Abstract
Ninety-seven female students, aged 18–20 yrs, were assigned to groups consisting of 55 infrequent (less than monthly) and 42 frequent (at least monthly) binge drinkers. The groups were compared on self-report measures of impulsivity, sensation seeking, and alexithymia. The groups were also compared on several objective measures relevant to neural and genetic mechanisms, such as brain activation during a time estimation task and selected genotypes. Analyses of stimulus-locked brain activity revealed a slow cortical potential generated over the right parietal cortex during time estimation that was more negative in the frequent binge drinking group. This group also showed a greater prevalence of a CHRM2 genotype previously associated with substance dependence and Major Depressive Disorder as well as a modest elevation on a non-planning impulsiveness self-report scale. We conclude that the enhanced brain activation shown by binge drinkers during time estimation compensates for an underlying deficit. That deficit may be reflected in poor planning skills and a genetic difference indicating increased risk for both externalizing and internalizing disorders in later life.
Keywords: alcohol, binge drinking, gene, CHRM2, contingent negative variation, evoked potential, electroencephalography, college students, time
1. Introduction
An impaired ability to estimate the passage of time has been linked to substance use. For example, Smart (Smart, 1968) found that alcoholics performed less well than social drinkers on the Future Time Perspective (Wallace, 1956) task, which measures the ability to conceptualize the future in terms of the timing and ordering of future events. Manganiello (Manganiello, 1978) described a similar finding from his comparison of 45 adult heroin addicts and 50 high school students. In addition, Petry and colleagues (Petry, Bickel, & Arnett, 1998) compared 34 active heroin addicts to 59 non-drug-using controls. Heroin addicts scored lower than the control group on both the Future Time Perspective task and the future orientation subscale of the Stanford Time Perception Inventory (TPI; Zimbardo, 1992). In all three studies, substance-abusing patients reported shorter time horizons than the control group. Petry and others have theorized that the shortened time horizons of substance abusers could contribute to delay discounting -- a disadvantageous preference for immediate, smaller rewards versus delayed, larger rewards.
Other research suggests that altered time perception and delay discounting are not only demonstrable in patients. Laghi and colleagues (Laghi, Liga, Baumgartner, & Baiocco, 2012) and Vuchinich and Simpson (Vuchinich & Simpson, 1998) have demonstrated similar findings in a subset of college students whose heavy drinking patterns suggest increased risk for future problems (Jennison, 2004), including dependence. Laghi and colleagues surveyed a total 1350, 17–19 year old participants of whom 422 met the study definition for frequent binge drinking. On the Stanford TPI, frequent binge drinkers exhibited lower scores than the control group on the positive future orientation subscale. Vuchinich and Simpson similarly found that heavy drinking college students reported low future time orientation scores on the TPI, as well as greater delay discounting of a hypothetical reward.
The present study was designed to extend these studies of altered time perception in heavy drinking college students. It offered at least four specific innovations. First, it examined time perception ability with a simple task in which the students used a button press response to register their estimate of the passage of a fixed interval of time. Because of its relative simplicity, a button press response offers the advantage of being less dependent than time perception questionnaires upon individual differences in reading skill, verbal comprehension, and motivation. It can also be argued that it is a more valid estimate of time estimation ability for actual events than are perceptions of time within fictional scenarios.
The second innovation was the use of a slow electroencephalographic potential (SP) as an indicator of neural differences underlying time estimation. Normal subjects with poor time estimation ability have been shown to exhibit SPs of a more negative amplitude and slope than normal subjects with accurate time estimation ability (Ladanyi & Dubrovsky, 1985). We have previously demonstrated more negative SP amplitudes indicating premature response preparation among cocaine-dependent adults with Antisocial Personality Disorder (Bauer, 2001) and HIV/AIDS patients with an obese body mass (Bauer, 2008). In the present study of college students, we improved upon on our previous methods. More specifically, in the present study, we adopted a new method in which we were able to differentiate and separately localize the SP associated with the complex process of estimating time [see (Bueti & Walsh, 2009) for a review] from the electroencephalographic change associated with executing a motor response, i.e., the motor potential.
The third innovative feature was the examination of covert genetic mechanisms that may contribute to binge drinking. Given the limited scope of this study, a short and a priori-specified list of candidate gene single nucleotide polymorphisms (SNPs) were tested. All of the candidates had previously been associated with risk for alcohol dependence in large cooperative studies, such as the Collaborative Study on the Genetics of Alcoholism. The genes studied presently were GABRA2 (Edenberg et al., 2004), CHRM2 (Wang et al., 2004), and ANKK1 (Dick et al., 2007). Accompanying the genetic assessment was an assessment of stable personality features that may also predict binge drinking (Moreno et al., 2012; Shin, Hong, & Jeon, 2012). Personality was measured with three self-report instruments that captured different aspects of the impulsivity construct--the Toronto Alexithymia Scale [TAS; (Taylor, Ryan, & Bagby, 1985)], Barratt Impulsiveness Scale [BIS-11;(Patton, Stanford, & Barratt, 1995)] and the Sensation Seeking Scale [SSS; (Zuckerman, Eysenck, & Eysenck, 1978)]
The final and most intriguing aspect of the study was its exclusive focus on women. This focus was suggested by evidence (McCabe, 2002) showing that women experience their heaviest drinking during the early years of college whereas the peak occurs during the later years for men. Because this sex difference may indicate the presence of other differences (Balodis, Potenza, & Olmstead, 2009; Randolph, Torres, Gore-Felton, Lloyd, & McGarvey, 2009) between women and men in the causes, correlates, and consequences of heavy drinking over time, including both sexes in the present study was not defensible. Instead, we recruited enough members of one sex to provide a powerful test of our hypotheses, which were the following:
In comparison to infrequent drinkers, binge drinkers will overestimate time passage (i.e., respond prematurely), exhibit a larger SP during the time estimation interval, and report higher scores on impulsivity scales (BIS-11, TAS, SSS).
The frequency of GABRA2, ANKK1, and CHRM2 SNP genotypes previously associated with substance dependence or impulsive personality features will be greater among binge drinkers in comparison to infrequent drinkers.
2. Methods
2.1. Participants
One-hundred-and-four female students, aged 18–20 years, were recruited from 4 private and 2 public universities in Connecticut. They were examined during their freshman or sophomore years. Students were contacted through a variety of methods, including newspaper and radio ads and posters. Interested parties were asked to call the study office for information and eligibility screening. Those who appeared eligible were invited to visit the Health Center on a subsequent day for further screening and evaluations. Students who reported no past year pregnancy, psychosis, or major medical disorders that would complicate their general health (HIV, thyroid disease) or evoked electroencephalographic responses (head injury, seizure disorder, heart disease, neurological disorders) were included. To ensure consistency of outside influence, they were also required to have full-time status, live on campus, and participate in the school’s food service meal plan. Ineligible volunteers were paid $30 for time and effort and dismissed. The students who completed the full evaluation were paid $150 each.
2.2. Laboratory Evaluation
At the time of their in-person visit to the laboratory, students reviewed and signed an IRB-approved informed consent and HIPAA agreements. They were then asked to complete questionnaires assessing alcohol (Saunders & Lee, 2000) and drug (McCabe, Boyd, Cranford, Morales, & Slayden, 2006) use. They provided medical history information by self-report and records obtained from the primary care physician. Sensation-seeking and alexithymic traits were assessed with the Sensation Seeking (Zuckerman et al., 1978) and Toronto Alexithymia Scales (Taylor, Bagby, Ryan, & Parker, 1990), respectively. In addition, self-ratings of impulsivity in the attention, motor, and non-planning categories were obtained from the BIS-11 (Patton et al., 1995) scale. A saliva sample was collected for DNA analysis.
To assess time estimation, we recorded electroencephalographic activity and response latency while the students performed a simple task consisting of 50 trials. Before the task, they were instructed to focus attention on a computer monitor which would display the letter “X” (retinal angle=2.8 deg) once every 4 seconds. They were asked to estimate the passage of 1.5 s after the onset of this stimulus and indicate their estimate by pressing a response key with the left index finger. Two seconds after each button press, the phrase “too slow”, “too fast”, or “on-target” was presented indicating that the estimate was slow, fast, or on-target (1.5 ± 0.25 s).
During the task, electroencephalographic activity was recorded from a cap (Electro-Cap International, Eaton, OH) containing 64 Ag/AgCl electrodes. The cap electrodes were referenced to linked ear electrodes. Similar electrodes placed above and below the right eye were used for eye movement and eyeblink detection. All signals were digitized at 500 Hz, amplified to a gain of 10K, and processed with a Synamps2 system (Compumedics Neuroscan, Charlotte, NC) running SCAN 4.4 software.
2.3. Data Reduction
2.3.1. Slow Potentials, Motor Potentials, and Task Performance
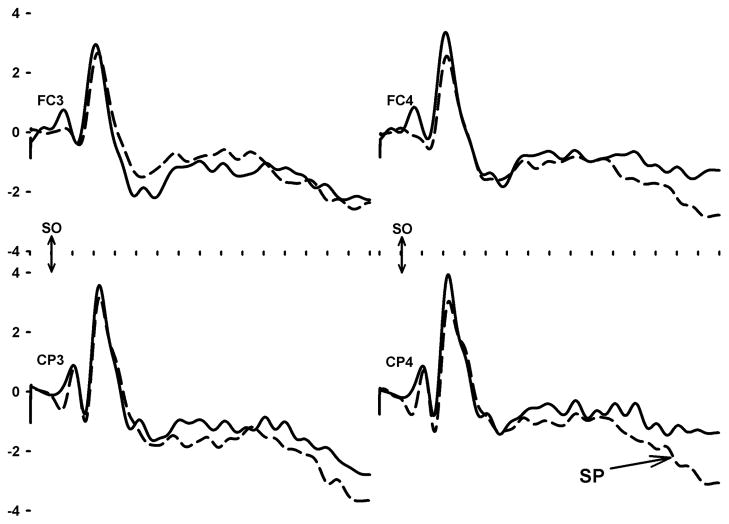
Slow potentials (SP) were derived from filtered (bandpass=0.5–12.0 Hz, 24 db/octave) epochs spanning a period from 100 ms preceding to 1500 ms following the onset of the time estimation cue, “X”. Each epoch was mathematically corrected for eye movement artifact (Semlitsch, Anderer, Schuster, & Presslich, 1986) and the average voltage of the pre-stimulus period. All epochs without A/D converter overflow or large voltage deviations (>20 μV) were formed into time-point averages. In keeping with the recommendations of Mordkoff and Gianaros (Mordkoff & Gianaros, 2000), the mean voltage of the SP was measured during a likely pre-onset period (502–1000 ms) and during a period within which it was likely to peak (1002–1500 ms). The amplitude change across these periods was then computed. It was the difference in voltage between the peak minus pre-onset periods at each of 4 electrode sites in the vicinity of pre-motor (FC3, FC4) and post-motor cortex (CP3, CP4).
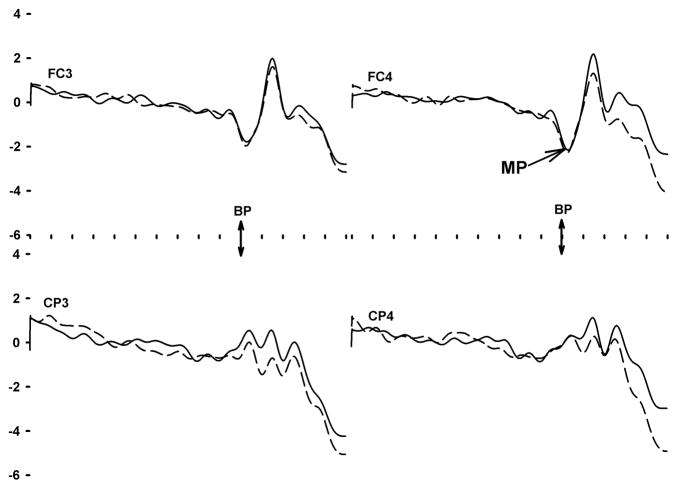
Motor potentials (MP) were similarly derived from filtered and artifact-corrected EEG epochs. In contrast to SPs which were derived from epochs aligned by stimulus onset, MPs were computed from epochs aligned by button press onset. Each epoch spanned a period from 1000 ms preceding to 500 ms following button press onset. All artifact-free epochs were formed into a time-point average. The amplitude of the large negative peak at response onset was measured at FC3, FC4, CP3, and CP4 sites relative to the average voltage within a pre-onset period (−1000 ms to −500 ms).
Behavioral estimates of time passage were also calculated. The principal estimate was button press latency measured to the nearest 2 ms. In addition, within-subject variability in time estimation was measured by the standard deviation of button press latency across all trials.
2.3.2. DNA
Genomic DNA was purified from saliva samples by the University of Connecticut’s Clinical Research Center core lab. DNA samples were placed in 96-well plates and genotyped using PCR based TaqMan 5′-nuclease allelic discrimination assay methods. Representative single nucleotide polymorphisms (SNP) were assayed within CHRM2 (rs12673281 and rs324650) and GABRA2 (rs279871) chromosomal regions. Within the ANKK1 region, the SNP at rs1800497 (also known as the Taq1a polymorphism) as well as a downstream SNP at rs17115439 (Dick et al., 2007) were assayed.
2.3.3. Group Assignment and Data Analysis Plan
In the analysis, the 55 students reporting infrequent (n=30 never plus n=25 less than monthly) episodes with >= 6 alcohol drinks consumed on the AUDIT were compared to the 42 students reporting frequent (n=28 monthly plus n=14 weekly) episodes on this scale.
Analyses of variance (ANOVAs) were used to compare these groups on background variables such as age, alcohol and drug use, BIS-11, sensation seeking, and alexithymia scale scores. For the analysis of electrophysiological data, separate ANOVAs were performed on SP amplitude change and MP peak amplitude across the 4 representative electrode sites. The ANOVAs tested binge drinking frequency as a grouping factor as well scalp distribution differences by region (anterior/posterior) and hemisphere (left/right). The significance criterion, alpha=0.05, was not corrected for the number of analyses performed.
Further analyses were performed on the electrophysiological data for the purpose of identifying the neuroanatomical sources of the SP and MP waveforms. For these analyses, Standardized Low Resolution Electromagnetic Tomographic Analysis (sLORETA) was applied to all 64 electrode sites as described below.
A third set of analyses attempted to identify genetic correlates of group membership. For these analyses, only students of self-identified European-American ancestry were considered (n=96). Also, for these analyses, genotypes were collapsed from 3 to 2 levels (major allele carriers versus minor allele homozygotes) to test a recessive model. Simple χ2 analyses were used to identify group differences in the frequencies of selected CHRM2, ANKK1, and GABRA2 genotypes.
A final set of analyses were used to address a persistent question in the binge drinking literature: are the differences in brain function found among frequent binge drinkers a cause or a consequence of their drinking? Because the present study does not have longitudinal data, one cannot answer this question definitively. However, one can provide a reasonable though tentative answer by examining the variance in brain function explained by measures of drinking versus measures of premorbid risk. To this end, we formally compared the correlations of a continuous measure of alcohol involvement, i.e., the total AUDIT score and a genotype with SP amplitude change. The choice of the specific genotype (CHRM2 @ rs324650) entered in these analyses was guided by the findings of the genetic analyses described above.
3. Results
Adequate artifact-free data were provided by 97 of the 104 students. These 97 students were included in the analysis.
3.1. Background Characteristics
Table 1 shows group means and standard deviations for age, alcohol and drug use, BIS-11, sensation seeking, and alexithymia scale scores, as well as the behavioral estimate and standard deviation of the behavioral estimate of time passage. In comparison to the group reporting infrequent binge drinking, the frequent binge drinking group reported greater AUDIT (F1,95=74.9, p<0.01) and DAST-10 (F1,95=4.3, p<0.04) scores. On specific subscales of the BIS-11 and SSS, they also differed. The latter group reported greater non-planning impulsivity (F1,95=4.7, p<0.03) and disinhibition (F1,95=23.7, p<0.001) scores, but did not differ significantly from their peers on attentional or motor impulsivity, boredom susceptibility, or experience or thrill seeking. No other group differences shown in the table were statistically significant.
Table 1.
Background characteristics and task performance sorted by group.
| Infrequent Binge Drinking N=55 |
Frequent Binge Drinking N=42 |
|
|---|---|---|
| Age in yrs (SD) | 19.5(1.3) | 19.4(1.1) |
| Alcohol Use Disorders Inventory—Total Score | 4.5(3.5) | 11.6(4.5)* |
| Drug Abuse Screening Test—Total Score | 1.0(1.4) | 1.7(1.7)* |
| BIS-11 Attentional Impulsiveness | 16.6(4.0) | 16.7(4.2) |
| BIS-11 Motor Impulsiveness | 22.1(4.5) | 23.5(3.7) |
| BIS-11 Non-planning Impulsiveness | 22.6(4.9) | 24.7(4.4)* |
| SSS Disinhibition | 4.4(2.6) | 6.7(1.7)* |
| SSS Boredom Susceptibility | 2.7(2.1) | 2.7(1.9) |
| SSS Experience Seeking | 6.0(2.1) | 6.0(2.0) |
| SSS Thrill Seeking | 7.1(2.6) | 7.2(2.5) |
| TAS--Difficulty Describing Feelings | 11.5(4.2) | 12.9(4.6) |
| TAS--Difficulty Identifying Feelings | 13.3(5.8) | 14.5(6.5) |
| TAS--Externally-Oriented Thinking | 17.7(4.1) | 18.2(4.0) |
| Response latency in ms | 1440.7(97.5) | 1434.0(78.9) |
| SD of response latency | 313.7(91.8) | 301.9(93.8) |
p<0.05.
3.2. Slow Potentials and Motor Potentials
The analyses of SP amplitude change and MP peak amplitude employed all available artifact-free epochs. Table 2 shows that the number of epochs contributing to the averaged SP and MP responses did not differ by group. Table 2 also shows that that the statistical effect of group was significant in the analysis of SP amplitude change. A more negative SP amplitude was found in the frequent binge drinking group versus the other group (F1,95=7.3, p<0.01). There were no significant main or interaction effects indicating differences by electrode site.
Table 2.
SP amplitude change and MP peak amplitude sorted by group.
| Infrequent Binge Drinking N=55 |
Frequent Binge Drinking N=42 |
|
|---|---|---|
| # of SP epochs averaged/subject (SD) | 45(2.3) | 44(2.6) |
| SP Amplitude Change in μV (SD) | ||
| FC3 | −0.45(1.35) | −1.09(1.63)* |
| FC4 | −0.33(0.93) | −1.05(1.57)* |
| CP3 | −0.95(2.32) | −0.96(2.47) |
| CP4 | −0.41(1.05) | −1.10(1.36)* |
| # of MP epochs averaged/subject (SD) | 43(2.8) | 40(3.2) |
| MP peak amplitude in μV (SD) | ||
| FC3 | −2.74(1.57) | −2.75(1.53) |
| FC4 | −3.09(1.68) | −2.98(1.87) |
| CP3 | −1.77(1.73) | −2.40(2.31) |
| CP4 | −1.60(1.43) | −1.77(1.59) |
p<0.05
In contrast to the SP analysis, the analysis of MP peak amplitude (Table 2) revealed no effect of group. However, in this analysis, scalp distribution did affect amplitude: the significant interaction of anterior/posterior location and hemisphere (F1,95=4.4, p<0.05) reflects the presence of a more negative MP at the FC4 electrode site versus other sites. This interpretation was confirmed by the results of Tukey post hoc tests (p<0.05).
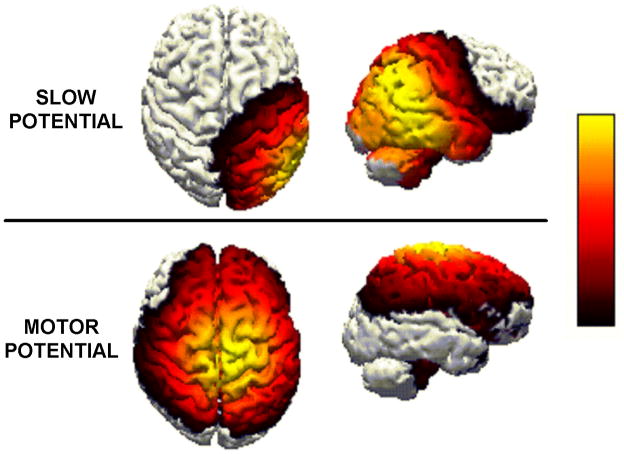
A separate set of analyses attempted to locate the neuroanatomical sources of the SP and MP using all available EEG data across all subjects. To accomplish this goal, we re-referenced the study-averaged SP and MP waveforms at each electrode site to the common averaged reference. With Curry version 5 software (Compumedics-Neuroscan, Charlotte, NC), principal components analysis was then used to find the major underlying constituents of the waveforms. For the SP waveform, the targeted latency range was 800–1500 ms relative to stimulus onset. For the MP waveform, the range was −100 to +100 ms relative to key press onset. The major constituents of the SP and MP respectively explained 65.2% (SNR=9.8) and 66.1% (SNR=5.1) of the variance. Within the constraints of a boundary element method model, Standardized Low Resolution Electromagnetic Tomographic Analysis (sLORETA) was then used to find sources (Figures 1 and 2). The source of the SP was located in the vicinity of the right parietal cortex. The MP was located within primary motor cortex medial and anterior to the source of the SP.
Figure 1.
Top: Group-averaged slow potential waveforms, spanning −100 to +1500 ms, sorted by group (solid line=infrequent binge drinking, dashed line=frequent binge drinking). The double-headed arrow indicates warning stimulus onset (SO). The horizontal axis is divided by ticks 100 ms apart. The emergence of the Slow Potential at the CP4 electrode is indicated by the other arrow.
Figure 2.
Group-averaged motor potential waveforms, spanning −1000 to + 500 ms, sorted by group (solid line=infrequent binge drinking, dashed line=frequent binge drinking). The horizontal axis is marked by ticks 100 ms apart. The double-headed arrow indicates button press (BP) onset. The associated Motor Potential at FC4 is indicated by the other arrow.
3.3. Genotypes
The analysis revealed a significant result involving one of the 5 SNPs (Table 3) —a CHRM2 SNP at rs324650 (χ2=9.4, p=0.002). This significant test χ2 indicates an excess prevalence of minor allele homozygotes among frequent binge drinkers (AA or AT: 57.1%, n=24; TT: 42.9%, n=18) in comparison to the infrequent binge drinkers (AA or AT: 85.2%, n=46; TT: 14.8%, n=8). Overall, the genotype frequencies at this SNP were consistent with Hardy-Weinberg equilibrium expectations (χ2=0.2).
Table 3.
Genotype frequencies (minor allele homozygotes, major allele carriers) sorted by group.
| Infrequent Binge Drinking N=54 |
Frequent Binge Drinking N=42 |
|
|---|---|---|
| ANKK1 @ rs1800497 | 21,33 | 12,30 |
| ANKK1 @ rs17115439 | 17,37 | 23,19 |
| GABRA2 @ rs279871 | 23,31 | 21,21 |
| CHRM2 @ rs12673281 | 15,39 | 15,27 |
| CHRM2 @ rs324650* | 8,46 | 18,24 |
p<0.01,
p<0.05.
3.4. Correlations
The correlations of SP amplitude change (averaged over the four electrodes) × AUDIT score and SP amplitude change × CHRM2 genotype were r=−0.182 (p=0.07) and r=0.296 (p=0.004), respectively. These correlation coefficients were converted to their z-score equivalents and compared. The difference was statistically significant, z=−3.34 (p<0.001), indicating that the association between the SP and genotype was significantly greater than the association between the SP and alcohol use.
4. Discussion
The goal of the present study was to reveal correlates of binge drinking among college women. This goal was achieved. In comparison to students reporting infrequent binge drinking episodes, frequent binge drinkers reported higher scores on the BIS-11 non-planning and SSS disinhibition subscales, exhibited larger (more negative) slow potentials during time estimation, and were more likely to be homozygous for a CHRM2 SNP genotype previously associated with risk for adult Alcohol Dependence and Major Depressive Disorder.
The demonstration of a group difference on the BIS-11 non-planning subscale is unsurprising. A failure to plan ahead has face validity for the apparent inability, or lack of interest, of binge drinkers in considering the adverse consequences of multiple episodes of intoxication. Differences on this subscale have been detected in other investigations of college student drinking (Moreno et al., 2012) but not consistently (Carlson, Johnson, & Jacobs, 2010). We attribute some of the variability in findings across studies to class year: in comparison to studies that sample college students across all years, studies similar to the present that focus on the early years of college are more likely to capture the high risk subset (Hollar & Moore, 2004).
The elevated scores of frequent binge drinkers on the SSS disinhibition scale is especially unsurprising. It is also tautological. The disinhibition scale includes items that ask directly about alcohol or drug use and attending parties where alcohol/drugs are available. The scale is therefore partially redundant with the criterion used to define the groups (Darkes, Greenbaum, & Goldman, 1998).
Of greater interest and innovation are the group differences in neurophysiology. Indeed, the present study departs from many other studies [for reviews, see (Jacobus & Tapert, 2013; Lopez-Caneda, Rodriguez Holguin, Cadaveira, Corral, & Doallo, 2013)] of brain function in at-risk drinkers by examining a cognitive process which is not under the principal control of the frontal brain. Although there have been a few small (<20 subjects) functional magnetic resonance imaging studies (Morillon, Kell, & Giraud, 2009) which implicate frontal regions in time estimation, the evidence from other studies (Lewis & Miall, 2006) also implicates the posterior parietal region. A remarkably thoughtful and scholarly review by Bueti and Walsh (Bueti & Walsh, 2009) discusses the parietal cortex as the neural region primarily responsible for the estimation of time passage. In their theory, it is viewed as a general purpose estimator of magnitude (time, number, and size).
Indeed, the evidence in support of the Bueti and Walsh theory is extensive and compelling. For example, tasks that require judgments of duration, number, and size interfere with each other. Similarly, transcranial magnetic stimulation of the right but not left parietal lobe has been found to disrupt judgments of both time (Alexander, Cowey, & Walsh, 2005) and number (Cohen Kadosh et al., 2007). The results of case studies of patients with parietal lobe lesions [see (Koch, Oliveri, & Caltagirone, 2009) for a review] are consistent with these notions.
In our analysis of group differences in time estimation, we showed that frequent binge drinkers exhibited an enhanced slow potential (Figure 1) that could be mapped to the right parietal region (Figure 3). The results of the correlation analyses involving the SP suggest that this enhancement may be more strongly related to a genetic predisposition than an acquired pharmacological effect of alcohol. Indeed, as noted previously, other studies (Bauer, 2001) have demonstrated an enhanced slow potential among impulsive groups who abuse other substances, such as cocaine. In the present study, the enhancement most likely reflects a compensatory over-activation of the circuit to correct for an underlying deficit. This over-activation plus the feedback about performance that followed each trial may have allowed frequent binge drinkers to provide estimates of time passage that were as accurate as the estimates provided by the other group (Table 1).
Figure 3.
sLORETA maps (aerial and right hemisphere views) of the probable generators of the SP (top) and MP (bottom). The color scale indicates current density.
Another innovative aspect of the present study was our attempt, via forward and backward averaging, to separate the neural activation associated with time estimation (Figure 1) from the overlapping activation associated with preparing and executing the motor response (Figure 2). We found that the groups differed in the generation of the parietally-focused SP but not in the generation of the frontally-focused MP. One could argue that other studies of time estimation do not adequately separate these component operations (Morillon et al., 2009). For this reason, some other studies may have overemphasized the role of the frontal brain in the execution of the task.
A final notable contribution of the present study is the demonstration of an association between binge drinking and CHRM2 genotype. Admittedly, this association is based on an analysis of a relatively small sample of 96 European-American students. A larger sample and/or a replication is clearly needed. Yet, we do have some confidence in this finding because it implicates a gene that has been connected to drinking [especially alcohol dependence (Luo et al., 2005; Wang et al., 2004)] risk and associating with antisocial or deviant peers (Latendresse et al., 2011) in large samples. It is noteworthy that CHRM2 has also been associated with risk for Major Depressive Disorder (Luo et al., 2005; Wang et al., 2004). The latter association may be more relevant to and more predictive of binge drinking risk in women than men. Unfortunately, we do not have a comparable sample of male college students with which we could test the hypothesis of a CHRM2 association with binge drinking that is sex specific.
4.1. Limitations
We would be remiss if we did not acknowledge that the present findings have modest significance. Although we successfully identified differences between frequent and infrequent binge drinkers in personality, neurophysiology, and perhaps also genetics, the implications and predictive value of these findings are unclear. Indeed, many female college students discontinue hazardous drinking upon graduation and are therefore only at-risk during college for the immediate consequences of drinking, including alcohol poisoning, assault, and pedestrian or vehicular accidents, among others. It would be interesting to follow these women over time to determine if the personality, neurophysiological, and genetic variables studied here predict future problems such as academic failure and abuse or dependence.
A second limitation pertinent to the SP data only was the task requirement to respond with the left index finger only. This requirement simplified the study design. But, it did not allow us to disentangle the accompanying motor potential, which would clearly and invariably appear over the right hemisphere, from the timing response or SP, which was also expected to originate from right hemisphere. In a better design, the responding hand would be counterbalanced across subjects, or varied systematically within a subject.
Acknowledgments
This research was supported by pilot study funds provided by the University of Connecticut Alcohol Research Center and NIAAA (P60AA03510).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander I, Cowey A, Walsh V. The right parietal cortex and time perception: back to Critchley and the Zeitraffer phenomenon. Cognitive Neuropsychology. 2005;22(3):306–315. doi: 10.1080/02643290442000356. [DOI] [PubMed] [Google Scholar]
- Balodis IM, Potenza MN, Olmstead MC. Binge drinking in undergraduates: relationships with sex, drinking behaviors, impulsivity, and the perceived effects of alcohol. Behavioral Pharmacology. 2009;20(5–6):518–526. doi: 10.1097/FBP.0b013e328330c779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO. Antisocial personality disorder and cocaine dependence: their effects on behavioral and electroencephalographic measures of time estimation. Drug and Alcohol Dependence. 2001;63(1):87–95. doi: 10.1016/s0376-8716(00)00195-2. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Psychiatric and neurophysiological predictors of obesity in HIV/AIDS. Psychophysiology. 2008;45(6):1055–1063. doi: 10.1111/j.1469-8986.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueti D, Walsh V. The parietal cortex and the representation of time, space, number and other magnitudes. Philosophy Transactions of the Royal Society of London. B Biological Sciences. 2009;364(1525):1831–1840. doi: 10.1098/rstb.2009.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SR, Johnson SC, Jacobs PC. Disinhibited characteristics and binge drinking among university student drinkers. Addictive Behaviors. 2010;35(3):242–251. doi: 10.1016/j.addbeh.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R, Cohen Kadosh K, Schuhmann T, Kaas A, Goebel R, Henik A, et al. Virtual dyscalculia induced by parietal-lobe TMS impairs automatic magnitude processing. Current Biology. 2007;17(8):689–693. doi: 10.1016/j.cub.2007.02.056. [DOI] [PubMed] [Google Scholar]
- Darkes J, Greenbaum PE, Goldman MS. Sensation seeking-disinhibition and alcohol use: Exploring issues of criterion contamination. Psychological Assessment. 1998;10(1):71–76. [Google Scholar]
- Dick DM, Wang JC, Plunkett J, Aliev F, Hinrichs A, Bertelsen S, et al. Family-based association analyses of alcohol dependence phenotypes across DRD2 and neighboring gene ANKK1. Alcoholism: Clinical and Experimental Research. 2007;31(10):1645–1653. doi: 10.1111/j.1530-0277.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. American Journal of Human Genetics. 2004;74(4):705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollar D, Moore D. Relationship of substance use by students with disabilities to long-term educational, employment, and social outcomes. Substance Use & Misuse. 2004;39(6):931–962. doi: 10.1081/ja-120030894. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Neurotoxic effects of alcohol in adolescence. Annual Review of Clinical Psychology. 2013;9:703–721. doi: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennison KM. The short-term effects and unintended long-term consequences of binge drinking in college: a 10-year follow-up study. American Journal of Drug and Alcohol Abuse. 2004;30(3):659–684. doi: 10.1081/ada-200032331. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philosophy Transactions of the Royal Society of London. B. Biological Sciences. 2009;364(1525):1907–1918. doi: 10.1098/rstb.2009.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladanyi M, Dubrovsky B. CNV and time estimation. International Journal of Neuroscience. 1985;26(3–4):253–257. doi: 10.3109/00207458508985622. [DOI] [PubMed] [Google Scholar]
- Laghi F, Liga F, Baumgartner E, Baiocco R. Time perspective and psychosocial positive functioning among Italian adolescents who binge eat and drink. Journal of Adolescence. 2012;35(5):1277–1284. doi: 10.1016/j.adolescence.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Latendresse SJ, Bates JE, Goodnight JA, Lansford JE, Budde JP, Goate A, et al. Differential susceptibility to adolescent externalizing trajectories: examining the interplay between CHRM2 and peer group antisocial behavior. Child Development. 2011;82(6):1797–1814. doi: 10.1111/j.1467-8624.2011.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. A right hemispheric prefrontal system for cognitive time measurement. Behavior Processes. 2006;71(2–3):226–234. doi: 10.1016/j.beproc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Lopez-Caneda E, Rodriguez Holguin S, Cadaveira F, Corral M, Doallo S. Impact of Alcohol Use on Inhibitory Control (and Vice Versa) During Adolescence and Young Adulthood: A Review. Alcohol and Alcoholism. 2013 doi: 10.1093/alcalc/agt168. [DOI] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Wang S, Blumberg HP, Gelernter J. CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: results from an extended case-control structured association study. Human Molecular Genetics. 2005;14(16):2421–2434. doi: 10.1093/hmg/ddi244. [DOI] [PubMed] [Google Scholar]
- Manganiello JA. Opiate addiction: a study identifying three systematically related psychological correlates. International Journal of Addiction. 1978;13(5):839–847. doi: 10.3109/10826087809039307. [DOI] [PubMed] [Google Scholar]
- McCabe SE. Gender differences in collegiate risk factors for heavy episodic drinking. Journal of Studies on Alcohol. 2002;63(1):49–56. [PubMed] [Google Scholar]
- McCabe SE, Boyd CJ, Cranford JA, Morales M, Slayden J. A modified version of the Drug Abuse Screening Test among undergraduate students. Journal of Substance Abuse Treatment. 2006;31(3):297–303. doi: 10.1016/j.jsat.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordkoff JT, Gianaros PJ. Detecting the onset of the lateralized readiness potential: a comparison of available methods and procedures. Psychophysiology. 2000;37(3):347–360. [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Estevez AF, Zaldivar F, Montes JM, Gutierrez-Ferre VE, Esteban L, et al. Impulsivity differences in recreational cannabis users and binge drinkers in a university population. Drug and Alcohol Dependence. 2012;124(3):355–362. doi: 10.1016/j.drugalcdep.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Morillon B, Kell CA, Giraud AL. Three stages and four neural systems in time estimation. Journal of Neuroscience. 2009;29(47):14803–14811. doi: 10.1523/JNEUROSCI.3222-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93(5):729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Randolph ME, Torres H, Gore-Felton C, Lloyd B, McGarvey EL. Alcohol use and sexual risk behavior among college students: understanding gender and ethnic differences. American Journal of Drug and Alcohol Abuse. 2009;35(2):80–84. doi: 10.1080/00952990802585422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Lee NK. Hazardous alcohol use: its delineation as a subthreshold disorder, and approaches to its diagnosis and management. Comprehensive Psychiatry. 2000;41(2 Suppl 1):95–103. doi: 10.1016/s0010-440x(00)80015-2. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shin SH, Hong HG, Jeon SM. Personality and alcohol use: the role of impulsivity. Addictive Behaviors. 2012;37(1):102–107. doi: 10.1016/j.addbeh.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart RG. Future time perspectives in alcoholics and social drinkers. Journal of Abnormal Psychology. 1968;73(1):81–83. doi: 10.1037/h0025449. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, Ryan DP, Parker JD. Validation of the alexithymia construct: a measurement-based approach. Canadian Journal of Psychiatry. 1990;35(4):290–297. doi: 10.1177/070674379003500402. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Ryan D, Bagby RM. Toward the development of a new self-report alexithymia scale. Psychotherapy and Psychosomatics. 1985;44(4):191–199. doi: 10.1159/000287912. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and Clinical Psychopharmacology. 1998;6(3):292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Wallace M. Future time perspective in schizophrenia. Journal of Abnormal Psychology. 1956;52(2):240–245. doi: 10.1037/h0039899. [DOI] [PubMed] [Google Scholar]
- Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Human Molecular Genetics. 2004;13(17):1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: cross-cultural, age, and sex comparisons. Journal of Consulting & Clinical Psychology. 1978;46(1):139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]