Abstract
Background:
Serum total human chorionic gonadotrophin β subunit (hCGβ) level might have prognostic value in urothelial transitional cell carcinoma (TCC) but has not been investigated for independence from other prognostic variables.
Methods:
We utilised a clinical database of patients receiving chemotherapy between 2005 and 2011 for urothelial TCC and an independent cohort of radical cystectomy patients for validation purposes. Prognostic variables were tested by univariate Kaplan–Meier analyses and log-rank tests. Statistically significant variables were then assessed by multivariate Cox regression. Total hCGβ level was dichotomised at < vs ⩾2 IU l−1.
Results:
A total of 235 chemotherapy patients were eligible. For neoadjuvant chemotherapy, established prognostic factors including low ECOG performance status, normal haemoglobin, lower T stage and suitability for cisplatin-based chemotherapy were associated with favourable survival in univariate analyses. In addition, low hCGβ level was favourable when assessed either before (median survival not reached vs 1.86 years, P=0.001) or on completion of chemotherapy (4.27 vs 0.42 years, P=0.000002). This was confirmed in multivariate analyses and in patients receiving first- and second-line palliative chemotherapy, and in a radical cystectomy validation set.
Conclusions:
Serum total hCGβ level is an independent prognostic factor in patients receiving chemotherapy for urothelial TCC in both curative and palliative settings.
Keywords: hCGβ, chemotherapy, cystectomy, prognosis, transitional cell carcinoma, urothelial carcinoma
Approximately 10 000 new bladder cancers are diagnosed annually in the United Kingdom and over 90% are transitional cell carcinomas (TCC) (Crabb and Wheater, 2010). Perioperative cisplatin-based chemotherapy provides a 5–6% absolute survival advantage for operable muscle invasive bladder TCC and a modest survival gain in metastatic disease (which may include TCC occurring in other parts of the urothelial tract) (Logothetis et al, 1990; von der Maase et al, 2000; Sternberg et al, 2001; Grossman et al, 2003; von der Maase et al, 2005; Advanced Bladder Cancer (ABC) Meta-analysis Collaboration, 2005a, 2005b; Crabb and Wheater, 2010; International Collaboration of Trialists et al, 2011). Improved prognostic characterisation to facilitate stratification for treatment would be valuable.
Various prognostic factors are established for urothelial tract TCC on treatment with chemotherapy. In the neoadjuvant setting (bladder TCC), favourable prognostic factors are pathological complete response in those undergoing cystectomy and lower T stage (Grossman et al, 2003). In advanced disease, performance status and disease extent are key prognostic factors (Mead et al, 1998; Bajorin et al, 1999). Bajorin et al (1999) described a retrospective cohort where Karnofsky performance status <80% and visceral metastases were independent poor prognostic factors. In a phase III trial comparing cisplatin-based regimens, good prognostic factors included Karnofsky performance status >70%, no M1 disease, low/normal alkaline phosphatase, ⩽3 disease sites and no visceral metastases (von der Maase et al, 2005). Predictive biomarker development for TCC has been unsuccessful to date (Stadler et al, 2011). Various prognostic or predictive molecular characterisation models in retrospective cohorts have been proposed warranting prospective validation of their potential for therapy selection (Dyrskjot et al, 2003; Takata et al, 2005; Sanchez-Carbayo et al, 2006; Mengual et al, 2009; Mitra et al, 2009; Smith et al, 2011).
Human chorionic gonadotrophin (hCG) is a heterodimeric glycoprotein secreted by trophoblastic cells during gestation with placental, uterine and fetal regulatory roles. The α subunit is common to hCG, LH, FSH and TSH, whereas the β subunit (hCGβ) is distinct to the variant hCG forms. Serum hCGβ levels are a key tumour marker for trophoblastic and germ cell cancers. In addition, hCGβ is elevated in various solid epithelial malignancies, including TCC, with links in some to poor prognosis (Iles, 2007).
We hypothesised that total hCGβ level would function as an independent prognostic marker in patients undergoing chemotherapy for urothelial TCC and report data to demonstrate this.
Materials and Methods
Patients and data collection
We undertook retrospective analysis of consecutive patients treated with systemic chemotherapy for invasive urothelial tract cancer at University Hospital Southampton NHS Foundation Trust, UK, between 2005 and 2011. Eligibility criteria were age ⩾18, confirmed pure or mixed histology TCC, and muscle invasive disease and/or nodal or metastatic spread (staged T2–4 and/or N1–3 and/or M1) at first use of chemotherapy. Data collection was through retrospective case note review with data lock on 5 January, 2013. An independent validation set of patients undergoing radical cystectomy for bladder TCC but not perioperative chemotherapy was also created with data lock of 2 August, 2013.
Patients receiving chemotherapy or surgery were managed by oncologists and urologists with specialist interests in urothelial cancer and consistent with regionally approved treatment guidelines. The treating institution undertook specialist multidisciplinary review of all diagnostic and staging investigations for all patients.
Chemotherapy analyses were undertaken in three prospectively defined patient cohorts. The ‘neoadjuvant cohort' received chemotherapy before either radical surgery or radiotherapy with curative intent for disease staged T2–4 N0 M0. The ‘first line cohort' either received chemotherapy for newly diagnosed disease staged Tany N1–3 M0 or Tany Nany M1 or previously received perioperative (adjuvant or neoadjuvant) chemotherapy and were then subsequently treated again at disease relapse. The ‘second line cohort' comprised all patients from the first-line cohort treated with subsequent chemotherapy at disease progression.
This research had UK National Research Ethics Service committee approval (10/H0405/99).
Statistical analyses
Overall survival was from the first day of the relevant course of chemotherapy, or the date of cystectomy in the validation set, to death. Progression free survival (first- and second-line cohorts) was from the first day of the relevant course of chemotherapy to disease progression or death from any cause. Relapse free survival (RFS) was from the first day of chemotherapy (neoadjuvant cohort), or cystectomy (validation set), to the first local, regional or distant recurrence or death from any cause. Statistical analysis was performed with SPSS, version 20.0 (IBM, Portsmouth, UK). Univariate analyses of survival outcomes were by the Kaplan–Meier method and log-rank tests. Statistically significant prognostic factors in univariate overall survival analyses were included in multivariate Cox regression analyses to determine hazard ratios as previously described (Crabb et al, 2008a, 2008b). P values <0.05 were considered statistically significant.
hCGβ measurement
Total hCGβ levels in serum samples were determined by an accredited UK National Health Service chemical pathology department using a quantitative chemiluminescent immunoassay on a Beckman Coulter DxI immunoassay system (product number 33500, Beckman Coulter, High Wycombe, UK). Blood samples used were those taken as part of routine clinical practice before and immediately following a course of chemotherapy. We prospectively dichotomised hCGβ levels at < vs ⩾2 IU l−1. hCGβ levels were the most recent before initiation, or the first following completion, of a course of chemotherapy within a 28-day window. Levels outside these time constraints were excluded.
Results
Chemotherapy cohorts and hCGβ levels
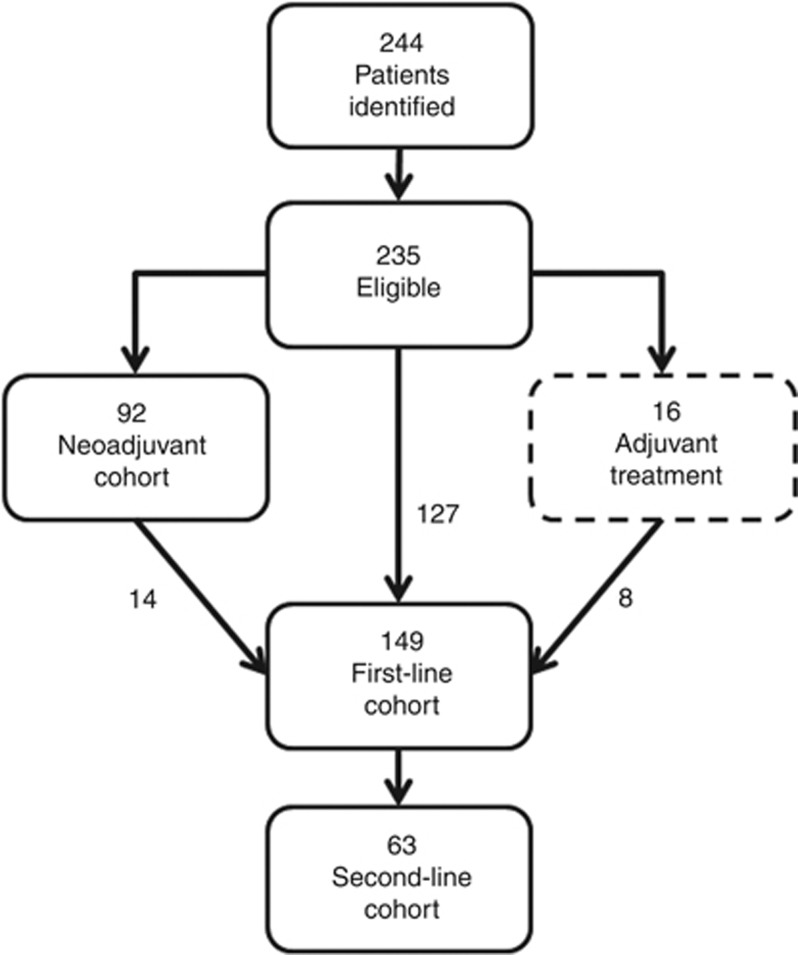
A total of 244 patients received chemotherapy for urothelial TCC between 2005 and 2011, of whom 235 met the inclusion criteria (Figure 1). A total of 92 and 149 received chemotherapy within the neoadjuvant and first-line cohorts, respectively. A total of 16 patients received adjuvant chemotherapy following radical cystectomy. A total of 14 and 8 patients receiving neoadjuvant or adjuvant chemotherapy, respectively, were also treated within the first-line cohort at disease relapse. A total of 63 patients had second-line chemotherapy.
Figure 1.
Flow diagram for chemotherapy cohorts used in this study.
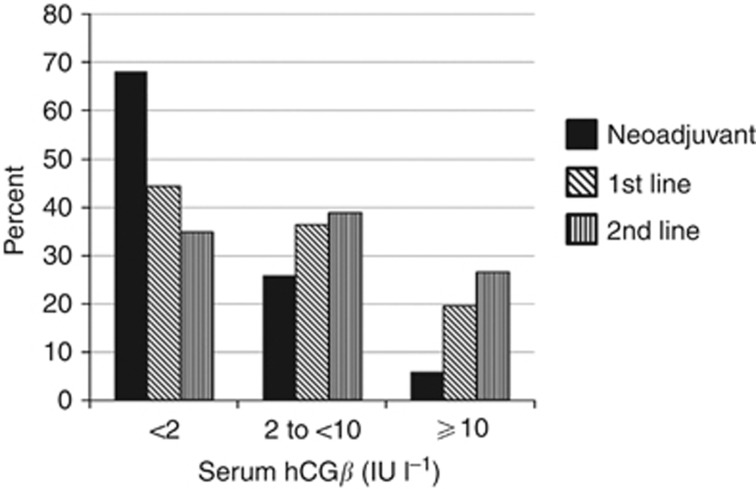
Table 1 shows clinico-pathological characteristics and hCGβ levels before, and on completion of, chemotherapy. In the neoadjuvant cohort, 90.2% received gemcitabine/cisplatin (GC) and the rest gemcitabine/carboplatin (GCarbo). For the first-line cohort corresponding figures were 51% and 33.6%, respectively, with the remainder mostly receiving gemcitabine alone due to poor performance status. hCGβ level, where available, was <2 IU l−1 before neoadjuvant, first-line and second-line chemotherapy in 68%, 44% and 35%, respectively. The percentage with low (<2 IU l−1) hCGβ levels fell with respect to line of treatment with a higher proportion with more advanced disease presenting with intermediate (2 to <10 IU l−1) or higher (⩾10 IU l−1) levels (Figure 2).
Table 1. Patient characteristics in the chemotherapy cohorts.
| Neoadjuvant chemotherapy cohort, n=92 | First-line chemotherapy cohort, n=149 | |
|---|---|---|
|
Age | ||
| Median | 69 | 69 |
| Range | 48–84 | 34–92 |
| ⩽70 | 55 (59.8%) | 77 (51.7%) |
| >70 |
37 (40.2%) |
72 (48.3%) |
|
Sex | ||
| Male | 65 (70.7%) | 109 (73.2%) |
| Female |
27 (29.3%) |
40 (26.8%) |
|
ECOG PS | ||
| 0 or 1 | 84 (91.3%) | 106 (71.1%) |
| ⩾2 | 5 (5.4%) | 28 (18.8%) |
| Unknown |
3 (3.3%) |
15 (10.1%) |
|
Hb | ||
| Median (g l−1) | 135 | 125 |
| Range | 94–177 | 80–170 |
| ⩾LLN | 61 (66.3%) | 69 (46.3% |
| <LLN | 29 (31.5%) | 74 (49.7%) |
| Unknown |
2 (2.2%) |
6 (4.0%) |
|
ALP | ||
| Median (Ul−1) | 88 | 104 |
| Range | 36–342 | 38–1181 |
| ⩽ULN | 79 (85.9%) | 96 (64.4%) |
| >ULN | 9 (9.8%) | 43 (28.9%) |
| Unknown |
4 (4.3%) |
10 (6.7%) |
|
LDH | ||
| Median (IU l−1) | 429 | 439 |
| Range | 321–703 | 272–2115 |
| ⩽ULN | 21 (22.8%) | 42 (28.2%) |
| >ULN | 8 (8.7%) | 23 (15.4%) |
| Unknown |
63 (68.5%) |
84 (56.4%) |
|
Grade | ||
| 2 | 10 (10.9%) | 14 (9.4%) |
| 3 | 81 (88.0%) | 122 (81.9%) |
| Unknown |
1 (1.1%) |
13 (8.7%) |
|
Primary tumour site | ||
| Bladder | 92 (100%) | 111 (74.5%) |
| Renal pelvis | — | 22 (14.8%) |
| Ureteric | — | 13 (8.7%) |
| Urethral |
— |
3 (2.0%) |
|
T stage | ||
| ⩽2 | 44 (47.8%) | 40 (26.8% |
| 3 | 35 (38.0%) | 48 (32.2%) |
| 4 | 13 (14.1%) | 16 (10.7%) |
| X |
0 |
43 (28.9%) |
|
N stage | ||
| 0 | 92 (100%) | 70 (47.0%) |
| 1 | — | 24 (16.1%) |
| 2 | — | 54 (36.2%) |
| 3 |
— |
1 (0.7%) |
|
M stage | ||
| 0 | 92 (100%) | 62 (41.6%) |
| 1 |
— |
87 (58.4%) |
|
Visceral metastases | ||
| No | — | 95 (63.8%) |
| Yes |
— |
54 (36.2%) |
|
Bajorin risk factors (Bajorin et al, 1999) | ||
| 0 | — | 70 (47.0%) |
| 1 | — | 47 (31.5%) |
| 2 | — | 17 (11.4%) |
| Unknown |
— |
15 (10%) |
|
Chemotherapy regimen | ||
| GC | 83 (90.2%) | 76 (51.0%) |
| GCarbo | 9 (9.8%) | 50 (33.6%) |
| Other |
0 |
23 (15.4%) |
|
Prior chemotherapy | ||
| No | — | 127 (85.2%) |
| Yes |
— |
22 (14.8%) |
|
Radical local therapy | ||
| Surgery | 48 (52.2%) | 10 (3.4%) |
| Radiotherapy | 28 (30.4%) | 5 (6.7) |
| None | 14 (15.2%) | 134 (89.9%) |
| Unknown |
2 (2.2%) |
0 |
|
hCGβ
level before chemotherapy | ||
| <2 IU l−1 | 58 (63.0%) | 61 (40.9%) |
| ⩾2 IU l−1 | 27 (29.3%) | 77 (51.7%) |
| Unknown |
7 (7.6%) |
11 (7.4%) |
|
hCGβ
level on completion of chemotherapy | ||
| <2 IU l−1 | 28 (30.4%) | 56 (37.6%) |
| ⩾2 IU l−1 | 5 (5.4%) | 40 (26.8%) |
| Unknown | 59 (64.1%) | 53 (35.6%) |
Abbreviations: ALP=alkaline phosphatase; ECOG PS=Eastern Cooperative Oncology Group performance status; GC=gemcitabine/cisplatin; GCarbo=gemcitabine/carboplatin; Hb=haemoglobin; LDH=lactate dehydrogenase; LLN=lower limit of normal for the treating institution's reference range; ULN=upper limit of normal for the treating institution's reference range.
Figure 2.
Total hCGβ levels before chemotherapy for patients undergoing neoadjuvant, first-line or second-line chemotherapy.
Neoadjuvant chemotherapy
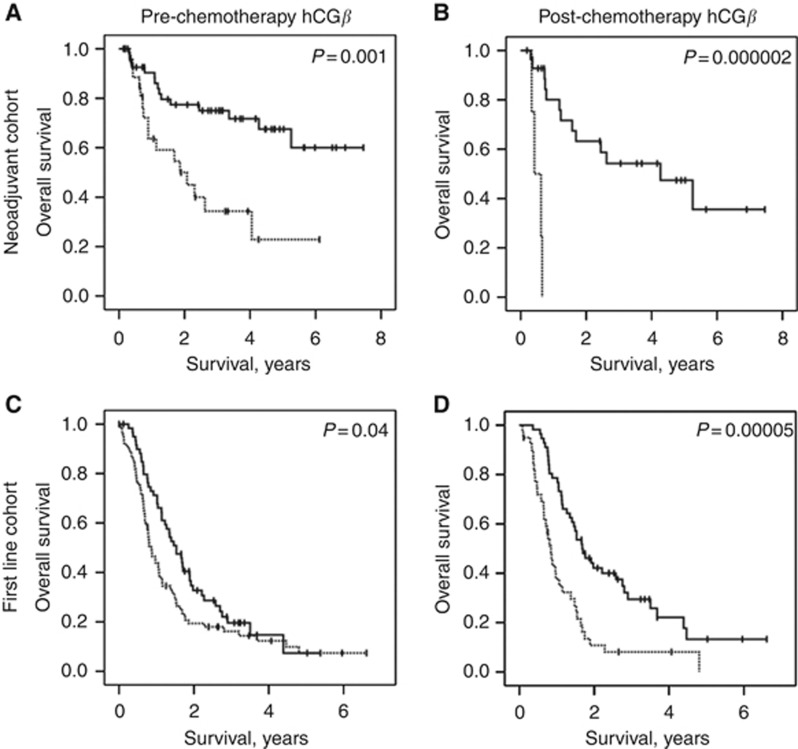
We assessed potential prognostic factors with regard to overall survival following neoadjuvant chemotherapy (Table 2). Favourable factors in univariate analyses for survival were ECOG performance status (0–1 vs ⩾2), haemoglobin (⩾ lower limit of normal, LLN), lower T stage (⩽2 vs 3 vs 4) and suitability for GC (vs GCarbo). In addition, hCGβ level <2 IU l−1 before neoadjuvant chemotherapy was associated with improved survival (median survival not reached vs 1.86 years, P=0.001, Figure 3A). Likewise, hCGβ level <2 IU l−1 following neoadjuvant chemotherapy was also associated with favourable median survival (4.27 vs 0.42 years, P=0.000002, Figure 3B).
Table 2. Univariate analyses to assess individual potential prognostic factors with respect to overall survival following chemotherapy.
| |
|
Neoadjuvant chemotherapy |
First-line chemotherapy | ||
|---|---|---|---|---|---|
| Factor | Division | Median OS, years (95% CI) | P-value | Median OS, years (95% CI) | P-value |
| Age | ⩽70 | NR | 0.08 | 1.13 (0.66–1.61) | 0.21 |
| |
>70 |
3.36 (0.91–5.81) |
|
0.98 (0.68–1.30) |
|
| Sex | Male | 5.26 (4.37–6.15) | 0.49 | 1.16 (0.79–1.53) | 0.84 |
| |
Female |
4.05 (0.88–7.23) |
|
1.02 (0.71–1.33) |
|
| ECOG PS | 0 or 1 | 5.26 (4.52–6.01) | 0.000008 | 1.49 (1.08–1.90) | 0.0000005 |
| |
⩾2 |
0.42 (0.33–0.50) |
|
0.63 (0.47–0.79) |
|
| Hb | ⩾LLN | NR | 0.00005 | 1.39 (0.97–1.82) | 0.15 |
| |
<LLN |
1.22 (0.94–1.51) |
|
0.96 (0.69–1.24) |
|
| ALP | ⩽ULN | NR | 0.08 | 1.35 (1.05–1.65) | 0.003 |
| |
>ULN |
1.14 (0.87–1.41) |
|
0.66 (0.52–0.80) |
|
| LDH | ⩽ULN | 4.27 (0.10–8.44) | 0.34 | 1.23 (0.80–1.66) | 0.58 |
| |
>ULN |
NR |
|
1.04 (0.53–1.55) |
|
| Grade | 2 | 4.05 (2.71–5.40) | 0.90 | 1.02 (0.44–1.90) | 0.87 |
| |
3 |
5.26 (4.49–6.03) |
|
1.14 (0.87–1.41) |
|
| Primary tumour site | Bladder | — | 1.07 (0.78–1.35) | 0.57 | |
| |
Other |
— |
|
1.07 (0.66–1.49) |
|
| T stage | ⩽2 | NR | 0.006 | — | |
| 3 | 5.27 (4.15–6.39) | — | |||
| |
4 |
1.09 (0.61–1.57) |
|
— |
|
| Visceral metastases | No | — | 1.49 (1.03–1.95) | 0.004 | |
| |
Yes |
— |
|
0.78 (0.67–0.88) |
|
| Chemotherapy regimen | GC | NR | 0.001 | 1.49 (1.05–1.93) | 0.00005 |
| GCarbo | 0.89 (0.48–1.31) | 0.80 (0.41–1.20) | |||
| |
Other |
— |
|
0.57 (0.40–0.74) |
|
| Bajorin risk factors (Bajorin et al, 1999) | 0 | — | 1.74 (1.41–2.08) | 0.0000001 | |
| 1 | — | 0.98 (0.78–1.17) | |||
| |
2 |
— |
|
0.47 (0.31–0.65) |
|
| Prior perioperative chemotherapy | No | — | 1.15 (0.90–1.41) | 0.001 | |
| |
Yes |
— |
|
0.60 (0.50–0.70) |
|
| hCGβ level before chemotherapy | <2 IU l−1 | NR | 0.001 | 1.53 (1.17–1.89) | 0.04 |
| |
⩾2 IU l−1 |
1.86 (0.51–3.21) |
|
0.86 (0.67–1.05) |
|
| hCGβ level on completion of chemotherapy | <2 IU l−1 | 4.27 (1.65–6.89) | 0.000002 | 1.68 (1.25–2.11) | 0.00005 |
| ⩾2 IU l−1 | 0.42 (0.14–0.70) | 0.84 (0.68–1.00) | |||
Abbreviations: ALP=alkaline phosphatase; CI=confidence interval; ECOG PS=Eastern Cooperative Oncology Group performance status; GC=gemcitabine/cisplatin; GCarbo=gemcitabine/carboplatin; Hb=haemoglobin; LDH=lactate dehydrogenase; LLN=lower limit of normal for the treating institution's reference range; NR=not reached; ULN=upper limit of normal for the treating institution's reference range.
Figure 3.
Kaplan–Meier plots to show overall survival according to total hCGβ level in the neoadjuvant (A, B) or first-line (C, D) chemotherapy cohorts either before (A, C), or on completion of (B, D) chemotherapy. Broken line – hCGβ level ⩾2; continuous line – hCGβ level <2.
We undertook multivariate analyses for overall survival incorporating factors reaching statistical significance in univariate analyses including hCGβ level either before, or on completion of, neoadjuvant chemotherapy. hCGβ level in each model remained a statistically significant factor (hazard ratios (HR) 3.41, 95% confidence interval (CI) 1.49–7.83, P=0.004 and 15.36, 95% CI 2.13–110.65, P=0.007, respectively), along with haemoglobin level in the first model (Table 3).
Table 3. Multivariate analyses of potential prognostic factors for overall survival in patients undergoing neoadjuvant chemotherapy incorporating hCGβ level either before, or on completion of, chemotherapy.
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|---|---|---|---|---|---|
|
ECOG PS | ||||||
| ⩾2 vs 0 or 1 |
2.10 |
0.41–10.89 |
0.38 |
2.14 |
0.31–14.95 |
0.44 |
|
Hb | ||||||
| <LLN vs ⩾LLN |
3.56 |
1.69–7.46 |
0.001 |
1.17 |
0.25–5.45 |
0.84 |
|
Chemotherapy regimen | ||||||
| GCarbo vs GC |
1.48 |
0.40–5.42 |
0.56 |
1.79 |
0.42–7.57 |
0.43 |
|
T stage | ||||||
| T3 vs T2 | 1.29 | 0.55–3.00 | 0.56 | 0.98 | 0.23–4.15 | 0.98 |
| T4 vs T2 |
1.76 |
0.47–6.61 |
0.40 |
0.66 |
0.07–6.04 |
0.71 |
|
hCGβ
level before chemotherapy | ||||||
| ⩾2 vs <2 |
3.41 |
1.49–7.83 |
0.004 |
— |
— |
— |
|
hCGβ
level on completion of chemotherapy | ||||||
| ⩾2 vs <2 | — | — | — | 15.36 | 2.13–110.7 | 0.007 |
Abbreviations: CI=confidence interval; ECOG PS=Eastern Cooperative Oncology Group performance status; GC=gemcitabine/cisplatin; GCarbo=gemcitabine/carboplatin; Hb=haemoglobin; HR=hazard ratio; LLN=lower limit of normal for the treating institution's reference range.
In addition, low hCGβ level in the neoadjuvant cohort, assessed before chemotherapy was associated with RFS of 7.38 vs 1.45 years, but this was not statistically significant (P=0.07). However, low hCGβ level on completion of neoadjuvant chemotherapy was associated with RFS of 7.37 vs 0.51 years, P=0.0003.
First-line chemotherapy
For first-line chemotherapy, favourable ECOG performance status, normal serum alkaline phosphatase (⩽ULN), absence of visceral metastases, receipt of GC (vs other regimens), absence of Bajorin risk factors (Bajorin et al, 1999) and no prior perioperative chemotherapy were each associated with longer survival in univariate analyses (Table 2).
In addition, hCGβ level <2 IU l−1 before, or on completion of, chemotherapy was associated with improved survival (median 1.53 vs 0.86 years, P=0.04 and 1.68 vs 0.84 years, P=0.00005, respectively, Table 2, Figure 3C and D).
We undertook multivariate models for overall survival including ECOG performance status and presence of visceral metastases as separate factors (and so omitting the Bajorin index). hCGβ level on completion of first-line chemotherapy remained a statistically significant prognostic factor (HR 3.47, 95% CI 1.97–6.10, P=0.00002, Table 4) along with performance status and the presence of visceral metastases. It did not retain statistical significance for hCGβ levels taken before chemotherapy (P=0.25, Table 4). We also assessed the impact of hCGβ level on progression free survival and found low levels to be associated with improved outcomes in univariate analysis both before, and on completion of, chemotherapy (0.86 vs 0.64 years, P=0.03 and 0.86 vs 0.50 years, P=0.0004, respectively).
Table 4. Multivariate analyses of potential prognostic factors for overall survival in patients undergoing first-line chemotherapy incorporating hCGβ level either before, or on completion of, chemotherapy.
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|---|---|---|---|---|---|
|
ECOG PS | ||||||
| ⩾2 vs 0 or 1 |
2.39 |
1.38–4.13 |
0.002 |
2.09 |
1.01–4.33 |
0.047 |
|
ALP | ||||||
| <LLN vs ⩾LLN |
0.98 |
0.59–1.60 |
0.92 |
1.44 |
0.78–2.68 |
0.24 |
|
Visceral metastases | ||||||
| Yes vs no |
2.01 |
1.29–3.13 |
0.002 |
2.65 |
1.49–4.70 |
0.001 |
|
Chemotherapy regimen | ||||||
| GCarbo vs GC |
0.83 |
0.83–0.66 |
0.11 |
1.86 |
0.52–6.66 |
0.34 |
|
Prior perioperative chemotherapy | ||||||
| Yes vs no |
2.16 |
0.81–5.79 |
0.82 |
1.07 |
0.60–6.66 |
0.34 |
|
hCGβ
level before chemotherapy | ||||||
| ⩾2 vs <2 IU l−1 |
1.28 |
0.84–1.96 |
0.25 |
— |
— |
— |
|
hCGβ
level on completion of chemotherapy | ||||||
| ⩾2 vs <2 IU l−1 | — | — | — | 3.47 | 1.97–6.10 | 0.00002 |
Abbreviations: ALP=alkaline phosphatase; CI=confidence interval; ECOG PS=Eastern Cooperative Oncology Group performance status; GC=gemcitabine/cisplatin; GCarbo=gemcitabine/carboplatin; HR=hazard ratio; LLN=lower limit of normal for the treating institution's reference range.
Second-line chemotherapy
In patients receiving second-line chemotherapy, low hCGβ level on completion of chemotherapy was associated with improved survival (median 1.78 vs 0.29 years, P=0.003), but not with levels before chemotherapy (P=0.3).
Validation cystectomy cohort
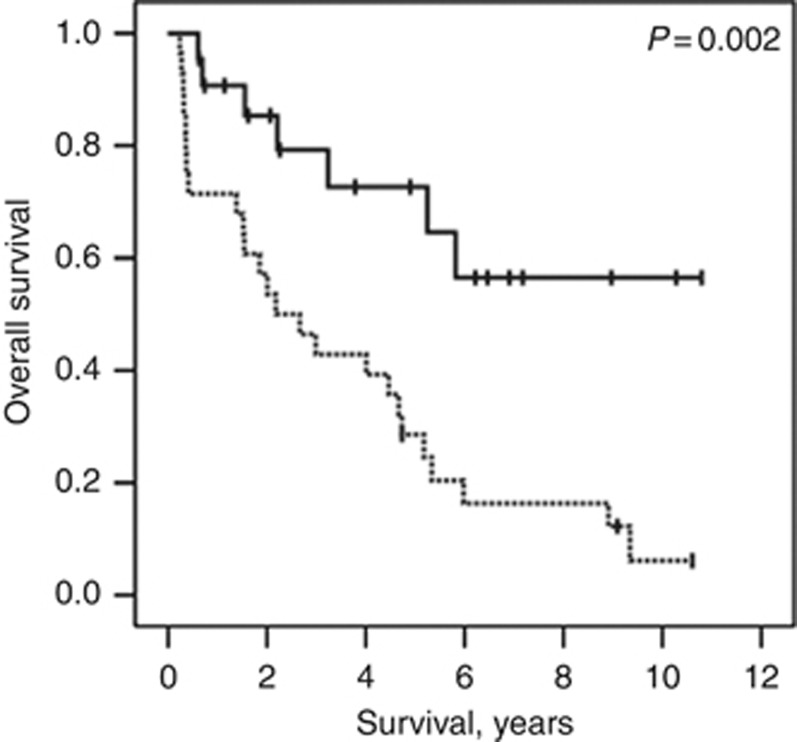
Finally we assessed hCGβ level in an independent sample set following radical cystectomy, but without chemotherapy, (Supplementary Table 1) and found that low levels were associated with improved overall survival (median not reached vs 2.18 years, P=0.002, Figure 4) and RFS (median not reached vs 0.87 years, P=0.00002).
Figure 4.
Kaplan–Meier plot to show overall survival according to total hCGβ level in a radical cystectomy cohort. Broken line – hCGβ level ⩾2; continuous line – hCGβ level <2.
Discussion
Chemotherapy for muscle invasive TCC improves cure rates in combination with radical treatment options (Grossman et al, 2003; Advanced Bladder Cancer (ABC) Meta-analysis Collaboration, 2005a, 2005b; International Collaboration of Trialists et al, 2011) and extends survival in metastatic disease (von der Maase et al, 2000, 2005). However, outcomes are poor following disease relapse or progression. We sought to extend the prognostic information available on initiation, or completion, of chemotherapy. In bladder cancer/TCC, previous studies indicate elevated hCGβ levels of 30–76% in serum, 35–73% in urine and 35% by immunohistochemistry, and possible associations to grade, stage and survival (Moutzouris et al, 1993; Iles, 2007). hCGβ-expressing TCC appears to act in a biologically aggressive manner with poor survival outcomes, increased risk of disease relapse and poor radiotherapy response (Martin et al, 1989; Marcillac et al, 1993; Moutzouris et al, 1993; Dobrowolski et al, 1994; Iles, 2007). Cook et al (2000) investigated hCGβ level within a tumour marker panel including carcino-embryonic antigen, CA125 and CA19.9, and response to chemotherapy in advanced bladder cancer. Neither clinical response nor survival differed between marker-negative and marker-positive patients, however clinical response was strongly related to marker response. Only 19 patients (24%) were evaluable for hCGβ response and so its relevance in this cohort remains uncertain (Cook et al, 2000). Urinary total hCG levels were found to be elevated in a subgroup of patients referred for cystoscopy who were found to have bladder cancer but none of those with benign conditions. It was a poor prognostic factor in those with muscle invasive disease (Iles et al, 1996).
Our work establishes raised total hCGβ level as a poor prognostic factor in chemotherapy-treated urothelial TCC and confirms independence from other established prognostic factors. To our knowledge this is the first time this has been demonstrated for a malignancy other than testis/germ cell cancer. We also demonstrated its association with a poor prognosis in patients who undergo cystectomy as a first step towards validation of hCGβ as a prognostic factor. We would now propose prospective validation in a chemotherapy-treated group of patients before clinical utilisation. Such development would be attractive as hCGβ level is routinely available to clinicians as a relatively cheap, commercially available, validated clinical test with known performance characteristics. Thus the path to establishing this biomarker for use in TCC may be less tortuous than other options. It is important to note that our assessment of hCGβ level utilised an automated, routine, clinically available immunoassay to detect total hCGβ. This is, in essence, a surrogate measurement of the free hCGβ presumed to be expressed by the tumour but will have included all forms of hCG present including intact, free, nicked and glycosylated forms. Future work should look to dissect the relevance of these using assays available with the sensitivity and specificity to do so (Cole and Butler, 2012).
Strengths of our study include that it represents a complete set of sequentially treated patients according to common management criteria, with all patients treated where clinically appropriate with cisplatin-based chemotherapy. These are ‘real world' data however (which we view as a strength) and so also include those unfit for cisplatin-based regimens who are frequently omitted from research in this disease despite representing 40–50% of the population. Thus our results are relevant to current standard-of-care management for this disease rather than the rarefied world of a clinical trial.
A number of questions arise from our data. We demonstrated a prognostic impact for hCGβ levels at completion of, as well as before, chemotherapy. This is despite higher numbers where hCGβ level was not recorded (64.1% neoadjuvant, 35.6% metastatic) which we acknowledge holds potential for bias. Thus there might be a role for hCGβ level as a predictive biomarker for chemotherapy benefit, either as an absolute level before, or following, chemotherapy, or in a dynamic sense as hCGβ ‘normalisation' during treatment. One could also consider what hCGβ dynamics might imply for required duration or type of chemotherapy or if subsequent hCGβ rise might be utilised to detect disease recurrence/relapse. Anecdotally we have experience of the latter representing early indication of disease activity. These are hypotheses however and should be tested prospectively. It would also be of interest to investigate the relevance of hCGβ level in other settings, for example in non-muscle invasive disease to test risk for relapse and progression. In this, and the neoadjuvant/adjuvant chemotherapy settings, the question of whether a raised hCGβ level reflects those with micro-metastases arises and warrants further prospective work, possibly with comparison with experimental imaging methodologies. A further question is the relevance of hCGβ expression in the primary tumour which we are investigating in our radical cystectomy cohort.
Our work raises the question of the biological role of hCGβ in TCC. hCGβ may have a functional role in cancer progression as a transforming growth factor, an immunosuppressive agent, an inducer of metastasis or as an angiogenic factor (Iles, 2007; Cole, 2010). hCGβ, but not intact hCG, hCGα or hCGβ core fragment, stimulated TCC cell line growth which could be inhibited by hCGβ antibodies (Gillott et al, 1996). There is some evidence to suggest that, in part, hCGβ might act in TCC, and potentially other malignancies, by inhibition of TGFβ-induced apoptosis by virtue of their structural homology (reviewed by Iles (Iles, 2007)). Whether these putative biological mechanisms are relevant, or if hCGβ simply acts as a surrogate for poorly differentiated, biologically aggressive disease remains uncertain and further investigation of the biological role in TCC is warranted. hCGβ might also represent a therapeutic target with vaccination strategies currently under development (Delves et al, 2007).
We utilised a prospectively defined hCGβ cut point of < vs ⩾2 IU l−1. This was in part pragmatic as, during the period in question, this was the lower level of quantification at our institution. It would be of interest to undertake future analysis either of other cut points to optimise a dichotomous variable or to analyse as a continuous variable.
Certain limitations of our data exist. First, these were retrospective analyses. Second, patients were included on the basis of receipt of chemotherapy for urothelial TCC. The study therefore holds bias for those suitable to commence chemotherapy and our patient cohorts are somewhat heterogeneous, which future prospective validation should seek to address and control for. Our validation cohort was a cystectomy-treated group. We chose this primarily for pragmatic reasons as, to our knowledge and after some effort to find an alternative, there is no current sample set available of chemotherapy-treated patients with hCGβ data available. Prospective validation in both treatment settings is therefore now required and warranted. Finally our cohort was not randomised to treatment and so we cannot establish whether a predictive factor role for hCGβ levels exists from this sample set.
In conclusion, serum hCGβ level is an independent prognostic factor for outcome in patients undergoing chemotherapy for TCC of the urothelial tract. Prospective validation is warranted to determine its value for patient stratification in this disease.
Acknowledgments
CC was part funded by the Southampton National Institute of Health Research (NIHR) Wellcome Trust Clinical Research Facility.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration Adjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol. 2005;48 (2:189–199. doi: 10.1016/j.eururo.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48 (2:202–205. doi: 10.1016/j.eururo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Bajorin DF, Dodd PM, Mazumdar M, Fazzari M, McCaffrey JA, Scher HI, Herr H, Higgins G, Boyle MG. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17 (10:3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8:102. doi: 10.1186/1477-7827-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LA, Butler S. Hyperglycosylated hCG, hCGbeta and hyperglycosylated hCGbeta: interchangeable cancer promoters. Mol Cell Endocrinol. 2012;349 (2:232–238. doi: 10.1016/j.mce.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Cook AM, Huddart RA, Jay G, Norman A, Dearnaley DP, Horwich A. The utility of tumour markers in assessing the response to chemotherapy in advanced bladder cancer. Br J Cancer. 2000;82 (12:1952–1957. doi: 10.1054/bjoc.2000.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb SJ, Bajdik CD, Leung S, Speers CH, Kennecke H, Huntsman DG, Gelmon KA. Can clinically relevant prognostic subsets of breast cancer patients with four or more involved axillary lymph nodes be identified through immunohistochemical biomarkers? A tissue microarray feasibility study. Breast Cancer Res. 2008;10 (1:R6. doi: 10.1186/bcr1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb SJ, Cheang MC, Leung S, Immonen T, Nielsen TO, Huntsman DD, Bajdik CD, Chia SK. Basal breast cancer molecular subtype predicts for lower incidence of axillary lymph node metastases in primary breast cancer. Clin Breast Cancer. 2008;8 (3:249–256. doi: 10.3816/CBC.2008.n.028. [DOI] [PubMed] [Google Scholar]
- Crabb SJ, Wheater M. Advances in chemotherapy and targeted systemic therapies for urothelial cancer. Curr Drug Ther. 2010;5 (1:17–28. [Google Scholar]
- Delves PJ, Iles RK, Roitt IM, Lund T. Designing a new generation of anti-hCG vaccines for cancer therapy. Mol Cell Endocrinol. 2007;260-262:276–281. doi: 10.1016/j.mce.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Dobrowolski ZF, Byrska B, Dolezal M. Prognostic value of beta human chorionic gonadotrophin in blood serum of patients with urinary bladder tumours. Int Urol Nephrol. 1994;26 (3:301–306. doi: 10.1007/BF02768213. [DOI] [PubMed] [Google Scholar]
- Dyrskjot L, Thykjaer T, Kruhoffer M, Jensen JL, Marcussen N, Hamilton-Dutoit S, Wolf H, Orntoft TF. Identifying distinct classes of bladder carcinoma using microarrays. Nat Genet. 2003;33 (1:90–96. doi: 10.1038/ng1061. [DOI] [PubMed] [Google Scholar]
- Gillott DJ, Iles RK, Chard T. The effects of beta-human chorionic gonadotrophin on the in vitro growth of bladder cancer cell lines. Br J Cancer. 1996;73 (3:323–326. doi: 10.1038/bjc.1996.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP, Jr., Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349 (9:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- Iles RK. Ectopic hCGbeta expression by epithelial cancer: malignant behaviour, metastasis and inhibition of tumor cell apoptosis. Mol Cell Endocrinol. 2007;260-262:264–270. doi: 10.1016/j.mce.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Iles RK, Persad R, Trivedi M, Sharma KB, Dickinson A, Smith P, Chard T. Urinary concentration of human chorionic gonadotrophin and its fragments as a prognostic marker in bladder cancer. Br J Urol. 1996;77 (1:61–69. doi: 10.1046/j.1464-410x.1996.82910.x. [DOI] [PubMed] [Google Scholar]
- International Collaboration of Trialists on behalf of the Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group), the European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, the Australian Bladder Cancer Study Group, the National Cancer Institute of Canada Clinical Trials Group, Finnbladder, Norwegian Bladder Cancer Study Group, and Club Urologico Espanol de Tratamiento Oncologico Group. International Collaboration of Trialists. Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29 (16:2171–2177. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis CJ, Dexeus FH, Finn L, Sella A, Amato RJ, Ayala AG, Kilbourn RG. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol. 1990;8 (6:1050–1055. doi: 10.1200/JCO.1990.8.6.1050. [DOI] [PubMed] [Google Scholar]
- Marcillac I, Cottu P, Theodore C, Terrier-Lacombe MJ, Bellet D, Droz JP. Free hCG-beta subunit as tumour marker in urothelial cancer. Lancet. 1993;341 (8856:1354–1355. doi: 10.1016/0140-6736(93)90872-e. [DOI] [PubMed] [Google Scholar]
- Martin JE, Jenkins BJ, Zuk RJ, Oliver RT, Baithun SI. Human chorionic gonadotrophin expression and histological findings as predictors of response to radiotherapy in carcinoma of the bladder. Virchows Arch A Pathol Anat Histopathol. 1989;414 (3:273–277. doi: 10.1007/BF00822032. [DOI] [PubMed] [Google Scholar]
- Mead GM, Russell M, Clark P, Harland SJ, Harper PG, Cowan R, Roberts JT, Uscinska BM, Griffiths GO, Parmar MK. A randomized trial comparing methotrexate and vinblastine (MV) with cisplatin, methotrexate and vinblastine (CMV) in advanced transitional cell carcinoma: results and a report on prognostic factors in a Medical Research Council study. MRC Advanced Bladder Cancer Working Party. Br J Cancer. 1998;78 (8:1067–1075. doi: 10.1038/bjc.1998.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengual L, Burset M, Ars E, Lozano JJ, Villavicencio H, Ribal MJ, Alcaraz A. DNA microarray expression profiling of bladder cancer allows identification of noninvasive diagnostic markers. J Urol. 2009;182 (2:741–748. doi: 10.1016/j.juro.2009.03.084. [DOI] [PubMed] [Google Scholar]
- Mitra AP, Pagliarulo V, Yang D, Waldman FM, Datar RH, Skinner DG, Groshen S, Cote RJ. Generation of a concise gene panel for outcome prediction in urinary bladder cancer. J Clin Oncol. 2009;27 (24:3929–3937. doi: 10.1200/JCO.2008.18.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutzouris G, Yannopoulos D, Barbatis C, Zaharof A, Theodorou C. Is beta-human chorionic gonadotrophin production by transitional cell carcinoma of the bladder a marker of aggressive disease and resistance to radiotherapy. Br J Urol. 1993;72 (6:907–909. doi: 10.1111/j.1464-410x.1993.tb16294.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24 (5:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- Smith SC, Baras AS, Dancik G, Ru Y, Ding KF, Moskaluk CA, Fradet Y, Lehmann J, Stockle M, Hartmann A, Lee JK, Theodorescu D. A 20-gene model for molecular nodal staging of bladder cancer: development and prospective assessment. Lancet Oncol. 2011;12 (2:137–143. doi: 10.1016/S1470-2045(10)70296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler WM, Lerner SP, Groshen S, Stein JP, Shi SR, Raghavan D, Esrig D, Steinberg G, Wood D, Klotz L, Hall C, Skinner DG, Cote RJ. Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. J Clin Oncol. 2011;29 (25:3443–3449. doi: 10.1200/JCO.2010.34.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg CN, de Mulder PH, Schornagel JH, Theodore C, Fossa SD, van Oosterom AT, Witjes F, Spina M, van Groeningen CJ, de Balincourt C, Collette L, European Organization for R, Treatment of Cancer Genitourinary Tract Cancer Cooperative G Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol. 2001;19 (10:2638–2646. doi: 10.1200/JCO.2001.19.10.2638. [DOI] [PubMed] [Google Scholar]
- Takata R, Katagiri T, Kanehira M, Tsunoda T, Shuin T, Miki T, Namiki M, Kohri K, Matsushita Y, Fujioka T, Nakamura Y. Predicting response to methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy for bladder cancers through genome-wide gene expression profiling. Clin Cancer Res. 2005;11 (7:2625–2636. doi: 10.1158/1078-0432.CCR-04-1988. [DOI] [PubMed] [Google Scholar]
- von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A, Lippert CM, Kerbrat P, Sanchez Rovira P, Wersall P, Cleall SP, Roychowdhury DF, Tomlin I, Visseren-Grul CM, Conte PF. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18 (17:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23 (21:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.