Abstract
Background
The Final Rule regulations were developed to allow exception from informed consent (EFIC) to enable clinical trial research in emergency settings where major barriers exist for informed consent. There is little known evidence of the effect of the Final Rule in minority enrollment in clinical trials, particularly in traumatic brain injury (TBI) trials. A clinical trial funded by the National Institute of Neurological Disorders and Stroke was conducted to study the effects of erythropoietin on cerebral vascular dysfunction and anemia in subjects with TBI. There were periods of time when EFIC was and was not available for enrollment into the study.
Purpose
To explore the effect of EFIC availability on TBI trial enrollment of minority versus non-minority subjects.
Methods
Minority status of screened (n=289) and enrolled (n=191) TBI subjects was determined for this study. We tested for the presence of a minority and EFIC availability interaction in a multiple logistic regression model after controlling for EFIC and minority group main effects and other covariates.
Results
An interaction between the availability of EFIC minority and non-minority enrollment was not detected (odds ratio: 1.22; 95% confidence interval: 0.29–5.16).
Limitations
Our study was conducted at a single site and the confidence interval for the EFIC and minority interaction term was wide. Therefore, a small interaction effect cannot be ruled out.
Conclusions
EFIC increased the odds of being enrolled regardless of minority status.
Keywords: Exception from informed consent, recruitment, race/ethnicity
Background
Informed consent is typically considered a fundamental aspect of ethical clinical research. Obtaining consent involves making potential subjects aware of the risks and potential benefits of the intervention and make a decision about their willingness or unwillingness to participate in the experiment based on this information.1, 2 Unfortunately, obtaining consent becomes impossible when subjects are severely impaired at the time of study entry, as is often the case in clinical trials in emergency settings.3–5 In these circumstances, study sites attempt to contact a legally authorized representative (LAR) to provide informed consent on behalf of the prospective participant. In emergency situations it is often critical that the representative be approached immediately due to time constraints for optimal interventions.6 However, it may not be possible to reach appropriate LARs in a short time frame. In these cases, the study may not be feasible if informed consent is required.
In response to the necessity of research in many emergency settings, the US Food and Drug Administration (FDA) and the Department of Health and Human Services (HHS) developed regulations in 1996 that allow approval of clinical trials in which prospective informed consent for critically ill or injured subjects is not attainable7. With the intention to ensure protection of vulnerable populations, these regulations, known as the Final Rule, have specific requirements that allow exception from informed consent (EFIC). For instance, the rule is only applicable if subjects have life-threatening medical conditions, if attempts to obtain proxy consent within the therapeutic window have been made, and if the local community has been consulted about the research.1, 2, 8, 9 Community consultation involves a dialog between investigators and the community to discuss the risks and benefits of the proposed investigation, providing the opportunity for feedback to determine whether the study should be conducted in that community.10, 11 For example, a series of public meetings could be held in communities where the clinical investigation will be conducted and from which the subjects will be drawn to discuss the study protocol. Despite the burden imposed by its application, several studies have used EFIC successfully.8, 12–15 A multicenter trial of hypothermia in subjects with acute brain injury designed to achieve 33 degrees Celsius within 8 hours after injury, concluded that the implementation of EFIC in the study increased enrollment and decreased both randomization time and time to achieve the target temperature.16
Fear and mistrust are two of the main reasons enrollment of women and diverse racial/ethnic groups in clinical trials has remained low throughout history. Other reasons for low participation include health care provider barriers, language barriers, socio-cultural barriers, feasibility, and costs.17, 18 Regulations and policies have been developed through time in order to protect humans in medical experiments and encourage participation of diverse groups. Notably, the National Institutes of Health released “The NIH Guidelines on Inclusion of Women and Minorities as Subjects in Clinical Research” in 1994 to ensure inclusion of these groups in clinical research. Despite the implementation of these guidelines by the US government, ethnic minorities in clinical trials still remain underrepresented. Dula 19 argued that minorities’ fears due to the historical abuse of informed consent might make researchers’ entrance into minority communities challenging and thus it is difficult to comply with the Final Rule’s requirement of community consultation. Additionally, research without consent could foster mistrust in minority populations since it could disproportionately affect this group. Institutional review boards have addressed these concerns by requiring representation of diverse communities in the community consultation process.
In 2006, the National Institute of Neurological Disorders and Stroke (NINDS) provided support to initiate a clinical trial in Houston, Texas to study the effects of erythropoietin on cerebral vascular dysfunction and anemia in subjects with Traumatic Brain Injury (TBI). Subjects with TBI were enrolled from 2006–2012. When this study started, subjects were enrolled using a prospective written consent from their family members. However, after IRB review and approval of the requirements for emergency research, the mechanism of enrollment was changed in August 2007 to allow EFIC. The advantage of having two modalities of enrollment in the same trial is the ability to compare these modalities in the same study population.
There is little known evidence of the effect of the Final Rule in minority enrollment in clinical trials, particularly in TBI trials. In this report, we studied whether there was a difference in minority enrollment in the Erythropoietin TBI trial between the periods of time when EFIC was available and when it was not.
Methods
Primary Hypothesis
Our primary hypothesis was that EFIC availability increases the likelihood of enrollment for minorities more than for non-minorities.
Study Population
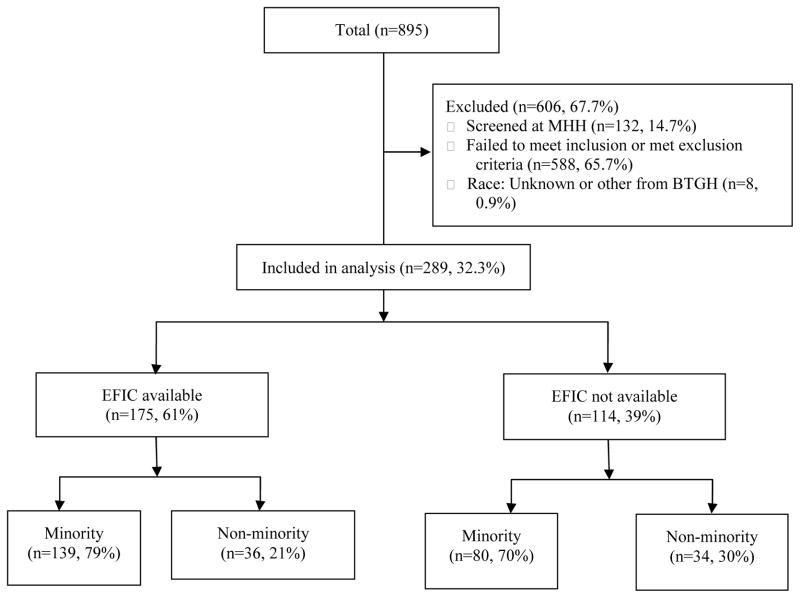
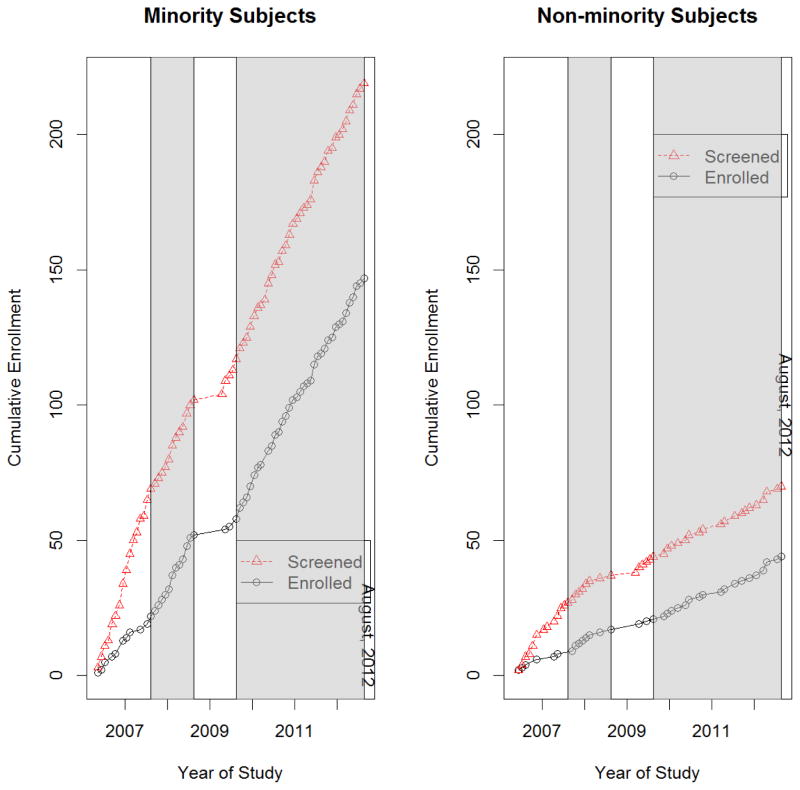
A total of 200 subjects were enrolled into the Erythropoietin TBI trial between 2006 and 2012. In order to be eligible for the study, subjects needed to have suffered a severe brain injury with a motor component of the Glasgow Coma Score (mGCS) <5, be at least 15 years of age, and be enrolled within 6 hours of injury. A CONSORT diagram of subjects who were included and excluded from this analysis is shown in Figure 1. A total of 895 subjects were screened in the emergency department at two sites: Memorial Hermann Hospital (MHH) and Ben Taub General Hospital (BTGH). The criterion for screening was a subject who was not following commands following a head injury. Because of the lack of demographic information available on the screened but not enrolled subjects at MHH as well as the small number of subjects enrolled from this site, all MHH subjects were omitted from this analysis (including 132 screened [14.8%], 8 enrolled [0.9%]). We also excluded 588 BTGH subjects (65.7%) who failed to meet trial inclusion criteria or who met exclusion criteria, with the exception of those excluded due to refusing consent and 8 BTGH subjects (0.9%) who had other or unknown race/ethnicity. For the screened subjects, minority status was determined by an assessment that was made on admission from appearance, information from any relatives, significant others and friends as well as from medical records. For enrolled subjects, minority status was revised for 1 subject (0.5%) based on the individual subject’s self-identified race/ethnicity and/or that of family members, depending on the status of the subject. All subjects were initially comatose, some died in the hospital and some never recovered to a stage where they could self-identify race/ethnicity. On the basis of the information received, subjects were categorized as African-American, Asian, White non-Hispanic, Hispanic, or Other. For the purposes of this paper, non-minority and minority groups were formed, where minority group was defined as Asian, Hispanic-Latino, or Black. White is defined as White non-Hispanic. Figure 2 illustrates the cumulative number (by month) of screened and enrolled subjects, with shaded regions indicating when EFIC was available. EFIC was not available at the beginning of the trial (May 2006 – July 11, 2007) and also for a short period of time in the middle of the trial (March 19, 2009 – August 9, 2009). Between September 15, 2008 and March 19, 2009 the FDA put a clinical hold on erythropoietin due to adverse events in its use for other medical conditions, but then allowed resumption of the treatment trial at a lower dose. Five months after the trial was restarted the FDA allowed EFIC to resume. Thus, in total, EFIC was available for 50 months and not available for 20 months.
Figure 1.
CONSORT diagram of screened subjects
Figure 2.
Cumulative enrollment of screened and enrolled subjects by month and minority status. Shaded and white regions indicate times when EFIC was and was not available, respectively.
Statistical Methods
To test the primary hypothesis, we assessed whether the probability of minority participant enrollment when EFIC is available is greater than the probability of minority participant enrollment when EFIC is unavailable. Hence, we tested for the presence of a minority and EFIC availability interaction in a multiple logistic regression model after controlling for EFIC and minority group main effects and other covariates. The main effects were coded as EFIC availability (1=yes, 0=no) and minority group (1=yes, 0=no). Other covariates included violent mechanism of injury (1=yes, 0=no), female (1=yes, 0=no) and age. The covariates to include in the final model were chosen using the model building process described below.
To determine distributional differences in demographic characteristics between enrolled and non-enrolled subjects, chi-square tests were performed for discrete variables and the t-test was used for continuous variables. To assess whether any differences detected in EFIC enrollment could be explained by the unavailability of the family at the time of enrollment, we compared, by minority status, time from injury until hospital arrival, from hospital arrival until enrollment, and from hospital arrival until family arrival using the Wilcoxon rank sum test due to non-normality.
We also compared data from the community consultation process to determine if minority and non-minority communities differed in willingness to have the study conducted in their community using Fisher’s exact test. Communities were classified into either non-minority, mixed, or predominantly minority. Willingness was defined as a response of “agree” or “strongly agree” to the question “Are you willing for this study to be done in your community?”. Eight community meetings were held in city multi-service centers or in schools distributed throughout Houston. The meetings were in association with other planned activities, such as health fairs and community meetings with the Houston Police department, and represented diverse racial and ethnic groups. Two hundred forty-three surveys were returned from these meetings. Four community leadership groups, classified as non-minority, were also included: the Houston Fire Department Emergency Medical Services, central nervous system basic scientists, Mothers Against Drunk Drivers, Gateway to Care, and Gulfcoast United Way. Fifty-two surveys were returned from these meetings.
Model Building
All covariates of interest were analyzed using univariate logistic regression models and tested at the alpha level of 0.25 to be entered into the multivariable model. The contribution of each variable in the multivariable model was determined by removing the variable of interest and comparing it to the full model using the likelihood ratio test at the 0.1 level of significance. A more detailed description of this approach is described in Hosmer and Lemeshow. 20 The area under the receiver operating characteristic curve (AUC) and the classification table were used to assess the adequacy of the logistic regression model.
All statistical analyses were performed using SAS version 9.2 (SAS Institute) with 2-sided statistical tests at a .05 significance level except the interaction term which was tested at the 0.1 significance level. A significance level 0.1 is generally used in assessing interactions, given the acknowledgement that interactions tests would have lower power than tests of the main effects.
Results
Of the 289 subjects included in this analysis, 191 (66%) were enrolled into the study (Table 1). Of these 289 subjects, 61% were screened during the time when EFIC was available and the majority were enrolled (81.2%; p<0.001). The number of Hispanic-Latino, Black, and Asian subjects was consistently higher than the White population among the enrolled (77%) and non-enrolled (73.5%) groups. Overall, the average age was 36 years, there were 75.8% minority, 85.5% male, and 9.7% who had violent injuries. There were no differences detected between enrolled versus screened but not enrolled by race, gender, mechanism of injury, or age.
Table 1.
Study characteristics of enrolled and not enrolled subjects
| Screened but not enrolled (N=98) | Enrolled (N=191) | P-Value | |
|---|---|---|---|
| Race | |||
| Minority* | 72 (73.5%) | 147 (77.0%) | 0.56 |
| White | 26 (26.5%) | 44 (23.0%) | |
| EFIC Availability | |||
| Available | 20 (20.4%) | 155 (81.2%) | <0.001 |
| Unavailable | 78 (79.6%) | 36 (18.9%) | |
| Gender | |||
| Male | 80 (81.6%) | 167 (87.4%) | 0.22 |
| Female** | 18 (18.4%) | 24 (12.6%) | |
| Mechanism of Injury | |||
| Violent | 10 (10.2%) | 18 (9.4%) | 0.84 |
| Non-Violent | 88 (89.8%) | 173 (90.6%) | |
| Mean±SD | |||
| Age | 34.5±12.9 | 34.0±13.6 | 0.74 |
Minority defined as Asian, Hispanic-Latino, and Blacks.
One subject coded as an anatomical male living as female. Subject analyzed as male in this analysis.
There were no statistically significant differences detected in the time to the subject’s hospital arrival between minorities and non-minorities (p=0.17). Minority families arrived significantly later than non-minority families (median= 3.8 hours for minorities, median=2.3 hours for non-minorities, p=0.02). The percentage of minority families that arrived later than 6 hours after the estimated injury time (estimated at the time of arrival to the hospital) was higher than for non-minority families (minority families - 42%; non-minority families - 24%; p=0.04).
Figure 2 displays the relationship between enrollment and EFIC availability, stratified by minority status. There are notable differences in the slopes of the cumulative enrollment line compared to the cumulative screened line when EFIC was not available (white, non-shaded areas). In contrast, the slopes are similar when EFIC was available (grey, shaded areas). This was consistent for both minority and non-minority groups.
Variables EFIC availability, minority status, and the interaction between EFIC availability and minority were included in all multiple logistic regression models. After the model building and variable selection process, the final model included EFIC availability, minority status, and the interaction between EFIC availability and minority status (violent mechanism of injury, female, and age variables were excluded from the model). The model fit the data well (sensitivity=81% and specificity = 80% for 0.5 posterior probability cutoff, AUC = 0.81).
Table 2a displays the results from the logistic regression analysis. An effect of the availability of EFIC on minority and non-minority enrollment was not detected (OR: 1.22; 95% CI: 0.29–5.16). When EFIC was not available, the log-odds of minority enrollment was 0.75. Alternatively, when EFIC was available, the log-odds of minority enrollment was 0.96. The logistic regression model was then fit without the interaction term (Table 2b). The odds of being enrolled during the time that EFIC was available were 17.1 times larger than the odds when EFIC was unavailable (OR: 17.1; 95% CI: 9.23–31.72).
Table 2.
| Table 2a. Logistic regression model estimates for likelihood of being enrolled into study including minority EFIC Availability Interaction.
| |||
|---|---|---|---|
| Odds Ratio | 95% CI | P-Value | |
| Minority | 0.78 | 0.34 – 1.84 | |
| EFIC Availability | 14.67 | 4.18 – 51.45 | |
| EFIC*Minority | 1.22 | 0.29 – 5.16 | 0.78 |
| Table 2b. Logistic regression model estimates for likelihood of being enrolled into study*
| |||
|---|---|---|---|
| Odds Ratio | 95% CI | P-Value | |
| Minority | 0.87 | 0.42 – 1.69 | 0.63 |
| EFIC Availability | 17.1 | 9.23 – 31.72 | <0.001 |
Since no interaction was detected, the model was refit without the interaction term.
In assessing the community consultation process, a difference in willingness for the study to be conducted in minority and non-minority communities was not detected (73/74=99% Agree or strongly agree in non-minority communities versus 203/215=94% in the mixed and minority communities, p=0.20, Fisher’s exact test). Separating the mixed community from the minority group, 80% (12/15) of the mixed group agreed or strongly agreed whereas 96% of the minority group was favorable to conducting the study in their community.
Discussion
TBI is one of the most common causes of brain damage in the United States where 1.7 million are affected every year, and of these, about 52,000 die.21 EFIC has been found to be important for TBI trials when the intervention effectiveness is contingent on using it within a short period of time after injury and when it is unreasonable to expect that the intermediate steps between surrogate availability and prospective consent can be shortened.
Minorities, defined by Humes et al. 22 as the entire U.S population excluding non-Hispanic Whites alone, account for 36.3% of the United States population and the percentage is projected to be 53.7% by 2050.23 Studies have found that minorities affected with TBI have different demographic and injury characteristics and poorer functional outcomes than injured non-minorities. 24–28 The discrepancy in functional outcomes has been related to less medical care provided to minorities 26, 28, 29 and to socio-demographic and injury factors.24, 25, 30 Because minorities and non-minorities afflicted with TBI differ in many aspects, it is necessary that TBI research studies consider an adequate representation of both groups in order to improve the outcomes of the whole population.
In the current study, we did not find any evidence to suggest that there were any differences in the odds of being enrolled into a TBI study between minorities and non-minorities. As expected, we observed a significant increase in the odds of being enrolled using the EFIC mechanism. While EFIC was available, 81% of subjects were enrolled whereas 19% of subjects were enrolled while EFIC was not available. Our primary objective was to test for an interaction between EFIC availability and minority enrollment. We did not detect a statistically significant interaction. Although minority families arrived at the hospital significantly later than non-minority families, had a higher percentage of families arriving outside of the six-hour window (disallowing prospective subject enrollment and at least partially increasing the percentage of minority subjects being enrolled under EFIC [Figure 1]), EFIC similarly increased the odds of being enrolled in both minority and non-minority populations. We were concerned that the EFIC process might enroll minority subjects who would otherwise not consent. If that was the case we would expect to see an interaction between minority status and the availability of EFIC. The data suggest that the increase in enrollment using EFIC was unlikely to come at the expense of unwilling minority subjects and their families. This is consistent with our own data from community consultation that showed little difference in willingness to have the study conducted in their communities.
Other studies have looked at a similar question. Using informed consent in the first 9 months of their trial and EFIC in the remaining 33 months, Clifton et al. 16 observed that EFIC increased minority enrollment of subjects with acute brain injury from 20% when it was not available to 26% when it was. The authors compared the distribution of race before and after the EFIC mechanism was implemented, only among those subjects enrolled into the study. Sugarman et al. 31 studied whether racial and ethnic minority enrollment was disproportionate using EFIC under emergency circumstances. No differences were found; however, historical controls were used and race information was not collected for those who were screened but did not enroll.
Our study has several strengths. Our trial has periods of time when EFIC was available and when it was not available; hence, we have a means of comparing the two. Also, there are two different time periods without EFIC, not just a single period before the trial starts. Unlike the Clifton et al. 16 and Sugarman et al. 31 studies, we have racial/ethnic information for all screened subjects, not only those that were enrolled into the study. Considering the screened and enrolled subjects allowed us to study changes in the probability of enrollment and thus the effect of the availability of EFIC on minority enrollment. An additional strength is the similarity of the minority distribution in the TBI trial compared to the population of subjects screened. Due to a relatively small sample size, the confidence interval for the EFIC and minority interaction term was wide. A small interaction effect cannot be ruled out. There are many challenges to obtaining a larger sample size to test this hypothesis. Additionally, this study uses data from one clinical site and thus may have uncertain generalizability. However, the BTGH site in Houston, Texas serves a very large and diverse population (32.2% Caucasian, non-Hispanic-Latino, 41.5% Hispanic-Latino, 19.5% Black-African American, 6.6% Asian, 2.8% other race/ethnicity, based on the 2010 population census).
Given that EFIC significantly increased enrollment and that there appears to be no adverse effects on minority recruitment, EFIC availability should be considered for TBI trials. While burdensome, EFIC requirements including community consultation are viable in the TBI setting.
Acknowledgments
We thank communities and subjects that enable much-needed ethical research to be conducted in their communities, sometimes without the possibility of obtaining prospective informed consent, in the name of research advances. We also thank Alesha Yamal for her editorial assistance.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health (grant No. P01NS38660).
Footnotes
Clinical Trial Registration: www.clinicaltrials.gov, NCT00313716
References
- 1.Adams JG, Wegener J. Acting without asking: an ethical analysis of the Food and Drug Administration waiver of informed consent for emergency research. Ann Emerg Med. 1999;33:218–223. doi: 10.1016/s0196-0644(99)70398-7. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt TA, Salo D, Hughes JA, et al. Confronting the ethical challenges to informed consent in emergency medicine research. Acad Emerg Med. 2004;11:1082–1089. doi: 10.1197/j.aem.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Olson CM. The Letter or the Spirit Consent for Research in CPR. JAMA: The Journal of the American Medical Association. 1994;271:1445–1447. [PubMed] [Google Scholar]
- 4.Lurie KG, Shultz JJ, Callaham ML, et al. Evaluation of active compression-decompression CPR in victims of out-of-hospital cardiac arrest. JAMA: the journal of the American Medical Association. 1994;271:1405–1411. [PubMed] [Google Scholar]
- 5.Prentice ED, Antonson L, Leibrock LG, et al. IRB review of a phase II randomized clinical trial involving incompetent patients suffering from severe closed head injury. IRB. 1993:1–7. [PubMed] [Google Scholar]
- 6.Sen Biswas M, Newby LK, Bastian LA, et al. Who refuses enrollment in cardiac clinical trials? Clinical trials. 2007;4:258–263. doi: 10.1177/1740774507079434. [DOI] [PubMed] [Google Scholar]
- 7.Offices of the Secretary, DHHS, FDA. Protection of Human Subjects: Informed Consent and Waiver of Informed Consent Requirements in Certain Emergency Research: Final Rules. 1996;61:51497–531. [Google Scholar]
- 8.Biros MH, Fish SS, Lewis RJ. Implementing the Food and Drug Administration’s final rule for waiver of informed consent in certain emergency research circumstances. Acad Emerg Med. 1999;6:1272–1282. doi: 10.1111/j.1553-2712.1999.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 9.Biros MH. Research without consent: current status, 2003. Ann Emerg Med. 2003;42:550–564. doi: 10.1067/s0196-0644(03)00490-6. [DOI] [PubMed] [Google Scholar]
- 10.Holcomb JB, Weiskopf R, Champion H, et al. Challenges to effective research in acute trauma resuscitation: consent and endpoints. Shock. 2011;35:107–113. doi: 10.1097/SHK.0b013e3181f7fd01. [DOI] [PubMed] [Google Scholar]
- 11.Contant C, McCullough LB, Mangus L, et al. Community consultation in emergency research. Crit Care Med. 2006;34:2049–2052. doi: 10.1097/01.CCM.0000227649.72651.F1. [DOI] [PubMed] [Google Scholar]
- 12.Blajchman MA, Carson JL, Eikelboom JW, et al. The role of comparative effectiveness research in transfusion medicine clinical trials: proceedings of a National Heart, Lung, and Blood Institute workshop. Transfusion. 2012;52:1363–1378. doi: 10.1111/j.1537-2995.2012.03640.x. [DOI] [PubMed] [Google Scholar]
- 13.Silbergleit R, Biros MH, Harney D, et al. Implementation of the exception from informed consent regulations in a large multicenter emergency clinical trials network: the RAMPART experience. Acad Emerg Med. 2012;19:448–454. doi: 10.1111/j.1553-2712.2012.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brasel KJ, Bulger E, Cook AJ, et al. Hypertonic resuscitation: design and implementation of a prehospital intervention trial. J Am Coll Surg. 2008;206:220–232. doi: 10.1016/j.jamcollsurg.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Wright DW, Clark PL, Pentz RD, et al. Enrolling subjects by exception from consent versus proxy consent in trauma care research. Ann Emerg Med. 2008;51:355–360. doi: 10.1016/j.annemergmed.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Clifton GL, Knudson P, McDonald M. Waiver of consent in studies of acute brain injury. J Neurotrauma. 2002;19:1121–1126. doi: 10.1089/08977150260337930. [DOI] [PubMed] [Google Scholar]
- 17.Schmotzer GL. Barriers and facilitators to participation of minorities in clinical trials. Ethn Dis. 2012;22:226–230. [PubMed] [Google Scholar]
- 18.Hussain-Gambles M, Atkin K, Leese B. Why ethnic minority groups are under-represented in clinical trials: a review of the literature. Health & social care in the community. 2004;12:382–388. doi: 10.1111/j.1365-2524.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 19.Dula A. Bearing the brunt of the new regulations: minority populations. Hastings Cent Rep. 1997;27:11–12. [Google Scholar]
- 20.Hosmer DW, Lemeshow S. Applied logistic regression. 2004. [Google Scholar]
- 21.Faul M, Xu L, Wald M, et al. Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 22.Humes K, Jones N, Ramirez R. Overview of race and Hispanic origin: 2010.2010 Census Briefs. US Census Bureau; 2011. [Google Scholar]
- 23.U.S. Census Bureau, Population Division. Table 4. Projections of the Population by Sex, Race, and Hispanic Origin for the United States: 2010 to 2050. Report no. NP2008-T4. [Google Scholar]
- 24.Arango-Lasprilla JC, Rosenthal M, Deluca J, et al. Traumatic brain injury and functional outcomes: Does minority status matter? Brain Injury. 2007;21:701–708. doi: 10.1080/02699050701481597. [DOI] [PubMed] [Google Scholar]
- 25.Arango-Lasprilla JC, Rosenthal M, Deluca J, et al. Functional outcomes from inpatient rehabilitation after traumatic brain injury: how do Hispanics fare? Arch Phys Med Rehabil. 2007;88:11–18. doi: 10.1016/j.apmr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Burnett DM, Silver TM, Kolakowsky-Hayner SA, et al. Functional outcome for African Americans and Hispanics treated at a traumatic brain injury model systems centre. Brain Injury. 2000;14:713–718. doi: 10.1080/026990500413731. [DOI] [PubMed] [Google Scholar]
- 27.Hart T, Whyte J, Polansky M, et al. Community outcomes following traumatic brain injury: impact of race and preinjury status. J Head Trauma Rehabil. 2005;20:158–172. doi: 10.1097/00001199-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal M, Dljkers M, Harrison-Felix C, et al. Impact of minority status on functional outcome and community integration following traumatic brain injury. J Head Trauma Rehabil. 1996;11:40–57. [Google Scholar]
- 29.Bazarian JJ, Pope C, McClung J, et al. Ethnic and racial disparities in emergency department care for mild traumatic brain injury. Acad Emerg Med. 2003;10:1209–1217. doi: 10.1111/j.1553-2712.2003.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 30.Sherer M, Nick TG, Sander AM, et al. Race and productivity outcome after traumatic brain injury: influence of confounding factors. J Head Trauma Rehabil. 2003;18:408. doi: 10.1097/00001199-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Sugarman J, Sitlani C, Andrusiek D, et al. Is the enrollment of racial and ethnic minorities in research in the emergency setting equitable? Resuscitation. 2009;80:644–649. doi: 10.1016/j.resuscitation.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]