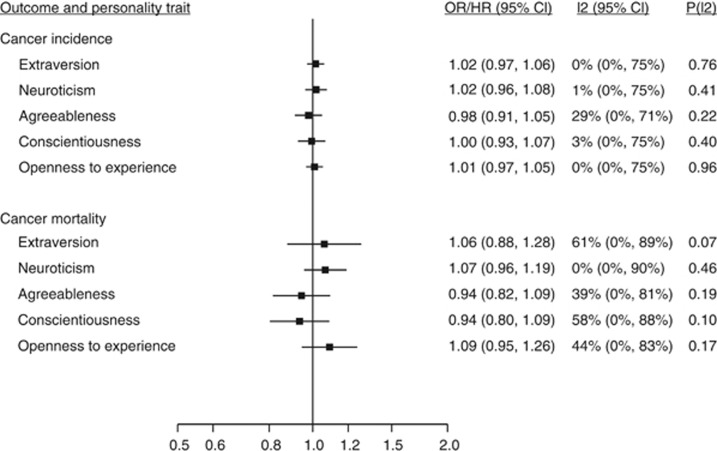
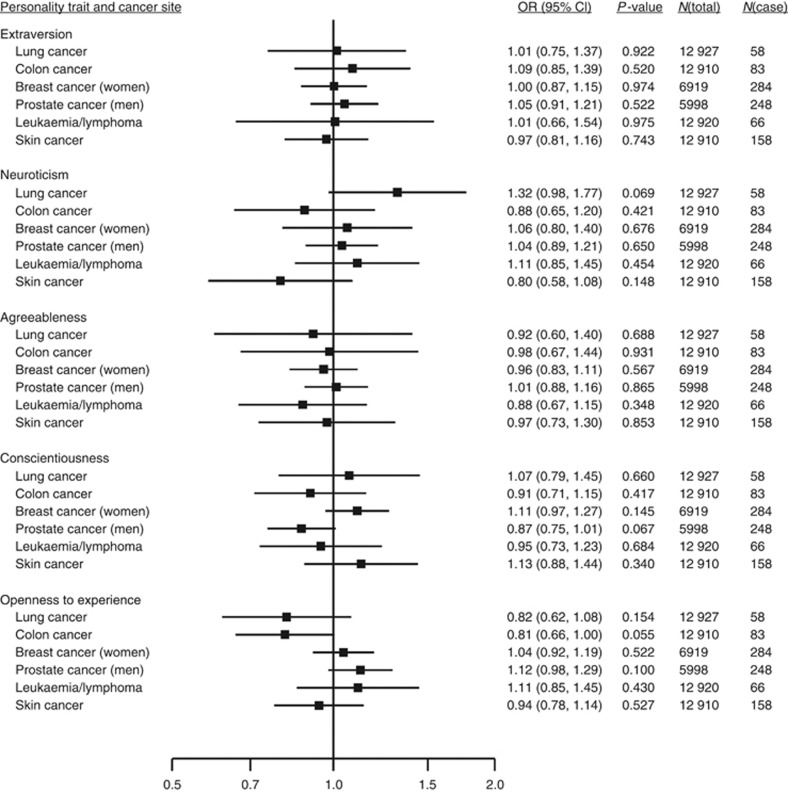
In 42 843 cancer-free participants from the six cohort studies, mean age was 52.2 years (range 16–104) and 55.6% were women. During a mean follow-up of 5.4 years, 2156 incident cancer cases were recorded. Personality traits were not associated with risk of diagnosed cancer (Figure 1) or with site-specific cancers (Figure 2). There were 421 cancer deaths among 21 835 participants in the three cohorts with available cause-specific mortality data. Again, none of the personality traits were associated with cancer mortality (Figure 1). Study-specific results are shown in Supplementary Figures 1–7.
Figure 1.
Pooled estimates for risk of cancer incidence (self-reported data) and cancer mortality associated with 1 s.d. difference in personality trait scores among individuals with no cancer at baseline. Meta-analysis of diagnosed cancer based on 2156 cancer cases in 42 843 participants of all 6 cohorts. Meta-analysis of cancer mortality based on 421 cancer deaths among 21 835 participants of the HRS, WLSG, and WLSS cohorts. I2 indicates the degree of heterogeneity in effect size across studies, and p(I2) gives the statistical significance for the heterogeneity. See Supplementary Figures 1 and 2 for study-specific results.
Figure 2.
Pooled estimates from meta-analyses of associations between personality traits and risk of diagnosed site-specific cancers among participants with no diagnosed cancer of any type at baseline. Total n=12 927 (5998 men and 6919 women) from MIDUS, WLSG and WLSS cohorts. For WLSG and WLSS, site-specific cancer deaths were also included as diagnosed cancers. See Supplementary Figures 3–7 for study-specific results.
The results for cancer mortality remained unchanged when excluding cancer deaths occurring within the first 2 years of personality assessment (Supplementary Figure 8). The null findings were replicated when the associations were adjusted for smoking, obesity, alcohol consumption, physical activity, and education (Supplementary Figures 9 and 10), and when only the cohort studies with a follow-up period of longer than 5 years (i.e., MIDUS, WLSG, and WLSS) were included in the analyses (Supplementary Figure 11). Finally, we examined all the possible two-way interactions between pairs of personality traits with all the six samples included in a single data set, adjusted for sample. Of the 10 possible trait combinations, two interaction effects were statistically significant (Supplementary Table 1), including an interaction between extraversion and agreeableness (P=0.04), and between extraversion and openness to experience (P=0.04). However, these interaction effects were not observed in any of the individual cohort studies (Supplementary Table 2) and these study-specific non-significant interaction effects did not demonstrate any consistent patterns across studies, suggesting that the two interaction effects in the pooled data were spurious rather than robust, which is why they are not described in detail here.
Discussion
In an individual-participant meta-analysis of six prospective cohort studies, none of the personality traits of the Five Factor Model were associated with overall risk of cancer incidence or with six site-specific cancers, including lung, breast, colorectal, prostate and skin cancers, or leukaemia/lymphoma. Analysis of cancer mortality in three cohorts replicated this null finding. These findings indicate that personality is not a risk factor for cancer.
The strengths of the present study include a large sample size pooled from six samples; assessment of personality using the standardised and widely accepted Five Factor Model that captures the most important dimensions of personality differences between individuals; prospective study design of cancer incidence and mortality among individuals with no diagnosed cancer at baseline; and the analysis of site-specific cancers. The main methodological limitation was the reliance on self-reported data in assessing diagnosed cancers. Self-reports are likely to underestimate the true prevalence of cancers, as people are not completely aware of their medical conditions and may not be able to correctly report all their earlier diagnoses (Nord et al, 2003). Moreover, individuals diagnosed of cancer may be more likely to drop out of longitudinal follow-up studies (Goldberg et al, 2001). Reporting bias could introduce error in the present results if personality was associated with decreased cancer risk but higher awareness of cancer, or vice versa. For example, individuals with higher conscientiousness might have lower cancer risk because of their healthier lifestyles, but this inverse association could be offset in self-reports by higher adherence to screenings and better knowledge of medical conditions. Currently, there are no enough data on personality and participation in cancer screenings to estimate the plausibility of this scenario. However, the null findings for all personality traits were very similar for cancer mortality for which data were derived from the US mortality registry. The mortality data were not subjected to the same selection bias (i.e., no selective attrition) and reporting bias (i.e., cause of death determined objectively) as self-reported cancer incidence. Therefore, the overall null findings of the present study were not limited to self-reported cancer incidence but were replicated with objective cancer-mortality data.
Our findings are in agreement with previous studies reporting no associations between personality traits of extraversion and neuroticism with cancer (Nakaya et al, 2010; Ranchor et al, 2010). Our current study extends these studies by investigating all traits of the more comprehensive Five Factor Model of personality. With respect to the five personality traits, it is particularly notable that there was no association between conscientiousness and cancer risk. Low conscientiousness has been consistently associated with all-cause mortality (Jokela et al, 2013a) and multiple chronic diseases, including obesity (Jokela et al, 2013b), diabetes (Jokela et al, 2014a), and coronary heart disease and stroke (Jokela et al, 2014b), among other health outcomes (Martin et al, 2007). This difference between cancer and other chronic diseases corresponds to findings on many other psychosocial risk factors, such as socioeconomic status (Liu et al, 2001), intelligence (Jokela et al, 2011), depression (Lemogne et al, 2013), and job-related stress (Heikkilä et al, 2013) that have been robustly associated with various chronic diseases but not with cancer.
In the Miyagi cohort of 30 277 Japanese participants (Nakaya et al, 2003), higher neuroticism was related to cancers diagnosed within 3 years of personality assessment but not after that, suggesting that reverse causality might confound associations between personality and cancer. In the current analysis of cancer mortality, the results remained unchanged when cancer deaths occurring within 2 years of personality assessment were excluded from the analysis, indicating that reverse causality between early cancer onset and personality change was unlikely to mask any associations. Given the adverse psychological impact of cancer (Reich, 2008), reverse causality should be considered carefully in studies of psychosocial risk factors and cancer (Lemogne et al, 2013).
In sum, together with earlier studies of extraversion and neuroticism (Schapiro et al, 2001; Lillberg et al, 2002; Nakaya et al, 2003, 2010; Hansen et al, 2005), this individual-participant meta-analysis based on the full Five Factor Model assessment of personality provides strong evidence to suggest that people's personality dispositions do not influence their risk of developing cancer.
Acknowledgments
This research was supported by the Academy of Finland (grant numbers: 124322, 124271, 132944, and 265977); the BUPA Foundation, UK; Medical Research Council (MRC, K013351); the US National Institutes of Health (R01HL036310; R01AG034454), the Finnish Cultural Foundation (CH), and the Finnish Work Environment Fund. The Centre for Cognitive Ageing and Cognitive Epidemiology is supported by the Biotechnology and Biological Sciences Research Council, the Engineering and Physical Sciences Research Council, the Economic and Social Research Council, the Medical Research Council, and the University of Edinburgh as part of the cross-council Lifelong Health and Wellbeing initiative. GDB is a Wellcome Trust Fellow. MK is an Economic and Social Research Council Professor.
Author contributions
MJ carried out statistical analysis; all authors contributed to the design of the study and drafting the manuscript. The corresponding author had full access to all individual- or study-level data in this meta-analysis and acts as the guarantor.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Antoni MH, Lutgendorf S. Psychosocial factors and disease progression in cancer. Curr Dir Psychol Sci. 2007;16:42–46. [Google Scholar]
- Arai S, Nakaya N, Kakizaki M, Ohmori-Matsuda K, Shimazu T, Kuriyama S, Fukao A, Tsuji I. Personality and gastric cancer screening attendance: a cross-sectional analysis from the Miyagi Cohort Study. J Epidemiol. 2009;19:34–40. doi: 10.2188/jea.JE20080024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleiker EMA, JHCL Hendriks, Otten JDM, Verbeek ALM, van der Ploeg HM. Personality factors and breast cancer risk: a 13-year follow-up. J Natl Cancer Inst. 2008;100:213–218. doi: 10.1093/jnci/djm280. [DOI] [PubMed] [Google Scholar]
- Bogg T, Roberts BW. Conscientiousness and health-related behaviors: a meta-analysis of the leading behavioral contributors to mortality. Psychol Bull. 2004;130:887–919. doi: 10.1037/0033-2909.130.6.887. [DOI] [PubMed] [Google Scholar]
- Butow PN, Hiller JE, Price MA, Thackway SV, Kricker A, Tennant CC. Epidemiological evidence for a relationship between life events, coping style, and personality factors in the development of breast cancer. J Psychosom Res. 2000;49:169–181. doi: 10.1016/s0022-3999(00)00156-2. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. Cancer, personality and stress: prediction and prevention. Adv Behav Res Ther. 1994;16:167–215. [Google Scholar]
- Goldberg M, Chastang JF, Leclerc A, Zins M, Bonenfant S, Bugel I, Kaniewski N, Schmaus A, Niedhammer I, Piciotti M, Chevalier A, Godard C, Imbernon E. Socioeconomic, demographic, occupational, and health factors associated with participation in a long-term epidemiologic survey: a prospective study of the French GAZEL cohort and its target population. Am J Epidemiol. 2001;154:373–384. doi: 10.1093/aje/154.4.373. [DOI] [PubMed] [Google Scholar]
- Hansen PE, Floderus B, Frederiksen K, Johansen C. Personality traits, health behavior, and risk for cancer. Cancer. 2005;103:1082–1091. doi: 10.1002/cncr.20871. [DOI] [PubMed] [Google Scholar]
- Heikkilä K, Nyberg ST, Theorell T, Fransson EI, Alfredsson L, Bjorner JB, Bonenfant S, Borritz M, Bouillon K, Burr H, Dragano N, Geuskens GA, Goldberg M, Hamer M, Hooftman WE, Houtman IL, Joensuu M, Knutsson A, Koskenvuo M, Koskinen A, Kouvonen A, Madsen IE, Magnusson Hanson LL, Marmot MG, Nielsen ML, Nordin M, Oksanen T, Pentti J, Salo P, Rugulies R, Steptoe A, Suominen S, Vahtera J, Virtanen M, Väänänen A, Westerholm P, Westerlund H, Zins M, Ferrie JE, Singh-Manoux A, Batty GD, Kivimäki M, IPD-Work Consortium Work stress and risk of cancer: meta-analysis of 5700 incident cancer events in 116 000 European men and women. BMJ. 2013;346:f165. doi: 10.1136/bmj.f165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänsel A, Hong S, Cámara RJA, Känel von R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev. 2010;35:115–121. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- John OP, Naumann LP, Soto CJ.2008Paradigm shift to the integrative big-five trait taxonomy: History, measurement, and conceptual issues Handbook of Personality: Theory and ResearchJohn OP, Robins RW, Pervin LA (eds) pp114–158.Guilford Press: New York, NY [Google Scholar]
- Jokela M, Batty GD, Deary IJ, Silventoinen K, Kivimäki M. Sibling analysis of adolescent intelligence and chronic diseases in older adulthood. Ann Epidemiol. 2011;21:489–496. doi: 10.1016/j.annepidem.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Jokela M, Batty GD, Nyberg ST, Virtanen M, Nabi H, Singh-Manoux A, Kivimäki M. Personality and all-cause mortality: individual-participant meta-analysis of 3947 deaths in 76 150 adults. Am J Epidemiol. 2013;178:667–675. doi: 10.1093/aje/kwt170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela M, Elovainio M, Nyberg ST, Tabák AG, Hintsa T, Batty GD, Kivimäki M.2014Personality and risk of diabetes in adults: pooled analysis of 5 cohort studies Health Psychol(in press) doi: 10.1037/hea0000003 [DOI] [PubMed]
- Jokela M, Hintsanen M, Hakulinen C, Batty GD, Nabi H, Singh-Manoux A, Kivimäki M. Association of personality with the development and persistence of obesity: a meta-analysis based on individual-participant data. Obes Rev. 2013;14:315–323. doi: 10.1111/obr.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela M, Pulkki-Råback L, Elovainio M, Kivimäki M.2014Personality traits as risk factors for stroke and coronary heart disease mortality: pooled analysis of three cohort studies J Behav Meddoi: 10.1007/s10865-013-9548-z [DOI] [PubMed]
- Kissen DM, Eysenck HJ. Personality in male lung cancer patients. J Psychosom Res. 1962;6:123–127. doi: 10.1016/0022-3999(62)90062-4. [DOI] [PubMed] [Google Scholar]
- Lemogne C, Consoli SM, Geoffroy-Perez B, Coeuret-Pellicer M, Nabi H, Melchior M, Limosin F, Zins M, Ducimetiere P, Goldberg M, Cordier S. Personality and the risk of cancer: a 16-year follow-up study of the GAZEL cohort. Psychosom Med. 2013;75:262–271. doi: 10.1097/PSY.0b013e31828b5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillberg K, Verkasalo PK, Kaprio J, Helenius H, Koskenvuo M. Personality characteristics and the risk of breast cancer: a prospective cohort study. Int J Cancer. 2002;100:361–366. doi: 10.1002/ijc.10484. [DOI] [PubMed] [Google Scholar]
- Liu L, Cozen W, Bernstein L, Ross RK, Deapen D. Changing relationship between socioeconomic status and prostate cancer incidence. J Natl Cancer Inst. 2001;93:705–709. doi: 10.1093/jnci/93.9.705. [DOI] [PubMed] [Google Scholar]
- Martin LR, Friedman HS, Schwartz JE. Personality and mortality risk across the life span: The importance of conscientiousness as a biopsychosocial attribute. Health Psychol. 2007;26:428–436. doi: 10.1037/0278-6133.26.4.428. [DOI] [PubMed] [Google Scholar]
- Nakaya N, Bidstrup PE, Saito-Nakaya K, Frederiksen K, Koskenvuo M, Pukkala E, Kaprio J, Floderus B, Uchitomi Y, Johansen C. Personality traits and cancer risk and survival based on Finnish and Swedish registry data. Am J Epidemiol. 2010;172:377–385. doi: 10.1093/aje/kwq046. [DOI] [PubMed] [Google Scholar]
- Nakaya N, Tsubono Y, Hosokawa T, Nishino Y, Ohkubo T, Hozawa A, Shibuya D, Fukudo S, Fukao A, Tsuji I, Hisamichi S. Personality and the risk of cancer. J Natl Cancer Inst. 2003;95:799–805. doi: 10.1093/jnci/95.11.799. [DOI] [PubMed] [Google Scholar]
- Nord C, Mykletun A, Fosså SD. Cancer patients' awareness about their diagnosis: a population-based study. J Public Health Med. 2003;25:313–317. doi: 10.1093/pubmed/fdg076. [DOI] [PubMed] [Google Scholar]
- Ranchor AV, Sanderman R, Coyne JC. Invited commentary: personality as a causal factor in cancer risk and mortality—time to retire a hypothesis. Am J Epidemiol. 2010;172:386–388. doi: 10.1093/aje/kwq210. [DOI] [PubMed] [Google Scholar]
- Reich M. Depression and cancer: recent data on clinical issues, research challenges and treatment approaches. Curr Opin Oncol. 2008;20:353–359. doi: 10.1097/CCO.0b013e3282fc734b. [DOI] [PubMed] [Google Scholar]
- Schapiro IR, Ross-Petersen L, Sælan H, Garde K, Olsen J, Johansen C. Extroversion and neuroticism and the associated risk of cancer: a Danish cohort study. Am J Epidemiol. 2001;153:757–763. doi: 10.1093/aje/153.8.757. [DOI] [PubMed] [Google Scholar]
- Stewart GB, Altman DG, Askie LM, Duley L, Simmonds MC, Stewart LA. Statistical analysis of individual participant data meta-analyses: a comparison of methods and recommendations for practice. PLoS One. 2012;7:e46042. doi: 10.1371/journal.pone.0046042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White VM, English DR, Coates H, Lagerlund M, Borland R, Giles GG. Is cancer risk associated with anger control and negative affect? Findings from a prospective cohort study. Psychosom Med. 2007;69:667–674. doi: 10.1097/PSY.0b013e31814d4e6a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.