Abstract
Pyrroloindoline and bispyrroloindoline are a subclass of alkaloid structural motifs that commonly exhibit biological activity. An enantioselective organocatalytic approach to the synthesis of pyrroloindoline architecture is described. The addition–cyclization of tryptamines with α,β-unsaturated aldehydes in the presence of imidazolidinone catalysts 1 and 8 provides pyrroloindoline adducts in high yield and excellent enantioselectivities. This transformation is successful for a wide range of tryptamine and α,β-unsaturated aldehyde substrates. This amine-catalyzed sequence has been extended to the enantioselective construction of furanoindoline frameworks. Application of this pyrroloindoline-forming reaction to natural product synthesis has been accomplished in the context of the enantioselective synthesis of (–)-flustramine B.
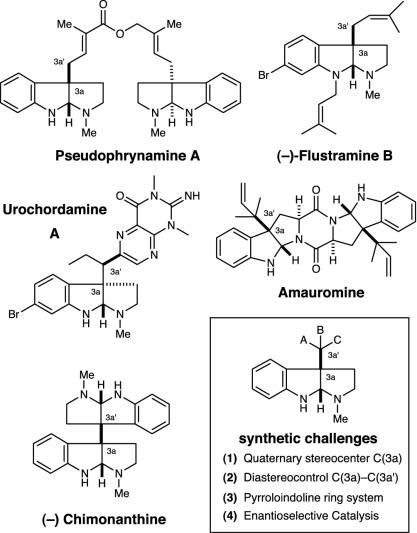
The pyrroloindolines and bis-pyrroloindolines represent a diverse family of structurally complex polyindoline alkaloids that have been isolated from a widespread series of natural sources (1), including amphibians, plants, and marine algae (Fig. 1). First described in the late 1930s, this alkaloid family has been found to exhibit remarkable biological properties across a broad spectrum of pharmacological screens. For example, several alkaloids isolated from fungal sources that comprise the C(3a)-bispyrroloindoline–diketopiperazine architecture have been shown to be powerful antagonists of cholecystokinin, substance P, and neurokinin 1 receptors (2–4). A related family of alkaloids that incorporates polythioketopiperazines has also been established to exhibit potent anticancer activities against lymphocytic leukemia cell lines (5) and cytotoxicity to HeLa cell lines (6).
Fig. 1.
Representative pyrroloindoline natural isolates.
Furthermore, McAlpine and coworkers (7–9) have reported that the pyrroloindoline 5-N-acetylardeemin demonstrates the ability to restore vinblastine sensitivity to tumor cell lines that manifest “operational resistance” to cytotoxic agents. The hydroxypyrroloindoline gypsetin has evoked interest as a potential inhibitor of the enzyme acyl-CoA:cholesterol acyltransferase and as such might find therapeutic use as a cholesterol-lowering agent (10–12). Psycholeine and quadrigemine C have been documented as the first nonpeptide antagonists of the somatostatin family of receptors (13). Several other polyindolines in this natural product family have also been shown to exhibit significant biological properties (13, 14–40).
A structural survey of this alkaloid family reveals a central cis-fused pyrroloindoline core that in all cases incorporates a quaternary center at the C(3a) site. A challenging structural feature with regard to developing a general strategy, the C(3a′) position has been shown to incorporate broad variation in substituents and stereogenicity. For example, whereas pseudophrynamine A and flustramine B are found to incorporate an unsubstituted methylene at C(3a′), urochordamine exhibits a tertiary carbon stereocenter at this site, whereas amauromine and chimonanthine contain vicinal fully substituted carbons between the C(3a)–C(3a′) positions. Indeed, chimonanthine presents a formidable synthetic challenge in the form of a vicinal quaternary carbon diastereochemical relationship.
The structural complexity of the pyrroloindolines makes them a particularly elusive and, at the same time, appealing target for total synthetic efforts. In this regard the Overman and Danishefsky groups have made seminal contributions in their design of new reaction methods that have enabled the rapid construction of many of these complex alkaloids. The Overman group has focused on the development of bis-Heck technology for the diastereoselective construction of a bisoxindole that is subsequently converted into the bispyrroloindoline core (41, 42). This highly innovative technology efficiently utilizes the stereochemical information of a conformationally locked cyclohexene substrate to generate the requisite vicinal C(3a)–C(3a′) quaternary carbon relationship. Link and Overman (43) have also reported a bis-enolate alkylation approach to bispyrroloindoline containing natural products. The Danishefsky group has used enantiopure tryptophan-based starting materials for their asymmetric synthesis of amauromine and the ardeemins (44, 45). Their strategy relies on stereochemical induction from a resident stereocenter derived from tryptophan in a cascade selenation–ring-closing protocol. The selenato functionality is then used in an ingenious radical addition step to introduce the “reverse prenyl” group as exhibited in amauromine. Both of these strategies are elegant, creative, and highly effective solutions to the construction of pyrroloindoline architecture. With these elegant approaches in mind, we sought to develop a complementary technology that would allow the enantioselective catalytic construction of pyrroloindoline architecture in one step with concomitant stereocontrolled generation of the requisite C(3a) and vicinal C(3a′) stereogenicity.
Methods
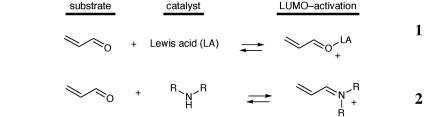
Iminium Catalysis. Our laboratory has recently disclosed (46–54) a strategy for asymmetric synthesis based on the capacity of chiral amines to function as enantioselective catalysts for a range of transformations that traditionally use Lewis acids. This catalysis concept was founded on the mechanistic hypothesis that the reversible formation of iminium ions from α,β-unsaturated aldehydes and amines (Scheme 2) might emulate the equilibrium dynamics and π-orbital electronics that are inherent to Lewis acid catalysis (Scheme 1). This new catalysis strategy has subsequently been applied to the development of a variety of enantioselective chemical processes, including cycloadditions (46, 47, 50), Mukaiyama–Michael additions (53), Friedel–Crafts alkylations (48, 49, 52), heteroconjugate additions, hydrogenations, and cascade reactions.
Schemes 1 and 2.
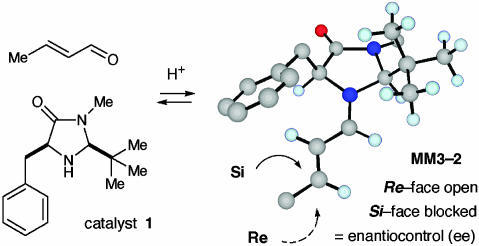
Central to these studies, we have identified a simple cyclic amine 1 that performs as a highly effective asymmetric catalyst for a broad range of new and traditional chemical transformations. The development of amine catalyst 1 was made on the basis of two design objectives, the requirement for (i) iminium ion geometry control and (ii) high levels of enantiofacial discrimination. As shown by the computational model MM3-2 (Fig. 2), the catalyst-activated iminium ion 2 was expected to be formed with (E)-isomer selectivity to avoid nonbonding interactions between the substrate olefin and the bulky tert-butyl group. In terms of enantiofacial discrimination, the calculated iminium structure MM3-2 also reveals that the benzyl and tert-butyl groups on the catalyst framework will effectively shield the Si-face of the substrate, leaving the Re-face exposed for enantioselective bond formation.
Fig. 2.
Iminium catalysis concept: Imidazolidinone catalyst and corresponding iminium structure.
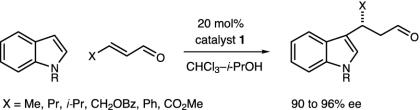
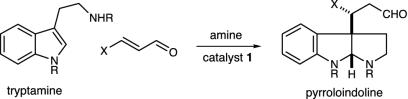
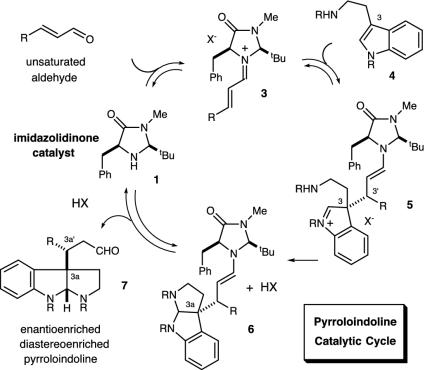
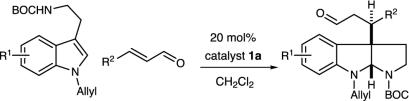
Pyrroloindoline Construction. In recent studies we have established the utility of lowest unoccupied molecular orbital-lowering iminium catalysis as a valuable platform for the development of enantioselective Friedel–Crafts alkylations by using anilines (52), pyrroles (48), oxazoles, furans, and thiophenes. Inclusive to this research, we have also demonstrated that the conjugate addition of indole substrates to α,β-unsaturated aldehydes can be achieved with excellent levels of enantiocontrol (Scheme 3) (49). Based on a variety of mechanistic considerations, we sought to explore whether this indole addition pathway might be manipulated to allow the cascade formation of pyrroloindoline architecture in lieu of substituted indole production (Scheme 4). As elaborated in Fig. 3, we envisioned that the addition of an indole derived from tryptamine 4 to the activated iminium ion 3 (arising from catalyst 1 and an α,β-unsaturated aldehyde) would generate the C(3)-quaternary carbon-substituted indolium ion 5. As a central design feature, this quaternary carbon-bearing indolium cannot undergo rearomatization by means of proton loss in contrast to the analogous 3-H indole addition pathway. As a result, we expected the prevailing reaction pathway to be partitioned toward a 5-exo-heterocyclization of the pendant ethylamine, thereby generating the tricyclic system 6. Subsequent hydrolysis of the tethered enamine moiety would provide the requisite pyrroloindoline framework and, in doing so, would reconstitute the imidazolidinone catalyst. In terms of molecular complexity development, this cascade sequence should allow the rapid and enantioenriched formation of stereochemically defined pyrroloindoline architecture from tryptamines and simple α,β-unsaturated aldehydes. Moreover, we hoped that the requisite C(3a) quaternary carbon would be forged with high levels of enantio- and diastereocontrol by using a simple amine catalyst.
Scheme 3.
Scheme 4.
Fig. 3.
Pyrroloindoline formation: Catalytic cycle.
Supporting Information. Experimental procedures, structural proofs, and spectral data for all new compounds are provided as supporting information on the PNAS web site.
Results
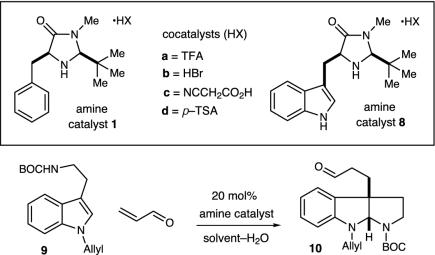
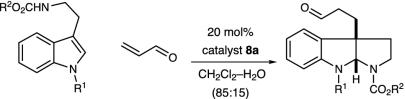
Our enantioselective organocatalytic pyrroloindoline construction was first evaluated by using N(10)-t-butoxycarbonyl (BOC)-N(1)-allyltryptamine with acrolein and a series of imidazolidinone catalysts (Table 1). In accord with our mechanistic postulate, we were delighted to find that the imidazolidinone catalysts 1a–d provided the desired pyrroloindoline architecture in good yield (entries 2–8). Although useful levels of enantioselectivity could be observed in this addition–cyclization sequence [entry 7, 84% enantiomeric excess (ee)], we were surprised to find large variations in enantioinduction as a function of reaction solvent. As delineated in Table 1, the use of high dielectric media (e.g., MeOH) led to the predominant formation of the (3aS)-pyrroloindoline enantiomer (entry 1, (+) 77% ee), whereas the use of low dielectric solvents provided the (3aR)-pyrroloindoline as the major antipode (entry 7, (–) 84% ee). Although reaction media are known to influence a variety of stereoselective processes, this apparent correlation between solvent dielectric and the magnitude of change in the absolute sense of enantiofacial discrimination is, to our knowledge, without precedent. The tryptophan-derived imidazolidinone salt 8a was found to exhibit the optimal levels of enantioinduction in the addition to acrolein in the presence of CH2Cl2–H2O. This increase in selectivity is postulated to arise from the more efficient stabilization by catalyst 8 of the α,β-unsaturated iminium ion by means of a favorable cation–π interaction. The superior levels of asymmetric induction and reaction efficiency exhibited by the trifluoroacetyl salt 8a to afford the pyrroloindoline (3aS)-10 in 89% ee and 85% yield prompted us to select this catalyst for further exploration.
Table 1.
| Entry | Solvent | Dielectric | Catalyst | Yield, % | [α]D | ee, *% |
|---|---|---|---|---|---|---|
| 1 | MeOH† | 32.6 | 1c | 9 | (+) | 77‡ |
| 2 | MeOH§ | 32.6 | 1d | 64 | (+) | 69¶ |
| 3 | Acetone§ | 20.2 | 1d | 58 | (+) | 60¶ |
| 4 | DME§ | 5.0 | 1d | 18 | (+) | 21¶ |
| 5 | CHCl3§ | 4.8 | 1d | 66 | (-) | 45¶ |
| 6 | Toluene§ | 2.4 | 1d | 60 | (-) | 59¶ |
| 7 | Toluene∥ | 2.4 | 1b | 50 | (-) | 84** |
| 8 | CH2Cl2†† | 9.1 | 1a | 79 | (+) | 70‡ |
| 9 | CH2Cl2†† | 9.1 | 8a | 85 | (+) | 89‡ |
p-TSA, para-tolunesulfonic acid; DME, 1,2-dimethoxyethane.
Product ratios determined by HPLC.
With no H2O.
Reaction performed at -85°C.
Solvent/H2O = 90:10.
Reaction performed at -40°C.
Solvent/H2O = 98:2.
Reaction performed at 4°C.
Solvent/H2O = 85:15.
Experiments that probe the scope of the tryptamine N(1) and N(10) substituents are summarized in Table 2. The reaction appears quite tolerant with respect to the steric contribution of the N(10) carbamate substituent (R = Et, allyl t-Bu, entries 1–5, ≥82% yield, 89–90% ee). As revealed in entries 2–5, the reaction can also accommodate a variety of electron donating N(1)-indole substituents (entry 2, N-allyl, 89% ee; entry 3, N-prenyl, 89% ee; entry 5, N-Bn, 90% ee). To demonstrate the preparative utility, the addition of N (10)-BOC-N (1)-benzyltryptamine to acrolein was performed on a 2 mmol scale with catalyst 8a to afford the corresponding pyrroloindoline (entry 5) in 90% ee and 82% yield.
Table 2.
Enantioselective pyrroloindoline formation with representative N(1)- and N(10)-substituted tryptamines
| Entry | R1 | R2 | Time, h | Yield, % | ee,* % |
|---|---|---|---|---|---|
| 1 | Allyl | t-Bu | 25 | 85 | 89 |
| 2 | Allyl | Et | 26 | 89 | 89 |
| 3 | Prenyl | Et | 24 | 89 | 89 |
| 4 | Benzyl | Allyl | 48 | 83 | 89 |
| 5 | Benzyl | t-Bu | 30 | 82 | 90† |
Product ratios determined by HPLC.
Absolute configuration determined by chemical correlation.
We next examined the utility of β-substituted α,β-unsaturated aldehydes in this enantioselective pyrroloindoline formation. The principal issue in this reaction is that of absolute and relative stereocontrol in the construction of the vicinal C(3a)–C(3a′) stereocenters. As revealed in Table 3, significant variation in the steric contribution of the olefin substituent (X = CO2Me, CH2OR, COPh), entries 1–3) is possible without loss in yield or enantiocontrol (66–93% yield, 91–94% ee). From the perspective of pyrroloindoline natural product synthesis, the requisite C(3a)–C(3a′) relationship is forged with excellent levels of diastereocontrol [entries 1–7, 13 to 50:1 diastereomeric ratio (dr)]. The electronic nature of the α,β-unsaturated aldehyde component also appears to have a broad scope. For example, the reaction can accommodate enals that do not readily participate in iminium formation (entry 1, R = COPh, 92% yield, 94% ee) and aldehydes that provide stable iminium intermediates (entry 2, R = CH2OBz, 66% yield, 91% ee). Although the combination of CH2Cl2 and H2O provides the optimal reaction medium for acrolein-derived pyrroloindoline formation (Tables 1 and 2), the use of dry CH2Cl2 was found to enhance enantioinduction in the addition to β-substituted α,β-unsaturated aldehydes (Table 3).
Table 3.
| Entry | R1 | R2 | Time, h | Yield, % | ee,* % | dr† |
|---|---|---|---|---|---|---|
| 1 | H | COPh | 64 | 92 | 94 | 13:1 |
| 2 | H | CH2OBz | 44 | 66 | 91 | 22:1 |
| 3 | H | CO2Me | 28 | 93 | 91 | 44:1‡ |
| 4 | 5-Me | CO2Me | 18 | 94 | 92 | 50:1 |
| 5 | 5-MeO | CO2Me | 20 | 99 | 90 | 10:1 |
| 6 | 6-Br | CO2Me | 36 | 86 | 97 | 31:1 |
| 7 | 7-Me | CO2Me | 30 | 97 | 99 | 17:1 |
Product ratios determined by chiral HPLC.
dr, diastereomeric ratio.
Absolute configuration determined by x-ray crystallography.
This amine-catalyzed tryptamine addition–cyclization is also general with respect to indole architecture (Table 3). Incorporation of alkyl and alkoxy substituents at the C(5)-indole position reveals that electronic and steric modification of the indole ring can be accomplished with little influence on reaction selectivity (entries 4 and 5, ≥90% yield, 90–92% ee, 10 to 50:1 dr). As revealed in entry 6, we have successfully used electron-deficient nucleophiles in the context of a 6-bromo-substituted tryptamine (86% yield, 97% ee, 31:1 dr). Such halogenated indole adducts should prove to be valuable synthons for use in conjunction with organometallic technologies [e.g., Buchwald–Hartwig (55, 56) and Stille couplings (57)]. The capacity of 6-bromotryptamine derivatives to participate in this process has direct implications for the synthesis of 6-bromopyrroloindoline natural products such as flustramine B (see below).
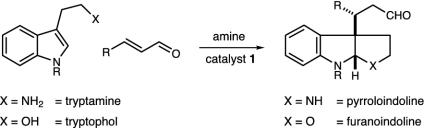
Furanoindoline Construction. Having successfully demonstrated the capacity of iminium catalysis to rapidly build complex pyrroloindoline systems, we sought to advance this enantioselective addition–cyclization technology to the realm of furanoindoline architecture. This oxygen-containing tricyclic synthon is also widely represented among natural isolates of biological relevance, including diazonamide A (58), physovenine (59), and picrinine (60). Given their structural similarity to the pyrroloindolines, we envisioned that a wide array of furanoindolines might be rapidly generated in enantioenriched format by the implementation of tryptaphol derivatives in this organocatalytic addition–cyclization sequence (Scheme 5).
Scheme 5.
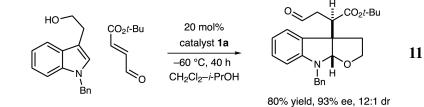
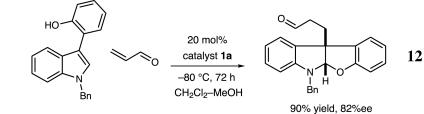
As demonstrated in Schemes 6 and 7, preliminary studies have demonstrated that the catalytic construction of furanoindolines can also be realized with valuable levels of enantioinduction. Exposure of N-benzyltryptophol to t-butyl-4-oxobutenoate in the presence of amine catalyst 1d results in the production of the desired tricycle in 93% ee and 80% yield (Scheme 6). Moreover, the generation of the C(3a)–C(3a′) stereochemical relationship is accomplished with high fidelity (12:1 dr), in accord with the analogous pyrroloindoline system. We have also found that 3-phenol-substituted indoles readily participate in this organocatalytic cascade sequence, thereby providing the basic furanoindoline core of the diazonamide family with excellent enantioselectivity (Scheme 7; 90% yield, 82% ee).
Scheme 6.
Scheme 7.
Natural Product Synthesis. The flustramines (34, 40, 61) and flustramides (62) are a small family of marine alkaloids isolated from the Bryozoa Flusta foliacea (L.). First described in the late 1970s, this alkaloid family has yet to undergo broad biological investigation; however, both flustramines A and B have been shown to block voltage-activated potassium channels (63) and exhibit skeletal and smooth muscle relaxant properties (64). An architectural survey of the flustramine class reveals they are a structurally unique subgroup of the pyrroloindoline isolate class because of the incorporation of a (C6)-bromine substituent on the indoline ring system. Although among the least complex of the pyrroloindoline natural products, the flustramines have not received broad synthetic attention. Indeed, at the present time, only two syntheses (65, 66) of flustramine B have been reported, both in racemic form. Given that the flustramine skeleton might represent an interesting template for a medicinal or diversity-oriented chemistry evaluation, we were prompted to undertake the enantioselective construction of flustramine B. As a prominent design feature, we sought to enantioselectively construct the central bromo-tricyclic ring system in one chemical step by using our organocatalytic pyrroloindoline technology.
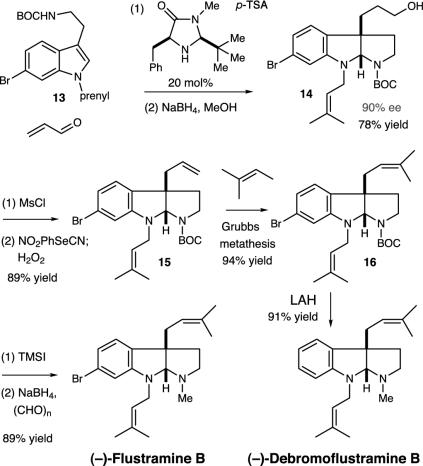
Our synthesis began with the exposure of the 6-bromotryptamine derivative 13 to acrolein in the presence of the imidazolidinone catalyst 1d (Fig. 4). To our delight, the key addition–cyclization cascade proceeded, as expected, to provide the central flustramine ring system 14 in 90% ee and 78% yield after reduction of the resulting formyl group. Conversion of the resulting C(3a)-3-hydroxy propyl adduct to the corresponding C(3a)-allyl pyrroloindoline 15 was accomplished cleanly in a three-step, two-pot sequence involving mesyl group formation–displacement, followed by peroxide-mediated selenoxide elimination to generate the requisite terminal olefin in 89% yield. Conversion of the resulting terminal allyl substituent to the requisite prenyl group was accomplished in high efficiency by exposure of 15 to 2-methyl-2-butene in the presence of Grubbs second-generation metathesis catalyst. The decision to use an allyl substituent as a synthetic precursor to a prenyl moiety was predicated on the elegant studies of Grubbs and coworkers (67) and Spessard and Stoltz (68). At this point, we envisioned that the requisite conversion of the N-BOC system to N-Me could be accomplished in one step by hydride reduction. Unfortunately, however, exposure of the bisprenylated adduct 16 to lithium aluminum hydride resulted in both carbamate reduction and dehalogenation, thereby providing the known isolate debromoflustramine B in 91% yield. This dehalogenation pathway was readily circumvented by selective removal of the BOC-protecting group from 16 followed by reductive N-methylation of the resulting pyrrolidine to afford (–)-flustramine B as a white crystalline solid in 89% yield for the two-step process. Flustramine B was found to be identical in all respects with the natural isolate (61).
Fig. 4.
Total synthesis of (–)-flustramine B
In summary, we have further established lowest unoccupied molecular orbital-lowering organocatalysis as a broadly useful concept for asymmetric synthesis in the context of pyrroloindoline construction. The rapid construction of this quaternary carbon architecture has potential uses in medicinal chemistry, natural product, and diversity-oriented synthesis. In this vein, we have used this organocatalytic technology toward the enantioselective synthesis of (–)-flustramine B. Application of this methodology to a variety of other pyrroloindoline and furanoindoline containing natural isolates is now under way.
Supplementary Material
Acknowledgments
We thank Dr. Claudia Roberson for insightful discussions and Dr. Larry Henling and Dr. Michael Day for x-ray crystallographic analysis. This work was supported by National Institute of General Medical Sciences Grant R01 GM66142-01 and kind gifts from Bristol-Myers Squibb, Johnson and Johnson, Eli Lilly, and Merck Research Laboratories. D.W.C.M. was supported by the Camille and Henry Dreyfuss Foundation and the Sloan Foundation and Research Corporation under the Cottrell Scholarship and Research Innovation programs. J.F.A. received a graduate fellowship from the Fannie and John Hertz Foundation, S.-G.K received postdoctoral fellowship support from Korean Science and Engineering Foundation, and C.J.S. received postdoctoral fellowship support from National Institutes of Health Grant 1-F32-CA91635-01.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: BOC, t-butoxycarbonyl; ee, enantiomeric excess; dr, diastereomeric ratio.
Data deposition: The atomic coordinates have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference nos. 197024 and 234570).
References
- 1.Kobayashi, J. & Ishibashi, M. (1992) Alkaloids 41, 41–124. [Google Scholar]
- 2.Barrow, C. J., Cai, P., Snyder, J. K., Sedlock, D. M., Sun, H. H. & Cooper, R. (1993) J. Org. Chem. 58, 6016–6021. [Google Scholar]
- 3.Oleynek, J. J., Sedlock, D. M., Barrow, C. J., Appell, K. C., Casiano, F., Haycock, D., Ward, S. J., Kaplita, P. & Gillum, A. M. (1994) J. Antibiot. 47, 399–410. [DOI] [PubMed] [Google Scholar]
- 4.Popp, J. L., Musza, L. L., Barrow, C. J., Rudewicz, P. J. & Houck, D. R. (1994) J. Antibiot. 47, 411–419. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi, C., Minoura, K., Yamada, T., Numata, A., Kushida, K., Shingu, T., Hagishita, S., Nakai, H., Sato, T. & Harada, H. (1995) Tetrahedron 51, 3483–3498. [Google Scholar]
- 6.Saito, T., Suzuki, Y., Koyama, K., Natori, S., Iitaka, Y. & Kinoshita, T. (1988) Chem. Pharm. Bull. 36, 1942–1956. [Google Scholar]
- 7.Hochlowski, J. E., Mullally, M. M., Spanton, S. G., Whittern, D. N., Hill, P. & McAlpine, J. B. (1993) J. Antibiot. 46, 380–386. [DOI] [PubMed] [Google Scholar]
- 8.Karwowski, J. P., Jackson, M., Rasmussen, R. R., Humphrey, P. E., Poddig, J. B., Kohl, W. L., Scherr, M. H., Kadam, S. & McAlpine, J. B. (1993) J. Antibiot. 46, 374–379. [DOI] [PubMed] [Google Scholar]
- 9.Takase, S. I. M., Ando, T., Okamoto, M., Yoshida, K., Horiai, H., Kohsaka, M., Aoki, H. & Imanaka, H. (1984) J. Antibiot. 37, 1320–1323. [DOI] [PubMed] [Google Scholar]
- 10.Fukuyama, T., Chen, X. Q. & Peng, G. (1994) J. Am. Chem. Soc. 116, 3127–3128. [Google Scholar]
- 11.Nuber, B., Hansske, F., Shinohara, C., Miura, S., Hasumi, K. & Endo, A. (1994) J. Antibiot. 47, 168–172. [DOI] [PubMed] [Google Scholar]
- 12.Shinohara, C., Hasumi, K., Takei, Y. & Endo, A. (1994) J. Antibiot. 47, 163–167. [DOI] [PubMed] [Google Scholar]
- 13.Guerittevoegelein, F., Sevenet, T., Pusset, J., Adeline, M. T., Gillet, B., Beloeil, J. C., Guenard, D. & Potier, P. (1992) J. Nat. Prod. 55, 923–930. [DOI] [PubMed] [Google Scholar]
- 14.Cui, C. B., Kakeya, H., Okada, G., Onose, R., Ubukata, M., Takahashi, I., Isono, K. & Osada, H. (1995) J. Antibiot. 48, 1382–1384. [DOI] [PubMed] [Google Scholar]
- 15.Houck, D. R., Ondeyka, J., Zink, D. L., Inamine, E., Goetz, M. A. & Hensens, O. D. (1988) J. Antibiot. 41, 882–891. [DOI] [PubMed] [Google Scholar]
- 16.Kamenecka, T. M. & Danishefsky, S. J. (1988) Angew. Chem. Int. Ed. Engl. 37, 2995–2998. [DOI] [PubMed] [Google Scholar]
- 17.Laycock, M. V., Wright, J. L. C., Findlay, J. A. & Patil, A. D. (1986) Can. J. Chem. Rev. Can. Chim. 64, 1312–1316. [Google Scholar]
- 18.Takase, S., Kawai, Y., Uchida, I., Tanaka, H. & Aoki, H. (1985) Tetrahedron 41, 3037–3048. [Google Scholar]
- 19.Tsukamoto, S., Hirota, H., Kato, H. & Fusetani, N. (1993) Tetrahedron Lett. 34, 4819–4822. [Google Scholar]
- 20.Wright, J. L. C. (1984) J. Nat. Prod. 47, 893–895. [DOI] [PubMed] [Google Scholar]
- 21.Verotta, L., Pilati, T., Tato, M., Elisabetsky, E., Amador, T. A. & Nunes, D. S. (1998) J. Nat. Prod. 61, 392–396. [DOI] [PubMed] [Google Scholar]
- 22.Duke, R. K., Allan, R. D., Johnston, G. A. R., Mewett, K. N., Mitrovic, A. D., Duke, C. C. & Hambley, T. W. (1995) J. Nat. Prod. Lloydia 58, 1200–1208. [DOI] [PubMed] [Google Scholar]
- 23.Saad, H. E. A., Elsharkawy, S. H. & Shier, W. T. (1995) Planta Med. 61, 313–316. [DOI] [PubMed] [Google Scholar]
- 24.Holst, P. B., Anthoni, U., Christophersen, C. & Nielsen, P. H. (1994) J. Nat. Prod. 57, 997–1000. [DOI] [PubMed] [Google Scholar]
- 25.Boyeskorkis, J. M., Gurney, K. A., Penn, J., Mantle, P. G., Bilton, J. N. & Sheppard, R. N. (1993) J. Nat. Prod. 56, 1707–1717. [Google Scholar]
- 26.Adjibade, Y., Weniger, B., Quirion, J. C., Kuballa, B., Cabalion, P. & Anton, R. (1992) Phytochemistry 31, 317–319. [Google Scholar]
- 27.Arai, K., Kimura, K., Mushiroda, T. & Yamamoto, Y. (1989) Chem. Pharm. Bull. 37, 2937–2939. [Google Scholar]
- 28.Spande, T. F., Edwards, M. W., Pannell, L. K., Daly, J. W., Erspamer, V. & Melchiorri, P. (1988) J. Org. Chem. 53, 1222–1226. [Google Scholar]
- 29.Keil, P., Nielsen, E. G., Anthoni, U. & Christophersen, C. (1986) Acta Chem. Scand. Ser. B Org. Chem. Biochem. 40, 555–558. [Google Scholar]
- 30.Laws, I. & Mantle, P. G. (1985) Phytochemistry 24, 1395–1397. [Google Scholar]
- 31.Tokuyama, T. & Daly, J. W. (1983) Tetrahedron 39, 41–47. [Google Scholar]
- 32.Hayashi, H., Furutsuka, K. & Shiono, Y. (1999) J. Nat. Prod. 62, 315–317. [DOI] [PubMed] [Google Scholar]
- 33.Fang, C. L., Horne, S., Taylor, N. & Rodrigo, R. (1994) J. Am. Chem. Soc. 116, 9480–9486. [Google Scholar]
- 34.Carle, J. S. & Chrisophersen, C. (1981) J. Org. Chem. 46, 3440–3443. [Google Scholar]
- 35.Fridrichsons, J., Mackay, M. F. & Mathieson, A. McL. (1974) Tetrahedron 30, 85–92. [Google Scholar]
- 36.Hendrickson, J. B., Goschke, R. & Rees, R. (1964) Tetrahedron 20, 565–579. [Google Scholar]
- 37.Birch, A. J. & Wright, J. J. (1970) Tetrahedron 26, 2329–2344. [DOI] [PubMed] [Google Scholar]
- 38.Steyn, P. S. (1971) Tetrahedron Lett. 36, 3331–3334. [Google Scholar]
- 39.Steyn, P. S. (1973) Tetrahedron 29, 107–120. [Google Scholar]
- 40.Carle, J. S. & Chrisophersen, C. (1980) J. Org. Chem. 45, 1586–1589. [Google Scholar]
- 41.Overman, A. E., Paone, D. V. & Stearns, B. A. (1999) J. Am. Chem. Soc. 121, 7702–7703. [Google Scholar]
- 42.Overman, L. E., Larrow, J. F., Stearns, B. A. & Vance, J. M. (2000) Angew. Chem. Int. Ed. Engl. 39, 213–215. [DOI] [PubMed] [Google Scholar]
- 43.Link, J. T. & Overman, L. E. (1996) J. Am. Chem. Soc. 118, 8166–8167. [Google Scholar]
- 44.Depew, K. M., Marsden, S. P., Zatorska, D., Zatorski, A., Bornmann, W. G. & Danishefsky, S. J. (1999) J. Am. Chem. Soc. 121, 11953–11963. [Google Scholar]
- 45.Schkeryantz, J. M., Woo, J. C. G., Siliphaivanh, P., Depew, K. M. & Danishefsky, S. J. (1999) J. Am. Chem. Soc. 121, 11964–11975. [Google Scholar]
- 46.Ahrendt, K. A., Borths, C. J. & MacMillan, D. W. C. (2000) J. Am. Chem. Soc. 122, 4243–4244. [Google Scholar]
- 47.Jen, W. S., Wiener, J. J. M. & MacMillan, D. W. C. (2000) J. Am. Chem. Soc. 122, 9874–9875. [Google Scholar]
- 48.Paras, N. A. & MacMillan, D. W. C. (2001) J. Am. Chem. Soc. 123, 4370–4371. [DOI] [PubMed] [Google Scholar]
- 49.Austin, J. F. & MacMillan, D. W. C. (2002) J. Am. Chem. Soc. 124, 1172–1173. [DOI] [PubMed] [Google Scholar]
- 50.Northrup, A. B. & MacMillan, D. W. C. (2002) J. Am. Chem. Soc. 124, 2458–2460. [DOI] [PubMed] [Google Scholar]
- 51.Northrup, A. B. & MacMillan, D. W. C. (2002) J. Am. Chem. Soc. 124, 6798–6799. [DOI] [PubMed] [Google Scholar]
- 52.Paras, N. A. & MacMillan, D. W. C. (2002) J. Am. Chem. Soc. 124, 7894–7895. [DOI] [PubMed] [Google Scholar]
- 53.Brown, S. P., Goodwin, N. C. & MacMillan, D. W. C. (2003) J. Am. Chem. Soc. 125, 1192–1194. [DOI] [PubMed] [Google Scholar]
- 54.Brown, S. P., Brochu, M. P., Sinz, C. J. & MacMillan, D. W. C. (2003) J. Am. Chem. Soc. 125, 10808–10809. [DOI] [PubMed] [Google Scholar]
- 55.Hartwig, J. F. (1998) Acc. Chem. Res. 31, 852–860. [Google Scholar]
- 56.Wolfe, J. P. & Buchwald, S. L. (1999) Angew. Chem. Int. Ed. Engl. 38, 2413–2416. [DOI] [PubMed] [Google Scholar]
- 57.Stille, J. K. (1986) Angew. Chem. Int. Ed. Engl. 25, 508–523. [Google Scholar]
- 58.Li, J., Jeong, S., Esser, L. & Harran, P. G. (2001) Angew. Chem. Int. Ed. Engl. 40, 4765–4770. [DOI] [PubMed] [Google Scholar]
- 59.Robinson, B. (1964) J. Chem. Soc., 1503–1506.
- 60.Grossman. E., Sefcovic, P. & Szasz, K. (1973) Phytochemistry 12, 2058–2058. [Google Scholar]
- 61.Carle, J. S. & Christophersen, C. (1979) J. Am. Chem. Soc. 101, 4012–4013. [Google Scholar]
- 62.Wulff, P., Carle, J. S. & Christophersen, C. (1982) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 71, 523–524. [Google Scholar]
- 63.Peters, L., Konig, G. M., Terlau, H. & Wright, A. D. (2002) J. Nat. Prod. 65, 1633–1637. [DOI] [PubMed] [Google Scholar]
- 64.Sjoblom, T., Bohlin, L. & Christophersen, C. (1983) Acta Pharm. Suec. 20, 415–419. [PubMed] [Google Scholar]
- 65.Hino, T., Tanaka, T., Matsuki, K. & Nakagawa, M. (1983) Chem. Pharm. Bull. 31, 1806–1808. [Google Scholar]
- 66.Morales-Rios, M. S., Suarez-Castillo, O. R., Trujillo-Serrato, J. J. & Joseph-Nathan, P. (2001) J. Org. Chem. 66, 1186–1192. [DOI] [PubMed] [Google Scholar]
- 67.Chatterjee, A. K., Sanders, D. P. & Grubbs, R. H. (2002) Org. Lett. 4, 1939–1942. [DOI] [PubMed] [Google Scholar]
- 68.Spessard, S. J. & Stoltz, B. M. (2002) Org. Lett. 4, 1943–1946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.