Abstract
Background:
Nicotine is able to activate mitogenic signalling pathways, which promote cell growth or survival as well as increase chemoresistance of cancer cells. However, the underlying mechanisms are not fully understood.
Methods:
In this study, we used immunoblotting and immunoprecipitation methods to test the ubiquitination and degradation of Bcl-2 affected by nicotine in lung cancer cells. Apoptotic assay was also used to measure the antagonising effect of nicotine on cisplatin-mediated cytotoxicity.
Results:
We demonstrated that the addition of nicotine greatly attenuated Bcl-2 ubiquitination and degradation, which further desensitised lung cancer cells to cisplatin-induced cytotoxicity. In this process, Bcl-2 was persistently phosphorylated in the cells cotreated with nicotine and cisplatin. Furthermore, Akt was proven to be responsible for sustained activation of Bcl-2 by nicotine, which further antagonised cisplatin-mediated apoptotic signalling.
Conclusions:
Our study suggested that nicotine activates its downstream signalling to interfere with the ubiquitination process and prevent Bcl-2 from being degraded in lung cancer cells, resulting in the increase of chemoresistance.
Keywords: nicotine, cisplatin, Bcl-2, Keap1, ubiquitination, Akt, chemoresistance
Cigarette smoke, as a major environmental risk factor, affects the development of many human diseases, especially of cancer. There are more than 4000 compounds in cigarette smoke, most of which are biohazards or carcinogens (Brunnemann and Hoffmann, 1991; Hecht, 2002; Karnath, 2002; Burns, 2003). Epidemiological studies provided a strong correlation between tobacco smoke and the development of human malignancies in various human organs, including the lung, breast, pancreas, or oesophagus (Manneckjee and Minna, 1990; Fuchs et al, 1996; Band et al, 2002; Pfeifer et al, 2002; Guo, et al, 2008). As an addictive substance, nicotine not only functions in the central nervous system but also affects non-neuronal cells via binding to its receptor, resulting in the promotion of cell survival or enhancement of chemoresistance (Guo et al, 2008; Hirata et al, 2010; Singh et al, 2011; Kunigai et al, 2012).
Nicotine was demonstrated to be able to activate several intracellular, mitogenic signalling pathways and further promote cell growth, survival and angiogenesis (Heeschen et al, 2002; Arredondo et al, 2006; Dasgupta and Chellappan, 2006a; Wessler and Kirkpatrick, 2008). Nicotine treatment affected XIAP and surviving, resulting in a negative impact on the chemosensitivity of human lung cancer A549 cells (Dasgupta et al, 2006b). In non-small-cell lung cancer (NSCLC) or breast cancer cells, nicotine treatment stimulated Src and Raf signalling, resulting in Rb phosphorylation and subsequent cell cycle progression (Nakayama et al, 2002; Dasgupta et al, 2006c; Nishioka et al, 2011). The expressions of various genes that are important in the regulation of cell migration and angiogenesis were altered in cultured cell lines, such as in lung, breast and pancreatic cancer cells following the treatment of nicotine (Manneckjee and Minna, 1990; Heeschen et al, 2003; Arredondo et al, 2006; Guo et al, 2008; Kunigai et al, 2012). Nicotine was shown to stimulate the secretion of several growth factors, including EGF, TGF-α and PDGF, in non-neuronal cells (Rakowicz-Szulczynska et al, 1994; Mathur et al, 2000; Kolmakova and Chatterjee, 2005). Moreover, the growth of nicotine-treated cancer cells was upregulated, in which EGFR and Src pathways were activated (Dasgupta et al, 2006c; Nishioka et al, 2011). In this process, Akt functioned directly downstream of Src, leading to the increase of cell survival or of drug resistance (Jull et al, 2001; West et al, 2003; Dasgupta et al, 2006c; Nishioka et al, 2011). It was also observed that the addition of nicotine activated β-arrestin/Src/Raf signalling for the development of human NSCLCs (Dasgupta et al, 2006c). The importance of nicotine in pathological angiogenesis has been demonstrated through its enhancement of the formation of atherosclerotic plaques in tumour messes (Heeschen et al, 2001, 2003, 2006).
Bcl-2 is a prosurvival factor. By regulating the membrane permeability of the mitochondria, Bcl-2 was shown to interfere with the release of the mitochondrial apoptotic factors (e.g., Bax or cytochrome c) to the cytosol, and thereby suppressed cell death process (Kluck et al, 1997; Kroemer, 1997; Rong and Distelhorst, 2008). The expression level of Bcl-2 in patients with lung, head or neck cancer was upregulated after a heavy exposure to tobacco smoke (Gallo et al, 1995; Borner et al, 2002; Zereu et al, 2003; Assis et al, 2005). Studies also showed that PKC in nicotine-treated cancer cells was able to stimulate Src, Akt or Bcl-2 and further antagonise apoptotic signals, suggesting that this prosurvival factor is one of the targets of nicotine in its promotion of cell survival or proliferation (Ruvolo et al, 1998; Assis et al, 2005; Resende and Adhikari, 2009; Nishioka et al, 2011). Cisplatin is a common drug to treat lung cancer. The sensitivity of lung cancer to cisplatin appeared to be partially dependent on Bcl-2, which indicates the importance of this prosurvival factor in the determination of the outcome of the chemotherapy (Zangemeister-Wittke et al, 1998; Losert et al, 2007).
The ubiquitin-mediated protein degradation is a key event in the regulation of various functions or activities of cells (Ciechanover, 1994; Hochstrasser, 1996; Weissman, 1997). The ubiquitination was proven to timingly control the amount of the expression of proteins involved in the regulation of each critical cellular activity, such as cell cycle checkpoints, tumour surveillances, cellular or DNA damage repair and duration of intracellular signal transduction. In the process of ubiquitination, proteins are covalently bound to ubiquitin that is a polypeptide with 76 amino acids and ubiquitously expressed in cells (Ciechanover, 1994; Hochstrasser, 1996). Keap1 is an adaptor protein and participates in Cul3-mediated degradation of Nrf2 during oxidative or radiation-induced stresses (Weissman, 1997; Cullinan et al, 2004; Zhang et al, 2004). The degradation of Bcl-2 in TNF-α or staurosporine-stimulated cells was reported to be through the ubiquitination (Dimmeler et al, 1999; Breitschopf et al, 2000). Upon stimulation, Bcl-2 was interacted with Keap1 and then rapidly degraded, leading to marked sensitisation of the cells to TNF-α- or staurosporine-induced apoptosis.
In this study, we investigated the effect of nicotine on the degradation of Bcl-2 in human or murine lung cancer cells. We identified that nicotine interfered with the association of Bcl-2 with Keap1 in cisplatin-treated cells, which attenuated the degradation process of this prosurvival factor. Characterisation of the signalling responsible for prolonging Bcl-2 degradation showed that nicotine might, by activating Akt, prolong the phosphorylation of Bcl-2, which delayed Bcl-2 degradation and desensitised cisplatin-treated lung cancer cells to apoptosis. Thus, the data suggested that the treatment of nicotine, by increasing Bcl-2 stability, rendered the lung cancer cells resistant to cisplatin.
Materials and methods
Cell lines and treatments
Human lung cancer H5800 cells were obtained from Dr Luo (Boston University School of Medicine, Boston, MA, USA), who originally purchased it from ATCC ((Manassas, VA, USA). LKR murine lung cells are a generous gift from Dr Jacks (MIT, Cambridge, MA, USA) and were also used by other groups (Johnson et al, 2001; Matsumura et al, 2010; Nishioka et al, 2010). The lung cancer cells were cultured in DMEM medium containing 10% fetal calf serum. Nicotine, cisplatin and inhibitors were purchased from Sigma (St Louis, MO, USA). Nicotine was dissolved in 1 × PBX and the concentration of 0.5 μM was used in previous studies (Nishioka et al, 2010, 2011).
Short hairpin (sh) RNA for bcl-2 was introduced into a lentiviral vector (OriGene, Rockville, MD, USA) that contains the 19 bp target sequence for bcl-2 (5′-GTGGATGACTGAGTACCTG-3′) (Wild-Bode et al, 2001; Kock et al, 2007), or scrambled sequence. The lentiviral construct was transfected into 293T cells. Forty-eight hours later, the supernatant was collected and tittered by serial dilutions. The supernatant containing optimal concentration of the viral particles was used for the knockdown experiments. For Bcl-2 overexpression, wt-bcl-2 was constructed in the PCDNA vector and subsequently transfected into the cells.
Annexin V-FITC apoptosis detection assay
After treatments cells were prepared and stained with Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences, San Jose, CA, USA) according to the manufacturer's instructions. Subsequently, 500 cells per treatment were counted for the positive staining cells.
Immunoprecipitation and immunoblotting analysis
After treatment, cell lysates were prepared and then separated by SDS–PAGE gels. Following the transfer, nitrocelluloses were incubated with the designated primary antibodies overnight in a cold room at 4 °C. Subsequently, bound primary antibodies were reacted with corresponding secondary antibodies for 2 h at room temperature and detected by chemiluminescence.
For immunoprecipitation, cell lysates were precipitated with an antibody first. The immunoprecipitates were then separated on a SDS–PAGE gel for immunoblotting.
For detecting protein degradation, cells were treated with cisplatin, nicotine or both. Subsequently, the cells were treated with cyclohexmide (CHX) and collected at various time points. After the preparation of cell lysates, immunoblotting was performed.
PCR and real-time PCR
Cells were treated with cisplatin, nicotine or both, and then exposed to actinomycin D for different time periods. Subsequently, total RNAs were extracted using RNease Mini Kit (Qiagen, Valencia, CA, USA) following the protocol provided by the manufacturer. One microgram of total RNA was reverse transcribed into the first-strand cDNA using cDNA Synthesis Kit (Promega, Madison, WI, USA). Primers for bcl-2 were designed as: 5′-CTGCGAAGAACCTTGTGTGA-3′ (sense) and 5′-CCGCATGCTGGGGCCGTACA-3′ (antisense). For real-time PCR (RT–PCR), QuantiTect SYBR Green RT–PCR Kit was used (Qiagen).
Statistical analysis
Three to five independent repeats were conducted in all experiments. Error bars represent these repeats. A Student's T-test was used and a P-value <0.05 was considered significant.
Results
Nicotine exposure reduced cisplatin cytotoxicity in lung cancer cells
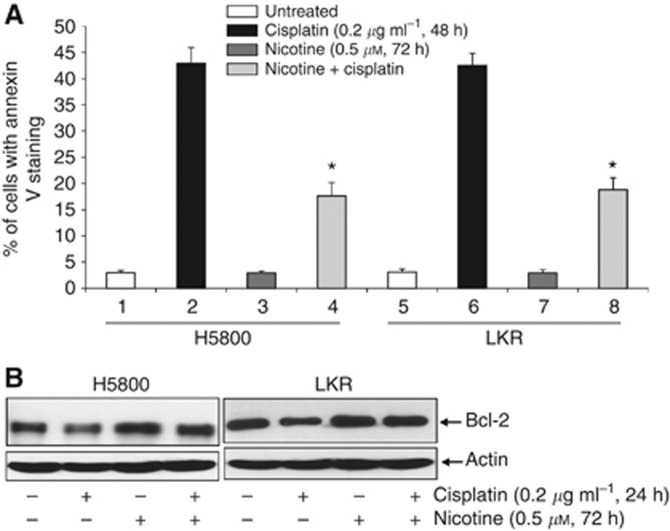
To explore the effect of nicotine exposure on apoptosis induced by anticancer drug cisplatin, human or murine lung cancer H5800, LKR cells were treated with cisplatin in the presence or absence of nicotine, and the induction of apoptosis was then analysed by Annexin V assay (Figure 1A). After the treatment of cisplatin for 24 h, only a few of the cancer cells were apoptotic (data not shown). Apoptosis became evident following 48 h of cisplatin treatment, in which more than 35% of the cancer cells were dead. The exposure of nicotine alone for 72 h had no role in cell viability. However, such prolonged nicotine treatment significantly attenuated the magnitude of cisplatin-mediated apoptosis to about two-fold reduction.
Figure 1.
Increase of prosurvival activity mediated by nicotine exposure. (A) H5800 and LKR cells were treated with nicotine (0.5 μM) for 72 h, cisplatin (0.2 μg ml−1) for 48 h or a combination of both (by exposing cells with nicotine for 24 h, before cisplatin for another 48 h). After cell lysates were prepared, Annexin V assay was performed. The error bars are s.d. from five independent experiments (n=5, *P<0.05). (B) The cells were treated with nicotine (0.5 μM) for 72 h, cisplatin (0.2 μg ml−1) for 24 h or a combination of both. Subsequently, cell lysates were prepared and subjected to immunoblotting for Bcl-2 expression. The blots were reprobed with anti-β-actin antibody to determine equal loading of total proteins per lane.
We previously reported that nicotine exposure desensitised cancer cells to apoptosis and Bcl-2 appeared responsible for the increase of cell survival. Therefore, the expression of Bcl-2 in H5800 and LKR cells was examined after the treatment of cisplatin for 24 h when the occurrence of apoptosis was not evident, of nicotine for 72 h or a combination treatment of both (Figure 1B). The level of Bcl-2 in cisplatin-treated cells was decreased in comparison with that of untreated cells. In nicotine-treated cells, the expression amount of Bcl-2 was increased to about 0.7-fold higher than that in the untreated controls, which was not further augmented by the cotreatment with cisplatin.
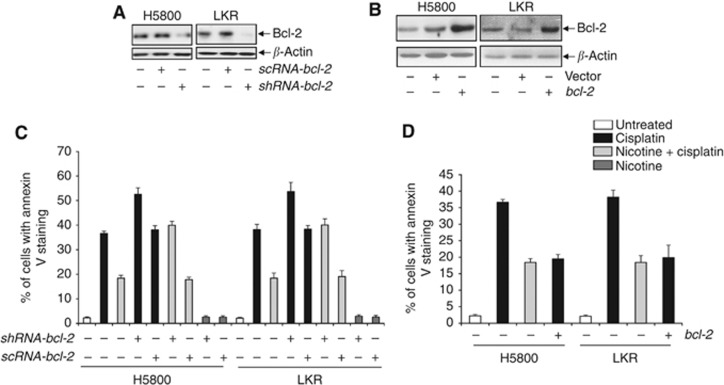
To further determine the role of Bcl-2 in cisplatin-induced cytotoxicity under the influence of nicotine, shRNA-bcl-2 or bcl-2 was introduced into the lung cancer cells. The shRNA, but not scRNA, efficiently knocked down Bcl-2 (Figure 2A). In bcl-2 transfectants, Bcl-2 protein was significantly overexpressed and the transfection of the vector alone had no role in the expression of this prosurvival factor (Figure 2B). Subsequently, the induction of apoptosis was analysed by Annexin V assay in the absence of bcl-2 (Figure 2C) or under the condition that bcl-2 was overexpressed (Figure 2D). About 40% of the cells became apoptotic after the treatment of cisplatin and the cotreatment with nicotine reduced the magnitude of the apoptotic process. The knockdown of bcl-2 by the shRNA increased the sensitivity of the cancer cells to cisplatin and the scRNA had no effect on the magnitude of cisplatin-induced cell death. In nicotine-treated cells, the introduction of the shRNA or scRNA did not have any influence on cell viability. Furthermore, after the overexpression of bcl-2, the lung cancer cells were more resistant to cisplatin, the magnitude of which was similar to that treated with cisplatin and nicotine. The data suggested that the negative influence of nicotine on the susceptibility of the lung cancer cells to cisplatin-induced cell death might be through Bcl-2.
Figure 2.
Effect of Bcl-2 on the resistance to cisplatin-induced apoptosis by nicotine. (A) After the knockdown of bcl-2 with the shRNA or scRNA, the expression of Bcl-2 in H5800 or LKR cells was analyzed by immunoblotting. (B) Following the transfection of wt-bcl-2 or vector into H5800 or LKR cells, lysates from the transfectants were prepared, and then subjected to immunoblotting for Bcl-2 expression. (C) Twenty-four hours after the introduction of shRNA-bcl-2 or scRNA-bcl-2, respectively, the cells were treated with cisplatin, nicotine or a combination of both for 48 h. Cell lysates were prepared and subjected to Annexin V assay. The error bars are s.d. from five independent experiments (n=5, P<0.05). (D) After the transfection of wt-bcl-2, the transfectants were treated as indicated. Subsequently, Annexin V assay was performed. The error bars are s.d. from five independent experiments (n=5, P<0.05).
Bcl-2 protein stability was affected in the cells treated with cisplatin or cotreated with nicotine
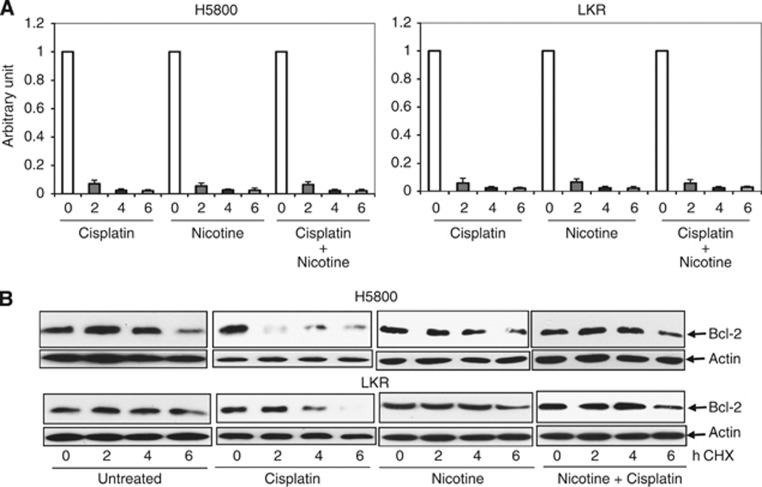
Bcl-2 is an important factor in the promotion of cell growth or survival. The block of cisplatin-mediated apoptosis by nicotine appeared to involve Bcl-2 (Figure 2) and the expression level of this prosurvival factor was changed upon treatment (Figure 1B). It led us to explore how Bcl-2 expression was being regulated in our experimental setting. First, RT–PCR analysis was used to test whether nicotine treatment altered bcl-2 expression at the transcriptional level. The amount of bcl-2 transcripts was not changed following each treatment in comparison with the untreated controls (data not shown). Next, the stability of bcl-2 was examined after the treatment (Figure 3A). The level of bcl-2 expression in untreated cells was served as the control for each treatment. After treatment, actinomycin D was added to the cell cultures to block the process of the gene transcription, the mRNAs were isolated every 2 h for a total of 6 h and then analysed by RT–PCR for bcl-2 expression. The transcripts of bcl-2 in cisplatin-, nicotine- and cotreated cells, 2 h after actinomycin D treatment, were markedly decreased in a similar magnitude to the baseline. Afterwards, the expression of bcl-2 remained undetectable at 4 and 6 h. It suggested that the alteration of Bcl-2 expression observed here was not regulated at the transcriptional level. Subsequently, the protein stability of Bcl-2 was examined by protein pulse-chase analysis (Figure 3B). After adding CHX to block protein synthesis, cell lystates were extracted at various time points and subjected to immunoblotting. The degradation of Bcl-2 in cisplatin-treated H5800 and LKR cells was faster than that in untreated cells. Nicotine treatment alone appeared to have no significant effect on Bcl-2 stability. Interestingly, Bcl-2 became relatively stable in cisplatin-treated cells in the presence of nicotine. Thus, it appeared that the addition of nicotine over-rode the effect of cisplatin, and further prevented Bcl-2 from being degraded.
Figure 3.
Delay of Bcl-2 protein degradation by nicotine. (A) The cells were treated with nicotine for 72 h, cisplatin for 48 h, or a combination of both. Subsequently, actinomycin D (80 ng ml−1) was added in the cell cultures and total RNAs from the cells were isolated every 2 h for RT–PCR analysis. The abundance of bcl-2 in treated cells was normalised to that of untreated cells. The units of the Y axis are arbitrary units. Error bars represent the standard deviation over three independent experiments. (B) After the treatment of nicotine, cisplatin or a combination of both, CHX (20 μM) was added into the cell cultures. Lysates were then prepared every 2 h and subjected for immunoblotting with anti-Bcl-2 antibody. The blot was reprobed with anti-β-actin antibody for the determination of equal loading of total proteins per lane.
Interaction of Bcl-2 and Keap1 was interfered by nicotine in cisplatin-treated cells
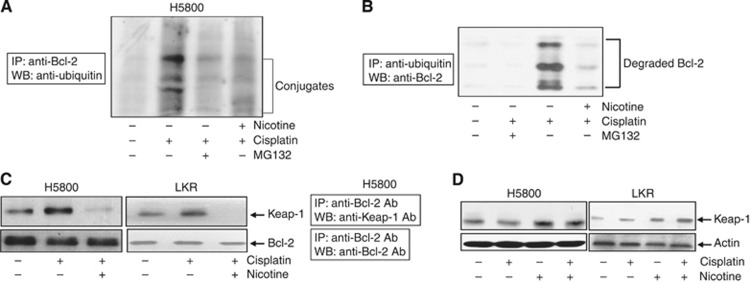
Ubiquitination pathway has a crucial role in protein degradation. In this process, ubiquitins are covalently conjugated with lysine residues of targeted proteins. Studies suggested that Bcl-2 underwent ubiquitination before being degraded (Dimmeler et al, 1999; Niture and Jaiswal, 2011). However, it was unclear if nicotine might affect the ubiquitination process and further targeted Bcl-2 degradation. Thus, the status of Bcl-2 ubiquitination in H5800 cells cotreated with cisplatin and nicotine was tested, by immunoprecipitation with anti-Bcl-2 antibody and immunoblotting with antiubiquitin antibody (Figure 4A). The addition of cisplatin rapidly triggered the ubiquitination of Bcl-2, which was suppressed by adding MG132 (an ubiquitination inhibitor). Cotreatment with nicotine also significantly attenuated Bcl-2 ubiquitination process. The reciprocal experiment was also performed using antiubiquitin antibody for immunoprecipitation and anti-Bcl-2 antibody for immunoblotting (Figure 4B); a similar result was obtained. The effect of nicotine exposure on Bcl-2 ubiquitination in LKR cells was also examined in the same experimental set-up and similar results were also obtained (data not shown).
Figure 4.
Interference of Keap1-mediated Bcl-2 ubiquitination by nicotine. (A) H5800 cells were treated with ciplatin or cotreated with nicotine in the presence or absence of MG132. The cell lysates were prepared and subsequently immunoprecipitated with anti-Bcl-2 antibody. Afterwards, the immunoprecipitates were subjected to immunoblotting with anti-ubiquitin antibody. (B) The cells were subjected to the treatment as indicated. The reciprocal immunoprecipitation with anti-ubiquitin antibody and immunoblotting with anti-Bcl-2 antibody were performed. (C) Following the treatment of cisplatin or cotreatment of nicotine, cell lysates were immunoprecipitated with anti-Bcl-2 antibody and then subjected to immunoblotting using an anti-Keap1 antibody. The blot was reprobed with anti-Bcl-2 antibody for the loading control. (D) After the treatment, immunoblotting was performed with anti-Keap1 antibody to test the Keap1 expression. The blots were reprobed with anti-β-actin antibody for the determination of equal loading of total proteins per lane.
Keap1, as an adaptor protein for Cul3-dependent protein ubiquitination, was shown to interact with Bcl-2 via its DGR (the C-terminal Kelch) domain, and further promote Bcl-2 ubiquitination and degradation (Niture and Jaiswal, 2011). Accordingly, the association of Bcl-2 and Keap1 in our experimental set-up was tested (Figure 4C). A moderate amount of the binding of these two proteins was detected in untreated H5800 or LKR cells. The association of Bcl-2 and Keap1 was markedly augmented following cisplatin treatment. However, cotreatment with nicotine suppressed the interaction of these two molecules. Next, immumoblotting was conducted to test if the expression level of Keap1 in untreated or treated H5800 or LKR cells was altered (Figure 4D). A similar amount of Keap1 was detected in treated or untreated H5800 and LKR cells. The data indicated that cisplatin, by affecting the Keap1-mediated ubiquitination, accelerated Bcl-2 degradation, and this process was interfered by nicotine.
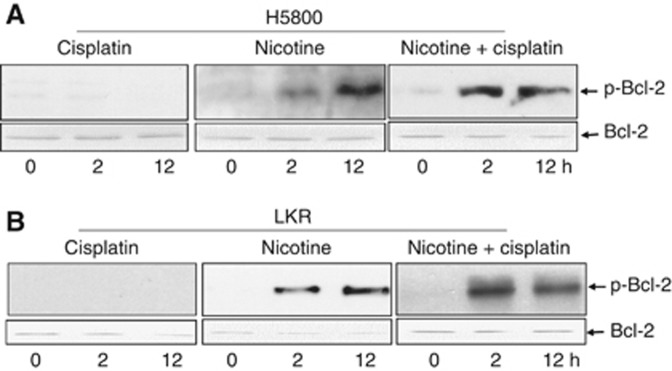
Nicotine treatment prolonged Bcl-2 phosphorylation
Protein phosphorylation and dephosphorylation are often required before undergoing ubiquitination-dependent degradation. The dephosphorylation of Bcl-2 was shown to be a necessary step for its ubiquitination (Dimmeler et al, 1999). On the basis of this information, the phosphorylation status of Bcl-2 in treated or untreated H5800 (Figure 5A) and LKR (Figure 5B) cells was analysed. This prosurvival factor was not phosphorylated in the cells after 2 or 12 h of cisplatin treatment. Bcl-2 phosphorylation was slightly detected in the cells treated with nicotine for 2 h, the level of which was increased at 12 h after the treatment. However, the kinetics of Bcl-2 phosphorylation were very different in the cotreated cells. A high amount of Bcl-2 was phosphorylated 2 h after the cotreatment of cisplatin and nicotine, which was significantly decreased at 12 h of the treatment.
Figure 5.
Bcl-2 phosphorylation. After the treatment at different time points, cell lysates from H5800 (A) or LKR (B) were prepared and then subjected to immunoblotting with the anti-phosphor-Bcl-2 antibody. Afterwards, the blots were reprobed with anti-Bcl-2 antibody for the determination of equal loading per lane.
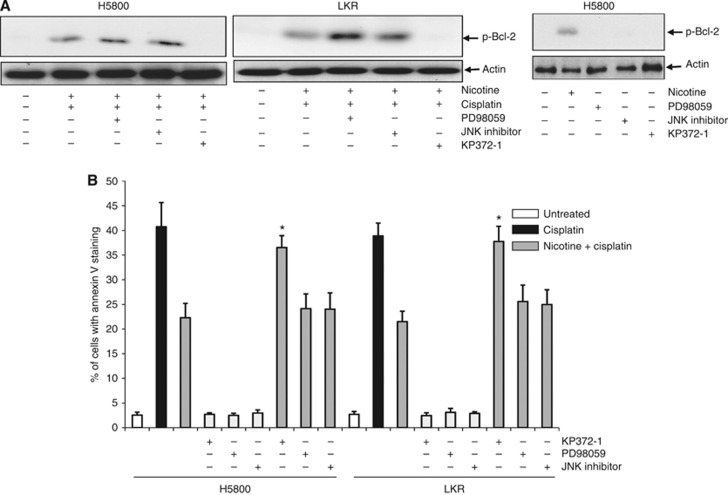
We reported that Akt functioned downstream of nicotine receptor (nAChR) and was responsible for the upregulation of Bcl-2. To test if Akt acted on Bcl-2 phosphorylation, and was responsible for nicotine-mediated increase of Bcl-2 stability, various kinase inhibitors were added to H5800 or LKR cell cultures, before the cotreatment of nicotine and cisplatin. Subsequently, Bcl-2 phosphorylation was examined by immunoblotting (Figure 6A). Two hours after treatment with PD98059 (an inhibitor of MAPK/ERK pathway) or JNK inhibitor, the phosphorylation form of Bcl-2 was still present in the cotreated cells. In comparison, the phosphorylated form of Bcl-2 was absent after the addition of KP372-1 (an Akt inhibitor). Immunoblotting was also performed to test if the addition of these inhibitors alone would have any influence on Bcl-2 phosphorylation. The treatment of PD98059, JNK inhibitor or KP372-1 alone had no role in Bcl-2 phosphorylation. To further link the signalling axis of nicotine, Akt and Bcl-2 to antagonise cisplatin-mediated apoptosis, Annexin V assay was conducted (Figure 6B). More than 40% of H5800 or LKR cells underwent apoptosis after cisplatin treatment, the magnitude of which was attenuated by the cotreatment with nicotine. The addition of PK372-1, but not of PD98059 or JNK inhibitor, abolished nicotine-mediated prosurvival activity and resensitised the cotreated cells to apoptosis. Taken together, the results suggested that nicotine, by activating Akt, prevented Bcl-2 to be dephosphorylated and thereby enhanced the resistance of the lung cancer cells to cisplatin treatment.
Figure 6.
Role of Akt in Bcl-2 activation. (A) The cells were treated with various inhibitors 30 min before the cotreatment of nicotine and cisplatin. Subsequently, cell lysates were prepared and then subjected to immunoblotting with the anti-phosphor-Bcl-2 antibody. β-Actin was used for the determination of equal loading of total proteins per lane. (B) The cells were treated as indicated. Lysates were prepared and subjected to Annexin V assay. The error bars are s.d. from five independent experiments (n=5, *P<0.05).
Discussion
It is estimated that more than 5 million mortalities annually worldwide in human beings are related to cigarette smoke (Brunnemann and Hoffmann, 1991; Karnath, 2002; Burns, 2003). Emerging evidence from epidemiological or biomedical investigations indicates that the carcinogens in cigarette smoke attack the integrity of the genome and promote cell survival activities for tumourigenesis (Brunnemann and Hoffmann, 1991; Hecht, 2002; Karnath, 2002; Burns, 2003). Nicotine, as a major component of cigarette smoke, was shown to influence directly cell growth-related activities in non-neuronal cells via the ligation with its receptor. nAChR not only exists in neurons and neuromuscular junctions but is also present in various non-neuronal organs, tissues or cells (Manneckjee and Minna, 1990; Guo et al, 2008; Singh et al, 2011). The engagement of nAChR has been shown to accelerate cell growth as well as to enhance drug resistance (Dasgupta and Chellappan, 2006a; Dasgupta et al, 2006b; Wessler and Kirkpatrick, 2008; Resende and Adhikari, 2009). Studies demonstrated that cigarette smoking or nicotine treatment, by upregulating Bcl-2, decreased the susceptibility of cancer cells to apoptosis or augmented the resistance of cancer cells to chemotherapies (Zangemeister-Wittke et al, 1998; Losert et al, 2007; Resende and Adhikari, 2009). However, the underlying mechanisms by which nicotine targets Bcl-2 for the establishment of chemoresistance remained enigma. In this study, we demonstrated that the engagement of nicotine in human and murine lung cancer cells might, by activating Akt, cause the prolonged phosphorylation of Bcl-2. Therefore, Bcl-2 was unable to associate with Keap1 in cisplatin-treated lung cancer cells, leading to a significant delay of Bcl-2 ubiquitination and degradation. As a result, the susceptibility of the lung cancer cells to cisplatin-mediated cytotoxicity was markedly reduced. Collectively, the data indicated the importance of Akt and Bcl-2 signalling, governed by nicotine, in the establishment of cisplatin resistance in lung cancer cells and identified these signalling molecules as potential targets for improving the efficacy of cisplatin-related chemotherapies.
Nicotine is commonly used for the cessation of cigarette smoking or relief of chronic pain. However, many studies demonstrated that this major cigarette smoke compound could facilitate tumourigenesis in various human organs or cells. Upon nicotine exposure, mitogenic-related signalling pathways (such as Akt) were activated and antiapoptotic factors (such as Bcl-2) were upregulated (Mai et al, 2003; Dasgupta et al, 2006c; Nishioka et al, 2011). Apoptotic stimuli were shown to regulate Bcl-2 stability via affecting the ubiquitination-dependent protein degradation (Dimmeler et al, 1999; Niture and Jaiswal, 2011). Keap1 is an adaptor for Cul3/Rbx1-mediated degradation of Nrf2 (Zhang et al, 2004; Niture and Jaiswal, 2011). The C terminus of Keap1 is critical for its binding to Nrf2 (Wakabayashi et al, 2004; Eggler et al, 2005; Niture et al, 2009). Upon association, Nrf2 underwent ubiquitination and further degradation during oxidation- or radiation-induced stresses. It has also been shown that Keap1 used the same domain to interact with Bcl-2 and further activated Cul3/Rbx1-dependent degradation (Niture and Jaiswal, 2011). The present study demonstrated that nicotine interfered with the binding of Bcl-2 to Keap1 in cisplatin-treated cancer cells, resulting in the suppression of the process of ubiquitination and further increasing the stability of Bcl-2. Thus, the association of these two molecules appears as a potential target of nicotine for antagonising the efficacy of cisplatin.
The protein modifications of Keap1 partner proteins are the important events in the regulation of their associations with Keap1 and subsequently ubiquitination and degradation (Weissman, 1997; Cullinan et al, 2004; Zhang et al, 2004). In TNFα-induced apoptosis, MAPK/ERK1/2 signalling was suggested to be responsible for Bcl-2 activation (Dimmeler et al, 1999). The treatment with MAPK inhibitor blocked Bcl-2 phosphorylation and subsequently triggered the degradation of this prosurvival factor. Consistently, our present study demonstrated that nicotine treatment delayed Bcl-2 phosphorylation, which probably prevented Bcl-2 from associating with Keap1. In addition, the magnitude of Bcl-2 phosphorylation became evident at the late time period of nicotine treatment, which did not prolong the pattern of its degradation (data not shown). It is possible that upon cisplatin treatment, nicotine specifically affects a particular residue of Bcl-2 that timely controls the process of ubiquitination. It is also conceivable that the existence of a phosphatase can be activated by cisplatin and negatively interfered by nicotine. The investigation of identifying Bcl-2 phosphorylation site and the involvement of other factor(s) in the regulation of Bcl-2 degradation is under way.
Nicotine is not a conventional carcinogen, but was able to induce the secretion of growth factors, resulting in the activation of Raf, Akt or PKC pathways to promote the growth of various epithelial or cancer cells (Rakowicz-Szulczynska et al, 1994; Mathur et al, 2000; Kolmakova and Chatterjee, 2005). Upon nAChR ligation, Src tyrosine kinase was activated and acted as a key regulator linking nAChR signalling to several mitogenic-related kinase pathways, such as Akt and ERK1/2 (Dasgupta et al, 2006c; Nishioka et al, 2011). We previously demonstrated that Akt acted downstream of nAChR/Src for the upregulation of Bcl-2, which accounted for nicotine-mediated antiapoptotic activity (Nishioka et al, 2010). In this study, we further demonstrated the possible underlying mechanism by which nicotine activates Akt that is responsible for the attenuation of Bcl-2 being dephosphorylated in cisplatin-treated cells. As a result, Bcl-2 was unable to bind to Keap1, which prevented it from being ubiquitinated and further degraded.
It is known that cisplatin resistance is established through different mechanisms, such as changes in the drug uptake, efflux, detoxification or response to drug-induced stress. It has been shown that aberrant activity of protein kinases had a pivotal role in the establishment of the resistance to anticancer drugs in human small-cell lung cancer cells (Biswas et al, 2004; Niture et al, 2009). Bcl-2 was suggested to be involved in regulating drug sensitivity or acquiring chemoresistance in cancer cells (Ruvolo et al, 1998; Biswas et al, 2004). The study also showed that the sensitivity of cancer cells to cisplatin was closely related to the activation or phosphorylation status of Bcl-2 (Ruvolo et al, 1998; Mai et al, 2003; Biswas et al, 2004). Our current study again proved the influence of nicotine on mitogenic signalling and Bcl-2 activation, which rendered lung cancer cells resistant to cisplatin.
In summary, the results presented here identified a novel nicotine-mediated prosurvival pathway that involves the attenuation of Bcl-2 ubiquitination and further degradation in cisplatin-treated lung cancer cells. In this process, nicotine appeared to prolong Bcl-2 phosphorylation status, probably via activating Akt. Thus, Bcl-2 was unable to interact with Keap1 and to be quickly degraded. As lung cancer is closely related to cigarette smoke and the patients often use nicotine for their needs of smoking or pain relief, the current study not only demonstrated the link between nicotine and the cisplatin resistance but also provided the information for making better clinical regimen.
Acknowledgments
We thank Dr T Jacks (MIT, Cambridge, MA, USA) for providing LKR cells and Dr Z Luo (Boston University School of Medicine, Boston, Ma, USA) for providing MCF10A cells. This study is supported by National Institutes of Health Grant R01 CA124490 (to CC) and by Flight Attendant Medical Research Institute Award 062450-CIA (to CC).
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Arredondo J, Chernyavsky AI, Grando SA. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol Ther. 2006;5:511–517. doi: 10.4161/cbt.5.5.2601. [DOI] [PubMed] [Google Scholar]
- Assis GF, Ceolin DS, Marques ME, Salvadori DM, Ribeiro DA. Cigarette smoke affects apoptosis in rat tongue mucosa: role of bcl-2 gene family. J Mol Histol. 2005;36:483–489. doi: 10.1007/s10735-006-9023-z. [DOI] [PubMed] [Google Scholar]
- Band PR, Le ND, Fang R, Deschamps M. Carcinogenic and endocrine disrupting effects of cigarette smoke and risk of breast cancer. Lancet. 2002;360:1044–1049. doi: 10.1016/S0140-6736(02)11140-8. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Huang J, Persaud S, Basu A. Down-regulation of Bcl-2 is associated with cisplatin resistance in human small cell lung cancer H69 cells. Mol Cancer Ther. 2004;3:327–334. [PubMed] [Google Scholar]
- Borner MM, Brousset P, Pfanner-Meyer B, Bacchi M, Vonlanthen S, Hotz MA, Altermatt HJ, Schlaifer D, Reed JC, Betticher DC. Expression of apoptosis regulatory proteins of the Bcl-2 family and p53 in primary resected non-small-cell lung cancer. Br J Cancer. 2002;79:952–958. doi: 10.1038/sj.bjc.6690152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschopf K, Haendeler J, Maichow P, Zeiher AM, Dimmeier S. Posttranlational modification of Bcl-2 facilitates its proteasome-dependent degradation: molecular characterization of the involved signaling pathway. Mol Cell Biol. 2000;20:1886–1896. doi: 10.1128/mcb.20.5.1886-1896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunnemann KD, Hoffmann D. Analytical studies on tobacco-specific N-nitrosamines in tobacco and tobacco smoke. Crit Rev Toxicol. 1991;21:235–240. doi: 10.3109/10408449109017910. [DOI] [PubMed] [Google Scholar]
- Burns DM. Tobacco-related diseases. Seminars in Oncology Nursing. 2003;19:244–249. doi: 10.1053/j.soncn.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin–proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Gordan JD, Jin J, Haper JW, Diehl JA. The Keap1BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta P, Chellappan SP. Nicotine-mediated cell proliferation and angiogenesis: new twists to an old story. Cell Cycle. 2006;5:2324–2328. doi: 10.4161/cc.5.20.3366. [DOI] [PubMed] [Google Scholar]
- Dasgupta P, Kinkade R, Joshi B, DeCook C, Haura E, Chellappan S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and surviving. Proc Natl Acad Sci USA. 2006;103:6332–6337. doi: 10.1073/pnas.0509313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappan S. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116:2208–2217. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Breitschopf K, Kaendeler J, Zeiher AM. Dephosphorylation targets Bcl-2 for ubiqutin-dependent degradation: a link between the apoptosome and the proteasome pathway. J Exp Med. 1999;189:1815–1822. doi: 10.1084/jem.189.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci USA. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs CS, Colditz GA, Stampfer MJ, Giovannucci EL, Hunter DJ, Rimm EB, Willett WC, Speizer FEA. Prostective study of cigarette smoking and the risk of pancreatic cancer. JAMA Intern Med. 1996;156:2255–2260. [PubMed] [Google Scholar]
- Gallo O, Bianchi S, Porfirio B. Bcl-2 overexpression and smoking history in head and neck cancer. J Natl Cancer Inst. 1995;87:1024–1025. doi: 10.1093/jnci/87.13.1024. [DOI] [PubMed] [Google Scholar]
- Guo J, Ibaragi S, Zhu T, Luo LY, Hu GF, Huppi P, Chen C. Nicotine promotes mammary tumor migration via a signaling cascade involving protein kinase C and cdc42. Cancer Res. 2008;68:8473–8481. doi: 10.1158/0008-5472.CAN-08-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Chang E, Aicher A, Cooke JP. Endothelial progenitor cells participate in nicotine-mediated angiogenesis. J Am Coll Cardiol. 2006;48:2553–2560. doi: 10.1016/j.jacc.2006.07.066. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Jang JJ, Weis M, Pathak a, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atheroscierosis. Nat Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest. 2002;110:527–536. doi: 10.1172/JCI14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeschen C, Weis M, Cooke JP. Nicotine promotes arteriogenesis. J Am Coll Cardiol. 2003;41:489–496. doi: 10.1016/s0735-1097(02)02818-8. [DOI] [PubMed] [Google Scholar]
- Hirata N, Sekino Y, Kanda Y. Nicotine increases cancer stem cell population in MCF-7 cells. Biochem Biophy Res Comm. 2010;403:138–143. doi: 10.1016/j.bbrc.2010.10.134. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. Protein degradation or regulation: Ub the judge. Cell. 1996;84:813–815. doi: 10.1016/s0092-8674(00)81058-2. [DOI] [PubMed] [Google Scholar]
- Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- Jull B, Plummer HK, Schuller H. Nicotinic receptor-mediated activation by the tobacco-specific nitrosamine NNK of a Raf-1/MAP kinase pathway, resulting in phosphorylation of c-myc in human small cell lung carcinoma cells and pulmonary neuroendocrine cells. J Cancer Res Clin Oncol. 2001;127:707–717. doi: 10.1007/s004320100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath B. Smoking cessation. Am J Med. 2002;112:399–405. doi: 10.1016/s0002-9343(01)01126-3. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Kock N, Kasmieh R, Weissleder R, Shah K. Tumor therapy mediated by lentiviral expression of shBcl-2 and S-TRAIL. Neoplasia. 2007;9:435–442. doi: 10.1593/neo.07223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmakova A, Chatterjee S. Platelet derived growth factor recruits lactosylceramide to induce cell proliferation in UDP Gal:GlcCer;beta1–4 galactosyltransferase mutant Chinese hamster ovary cells. Glycol J. 2005;22:401–407. doi: 10.1007/s10719-005-3351-1. [DOI] [PubMed] [Google Scholar]
- Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- Kunigai S, Ponnusamy MP, Momi N, Batra SK, Chellappan SP. Nicotine, IFN-gama and retinoic acid mediated induction of MUC4 in pancreatic cancer requires E2F1 and STAT-1 transcription factors and utilize different signaling cascades. Mole Cancer. 2012;11:24. doi: 10.1186/1476-4598-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losert D, Pratscher B, Soutschek J, Geick A, Vornlocker HP, Muller M, Wacheck V. Bcl-2 downregulation sensitizes nonsmall cell lung cancer cells to cisplatin, but not to docetaxel. Anitcancer Drug. 2007;18:755–761. doi: 10.1097/CAD.0b013e3280adc8c8. [DOI] [PubMed] [Google Scholar]
- Mai H, May WS, Gao F, Jin Z, Deng X. A functional role for nicotine in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem. 2003;278:1886–1891. doi: 10.1074/jbc.M209044200. [DOI] [PubMed] [Google Scholar]
- Manneckjee R, Minna JD. Opioid and nicotine receptor affect growth regulation of human lung cancer cell lines. Proc Natl Acad Sci USA. 1990;87:3294–3298. doi: 10.1073/pnas.87.9.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur RS, SPk Mathur, Young RC. Up-regulation of epidermal growth factor-receptors by nicotine in cervical cancer cell lines: this effect may be mediated by EGF. Am J Reprod Immunol. 2000;44:114–120. doi: 10.1111/j.8755-8920.2000.440207.x. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Opiekun M, Oka H, Vachani A, Albelda SM, Yamazaki K, Beauchamp GK. Urinary volatile compounds as biomarkers for lung cancer: a proof of principle study using odor signatures in mouse models of lung cancer. PLoS One. 2010;5:e8819–e8824. doi: 10.1371/journal.pone.0008819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Numakawa T, Ikeuchi T. Nicotine-induced phosphorylation of Akt through epidermal growth factor receptor and Src in PC12h cells. J Neurochem. 2002;83:1372–1379. doi: 10.1046/j.1471-4159.2002.01248.x. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Guo J, Yamamoto D, Chen L, Huppi P, Chen C. Nicotine, through upregulating pro-survival signaling, cooperates with NNK to promote transformation. J Cell Biochem. 2010;109:152–161. doi: 10.1002/jcb.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka T, Kim Y, Luo L-Y, Huang Y, Guo J, Chen C. Sensitization of epithelial growth factor receptor by nicotine exposure to promote breast cancer cell growth. Breast Cancer Res. 2011;13:R113. doi: 10.1186/bcr3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niture SK, Jain AK, Jaiswal AK. Antioxidant-induced modificationof INrf2C151 and PKC delta-mediated phosphorylation of Nrf2S40 both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. J Cell Sci. 2009;122:4452–4464. doi: 10.1242/jcs.058537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Niture SK, Jaiswal AK. INrf2 (Keap1) targets Bcl-2 degradation and controls cellular apoptosis. Cell Death Differ. 2011;18:439–451. doi: 10.1038/cdd.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- Rakowicz-Szulczynska EM, Mclntosh DG, Smith M. Growth factor-mediated mechanisms of nicotine-dependent carcinogenesis. Carcinogenesis. 1994;15:1839–1846. doi: 10.1093/carcin/15.9.1839. [DOI] [PubMed] [Google Scholar]
- Resende RR, Adhikari A. Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun Signal. 2009;7:1–20. doi: 10.1186/1478-811X-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y, Distelhorst CW. Bcl-2 protein family members versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- Ruvolo PP, Deng X, Carr BK, May WS. A functional role for mitochondrial protein kinase Calpha in Bcl-2 phosphorylation and suppression of apoptosis. J Biol Chem. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol. 2011;2011:1–11. doi: 10.1155/2011/456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Dinkova-Kostova AT, Holzdaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman AM. Regulating protein degradation by ubiquitination. Immunol Today. 1997;18:189–198. doi: 10.1016/s0167-5699(97)84666-x. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmcol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild-Bode C, Weller M, Wick W. Molecular determinats of glioma cell migration and invasion. J Neurosurg. 2001;94:978–984. doi: 10.3171/jns.2001.94.6.0978. [DOI] [PubMed] [Google Scholar]
- Zangemeister-Wittke U, Schenker T, Luedke GH, Stahel RA. Synergistic cytotoxicity of bcl-2 antisense oligodeoxynucleotides and etoposide, doxorubicin and cisplatin on small cell lung cancer cell lines. Br J Cancer. 1998;78:1035–1042. doi: 10.1038/bjc.1998.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zereu M, Vinholes JJF, Zettler GC. P53 and Bcl-2 protein expression and its relationship with prognosis in small cell lung cancer. Clin Lung Cancer. 2003;4:298–302. doi: 10.3816/clc.2003.n.010. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]