Abstract
Internal action models refer to sensory-motor programs that form the brain basis for a wide range of skilled behavior and for understanding others’ actions. Development of these action models, particularly those reliant on visual cues from the external world, depends on connectivity between distant brain regions. Studies of children with autism reveal anomalous patterns of motor learning and impaired execution of skilled motor gestures. These findings robustly correlate with measures of social and communicative function, suggesting that anomalous action model formation may contribute to impaired development of social and communicative (as well as motor) capacity in autism. Examination of the pattern of behavioral findings, as well as convergent data from neuroimaging techniques, further suggests that autism-associated action model formation may be related to abnormalities in neural connectivity, particularly decreased function of long-range connections. This line of study can lead to important advances in understanding the neural basis of autism and, more critically, can be used to guide effective therapies targeted at improving social, communicative, and motor function.
Keywords: action models, autism, cerebral connectivity, EEG, motor function
Autism is a neurodevelopmental disorder that can have substantial psychosocial consequences for both the affected individual and his or her family (Hofvander and others 2009; Montalbano and Roccella 2009). The diagnosis is made based on the presence of deficits in social interaction and communication, as well as restricted, repetitive interests (American Psychiatric Association 2000). Autism represents a spectrum, with a range of severity. Also, different aspects of the autism phenotype can predominate at different ages, with delayed language/communication being a primary presenting feature in toddlerhood, impaired social interaction during school age, and impaired independent functioning during adulthood. That said, the core distinctive feature of autism is a pervasive impairment in the ability to engage in reciprocal social interaction with peers. Two recent studies place the incidence of autism around 1 in 110 (Centers for Disease Control and Prevention 2009; Kogan and others 2009); the combination of high prevalence and severe consequences of this disorder has spurred extensive research into the causes and possible treatments of autism. These efforts have led to important advances, but the neural basis of the core behavioral features remains obscure, and effective treatments for these core features remain elusive for many children with the disorder.
Autism may be understood on levels of analysis from the molecular to the behavioral, but the neural systems level may be particularly critical for the purpose of developing postnatal treatments. Autism has already been linked to a number of genetic causes, which suggests that the etiology of the disorder is diverse (Moss and Howlin 2009). It is nevertheless quite possible that these various etiologies lead to a common impairment in neural system function that results in core impairments in social, communicative, and behavioral capacity. Defining a neural system dysfunction characteristic of autism can lead to the identification of endophenotypes that provide advances in detection and diagnosis. Of even greater clinical importance, a systems-level description can also provide a foundation for advances in therapeutic intervention; direct evidence of this will be discussed later.
Recent theoretical and empirical developments in the neural basis of skill learning have shed a new and compelling light on how the autistic phenotype develops. Data from our and others’ laboratories substantiate many of the predictions made by these theories as they relate to autism. Social and communicative competence depends on development of skilled behaviors. These skilled behaviors reside in the brain as internal action models (Shadmehr and Mussa-Ivaldi 1994); the autism phenotype may therefore arise from anomalous formation of internal action models. Furthermore, there is evidence that these internal action models are used in a “feed-forward” fashion to extrapolate and understand the actions of others (Klin and others 2003)—a capacity that has been termed theory of mind (Baron-Cohen and others 1985). One potential neurobiological explanation for the autistic impairment in acquisition and execution of internal action models is decreased connectivity between the relevant cerebral regions; this may be a specific example of the underconnectivity hypothesis of autism that has been demonstrated under a number of other experimental conditions. In the current review, we examine the theory and evidence for internal action models as a basis for social and communicative development. We review the extensive evidence that demonstrates the power of this view in the study of autism via the examination of the motor system and correlation with social/communicative function. We consider the impact of altered procedural learning in autism in the development of anomalous internal action models and show how results from these studies support the view that altered cerebral connectivity may play a key pathogenic role in the autistic abnormalities of action models.
Internal Action Models as a Basis for Social/Communicative Skill Development
The concept of action being the basis for various cognitive faculties dates at least as far back as Piaget (Beilin and Fireman 1999), who not only proposed a sensory-motor stage of cognitive development but also placed action as a fundamental mechanism for cognitive development throughout the life span. In this view, individuals learn specific skills and develop cognitive faculties by performing actions and interpreting the sensory feedback that results. A more recent (and brain-based) formulation refers to discrete action plans and their resulting sensory feedback as internal action models (Shadmehr and Krakauer 2008; Shadmehr and Mussa-Ivaldi 1994). Internal action models are conceived of in their most basic sense as relating to motor skills, but both theory and evidence also suggest they are involved in the development of a wide range of human behavior, including those actions necessary to social interaction (Gidley Larson and Mostofsky 2006). The manner in which action models are employed as the basis of social skills is perhaps more complex than what is seen with motor skills. Specifically, as social skills depend on both engaging in a series of movements as well as understanding others’ movements (and associated intentions) (Beilin and Fireman 1999), the internal action models are used via feedforward mechanisms for the purpose of intention understanding (Klin and others 2003).
Autism is, at its core, a disorder of social and communicative development and function. If the model explaining autistic deficits through anomalous formation of action models validly applies to social skill development, then we would expect to see impairments in motor skills, which also function via internal action models. This is indeed the case. Motor deficits have been recognized in autism since Kanner's (1943) original description. Abnormalities of basic motor skills, such as gait, posture, balance, speed, and coordination, are recognized clinically and have been demonstrated repeatedly in studies of individuals with autism (Ghaziuddin and Butler 1998; Jansiewicz and others 2006; Noterdaeme and others 2002; Rinehart, Bellgrove, and others 2006; Rinehart, Tonge, and others 2006). Of greater relevance, clinical histories of children with autism reveal a particular difficulty with learning skilled motor tasks (Gidley Larson and Mostofsky 2006) in which internal action models are implicated. Although it is common for children with autism to have intact acquisition of early motor milestones that involve innate reflexive capacities (e.g., sitting up and walking), nearly all children with autism show delays and abnormalities in the acquisition of a wide range of learned skilled gestures, including those necessary for motor/adaptive functioning (e.g., peddling, pumping legs on a swing, various dressing skills and handwriting; Fuentes and others 2009), as well as social/communicative function (e.g., waving goodbye).
Numerous studies over the past several decades have directly observed impairments in skilled motor behaviors, specifically praxis and imitation (DeMeyer and others 1972). Both imitation and praxis depend on sensory-motor circuits necessary for action model formation—specifically, connectivity between posterior parietal regions necessary for formation and storage of spatial/temporal representations of action and premotor regions necessary for selection and sequencing of the resulting motor programs (Heilman and Valenstein 2003; Iacoboni and Mazziotta 2007; Wheaton and Hallett 2007). Motor imitation reflects online development of internal action models, whereas praxis—the performance of skilled, goal-directed motor behaviors—reflects the execution of internal action models but is also dependent on prior development of internal action models. Considerable emphasis has been given to impairments of imitation in the autism literature (Iacoboni and others 2005; Rogers and Pennington 1991; Williams and others 2004). However, a view of the pathogenesis of autism that posits anomalous formation of internal action models would suggest that both imitation and praxis should be involved. Indeed, several studies from our and others’ laboratories demonstrate this to be the case (Dewey and others 2007; Dowell and others 2009; Dziuk and others 2007; Ham, Bartolo, Corley, Rajendran, and others 2010; Ham, Bartolo, Corley, Swanson, and others 2010; Mostofsky and others 2006). It appears that these impairments in praxis are specific to autism, as children with attention-deficit/hyperactivity disorder (ADHD) and developmental coordination disorder show impairments in basic motor control but not in praxis (Dewey and others 2007; Mostofsky and others 2010). Furthermore, we have demonstrated that praxis impairment correlates with social/communicative impairment (Dziuk and others 2007). This strongly suggests that similar mechanisms (i.e., internal action models) may underlie impaired development and execution of both motor skills and social/communicative skills in autism. In this sense, it is plausible to consider that autism reflects a developmental dyspraxia of social/communicative skills.
Although acquisition and subsequent control of both motor and social skills rely on sensory feedback, social skill development is particularly dependent on incorporating feedback from the external world. We acquire social skills through observation and imitation of other people's actions and, through this process, develop internal models of action that allow us to infer others’ intentions. This conceptualization has been articulated in recent theories of social development under the rubric of “embodied cognitive science,” a field that originated with the study of normal social development (rather than the study of autism specifically). Current theories of embodied cognition (Grafton 2009; Williams 2008), enactive mind (Klin and others 2003), and embodied simulation (Gallese 2007) stress an individual's modeling of his or her own behaviors to form an understanding of the intentions or emotional state of the other person (Boria and others 2009; Grafton 2009). This understanding of others’ perspectives is often referred to in the autism and social cognition literature as “theory of mind” (Baron-Cohen and others 1985).
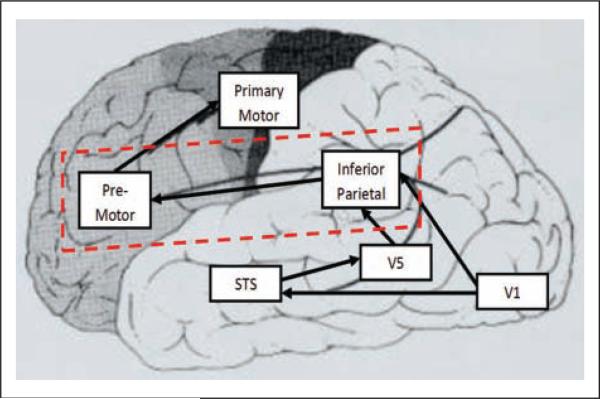
The neurobiological cognate of intention understanding may lie within the parietal-premotor circuitry necessary for both imitation and formation of action models. This circuitry is a central component of the so-called mirror neuron system (MNS) (Gallese 2007; Williams 2008), which is active both when an individual is performing a task and also when he or she observes the task being performed by others. This system is hypoactive by fMRI and electroencephalography (EEG) in autism (Dapretto and others 2006; Martineau and others 2008), and MNS dys-function has been emphasized in some recent theoretical models of autism (Gallese 2007; Williams 2008). Parietalpremotor circuitry outlined within recent MNS models has also long been recognized (since the early 1900s) to be necessary for praxis (Geschwind 1965; Heilman and Valenstein 2003; Wheaton and Hallett 2007) (Figure 1). These parietal-premotor circuits—together with inputs from the basal ganglia/cerebellum and thalamus—are critical to procedural learning and subsequent control of motor actions. The observed overlap between mirror neuron and praxis systems highlights the central contribution of parietal-premotor connectivity in sensory-motor coupling necessary for engaging in “online” imitation as well as forming sustainable internal models critical to the development of skilled actions (praxis) and the ability to interpret those actions when performed by others.
Figure 1.
Brain regions associated with praxis and imitative function include the primary visual cortex (V1); the visual region associated with recognition of movement (V5), the superior temporal sulcus (STS), which is associated with the perception of biological movement; the inferior parietal region, which is associated with storage of motor programs; the premotor regions, which are associated with transcoding of motor programs; and the primary motor cortex, which is associated with execution of motor programs. Connectivity between inferior parietal cortex and premotor cortex (red box), when lesioned, can lead to apraxia.
Abnormalities in parietal-premotor connectivity may thereby contribute to core features of autism: impaired ability to engage in social skills (i.e., the skilled actions necessary to engage in social interaction) as well impaired ability to interpret others’ actions (i.e., theory of mind). The contention that internal action models play a role in perceptual understanding of others’ intentions is supported by evidence. Similarly, at the motor level, children with autism not only demonstrate impaired ability to perform skilled gestures (praxis) but also show impaired interpretation of gestures in others. In a recently published study (Dowell and others 2009), we assessed the performance of children with autism on a test of “postural knowledge.” Participants examined drawings of a person performing a skilled action (e.g., hammering a nail, waving hello) in which the hand is missing; for each picture, they had to select (from three options) the correct representation of hand posture to fit the action. Children with autism performed less well than did typically developing children (Dowell and others 2009), and postural knowledge skill correlated significantly with praxis performance skill. This correlation suggests a single underlying mechanism that accounts for both “expressive” praxis skill and “receptive” understanding of others’ performance of the skill. Furthermore, data recently gathered from our laboratory reveal that children with ADHD show equivalent performance compared with typically developing children (Mostofsky and others 2010), suggesting that impairment in both recognition and performance of skilled gestures appears to be specific to autism.
Studies of Internal Action Model Development
Given that autism is a uniquely developmental disorder, it follows that investigation of differences in the acquisition or learning of skilled actions may be particularly relevant to autism. A model of anomalous skill-based (“procedural”) learning may therefore be a key component to mapping the neural basis of autism and for identifying effective therapies. Examination of procedural learning at the level of motor behavior, rather than “more complex” social/communicative behavior, is advantageous. Motor behaviors can be carefully controlled experimentally and easily quantified. Furthermore, we have a better understanding of the neurobiology of motor learning than we do of social and communicative learning, as we try to understand the brain correlates and possible therapies.
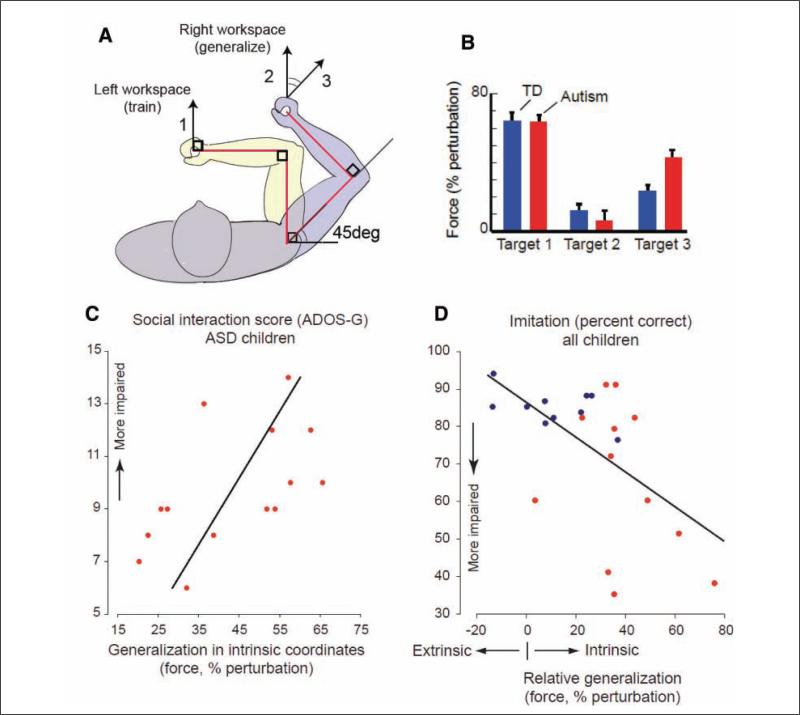
Our laboratory has examined the development of internal action models, in part, through the study of motor adaptation (Haswell and others 2009). An experimental design that has proven particularly revealing involves children with autism and control subjects using a novel tool to learn to compensate for force perturbations produced by a robotic device. In this task, the brain builds an association between self-generated motor commands and the sensory consequences of their arm position being perturbed. The perturbations are made in two different fashions: one in which the “discrepancy” is visual (the subjects’ arm position in space differs from the anticipated trajectory) and proprioceptive (“feeling” a change in joint position/relationship that differs from what is anticipated). The strength of each association (visual-motor and proprioceptive-motor) can be inferred by how the brain generalizes the errors from the trained movements to novel movements (Shadmehr 2004). To accomplish this, we used a “robot” that consists of a movable arm that controls a cursor on a screen. The robotic arm is attached to a motor that can push the arm perpendicular to the direction in which the subject moves it; overcoming this additional force while moving the cursor in the appropriate fashion represents the motor skill that needs to be learned. The screen covers the robot arm, so that the subject is unable to see his or her hand. The subject is directed to move the cursor on the screen to “capture an animal that has escaped from the zoo.” First there is a training phase to form the internal action model; the success of this formation is then measured when the subject's skill is tested in different ways. The training took place in the left workspace (i.e., both the subject's hand and the visual display are on the left) (Figure 2). We then tested the function of the internal action model by examining performance in two different ways. For one, the extrinsic coordinates matched those of the initial task; for the other, the intrinsic coordinates of the arm matched those of the initial task. This allowed us to determine the degree to which each subject relied on proprioceptive or visual feedback during motor learning. Our findings revealed that in learning an internal model of the novel tool, the children with autism placed an excessive association between self-generated motor commands and proprioception with relatively less reliance on the same motor commands and visual feedback. That is, for children with autism, the sense of proprioception and its association with motor commands, which is mediated by connections between primary motor and somatosensory cortices, appeared to be abnormally up-regulated. In contrast, the association between visual input and motor commands, which is mediated by longer range connections between premotor and posterior parietal cortices, appeared to be abnormally down-regulated.
Figure 2.
(a) Subjects undergoing testing using the upper extremity robot first develop internal action models by training to the task in the left workspace (1). They then demonstrate generalization of the internal action models by performing the task in the right workspace. The performance of the task may be done either in visual coordinates that were similar to the training (2) or in proprioceptive coordinates that were similar to the training (3), allowing differential examination of the contribution of visual and proprioceptive feedback during the development of the internal action model. (b) Children with autism and typically developing (TD) children have similar performance during the training (target 1). Children with autism show significantly more proprioceptively guided generalization (to target 3) in contrast to visually guided generalization (target 2). (c) Generalization to proprioceptive coordinates (target 3) correlates with Autism Diagnostic Observation Schedule–General (ADOS-G) score in autistic subjects (r = 0.572, P = .032). ASD = autism spectrum disorder. (d) In all children (blue dots = TD children; red dots = autistic children), relative generalization to proprioceptive coordinates (target 3) versus visual coordinates (target 2) correlates negatively with imitative function (r = -0.57, P = .006). This figure first appeared in a modified form in Nature Neuroscience (Haswell and others 2009); reprinted with permission.
Given that much of the sensory feedback for social interaction comes from the visual modality, the consequence of a weaker than normal association between motor commands and visual feedback is that children with autism may develop a “dyspraxia” for social (in addition to motor) skills. The conceptualization of the role of internal action models in both social as well as motor skill development is strongly supported by our published findings that reveal that the degree of bias toward proprioceptive feedback (and away from visual) during motor learning robustly predicts social and communicative deficits in autism, in addition to praxis and imitation impairment. Follow-up studies have replicated these findings in larger sample sizes and, critically, have provided evidence that this pattern of anomalous learning is specific to autism (Mostofsky and others 2010).
Cerebral Connectivity and Brain Mechanisms Underlying Anomalous Formation of Action Models
The evidence suggesting that children with autism place a greater than normal reliance during motor learning on their own proprioception while discounting visual consequences in the extrinsic world has a strong implication for understanding the brain basis for anomalous formation of internal action models. The formation of action models through proprioceptive feedback, the influence of which is increased in autism, can rely on short-range connections between adjacent primary somatosensory and motor cortices. The formation of action models through visual feedback, on the other hand, necessarily relies on much longer range interactions between visual and frontal motor regions. This disassociation between the efficacy of short-range (proprioceptive-motor) interactions and long-range (visual-motor) interactions may thereby be explained by altered cerebral connectivity. There is a well-substantiated theory that posits that aspects of the autism phenotype are caused by alterations in cerebral connectivity. The most extensively substantiated version of this altered connectivity theory states that the autistic phenotype is related to global cerebral underconnectivity and local overconnectivity. Three types of connectivity are routinely distinguished in the current research: structural (or anatomical) connectivity, functional connectivity, and effective connectivity. Structural connectivity refers to examination of anatomical connections between cerebral regions, such as via diffusion tensor imaging (DTI) and quantification of white matter volumes from anatomical MRI images. Functional connectivity, on the other hand, refers to physiological evidence of coupling (“synchronized firing”) between regions. Functional MRI-based techniques and EEG-based techniques are used to assess functional connectivity. The term effective connectivity, when applied to systems-level neuroscience, is used differently by different authors, but one definition involves artificial perturbation of the system (e.g., by a transcranial magnetic stimulation [TMS] pulse). The study of effective connectivity in autism will not be discussed, as there is a dearth of evidence.
Measurements of white matter through quantitative anatomical MRI have shown an autism-associated increase in “radiate” white matter that is immediately adjacent to the cortex and presumably is principally composed of “U-fiber” connections between adjacent cortical regions (Herbert and others 2004). It is thought that the early overgrowth of superficial white matter may result from incomplete pruning of adventitial synapses (Belmonte and others 2004; Courchesne 2004). The structural evidence for decreased global connectivity comes primarily from measurements of the mid-sagittal area of the corpus callosum, which is used as a proxy for the number of interhemispheric fibers. Several studies reveal that children with autism show reduced corpus callosum size (Frazier and Hardan 2009). In at least one study, the area of a relevant region of the corpus callosum was shown in autistic subjects to correlate with measurements of functional connectivity (Just and others 2007). DTI data to date have demonstrated alterations of white matter microstructure in autistic subjects (Cheng and others 2010; Fletcher and others 2010; Lee JE and others 2007), with data from one study correlating intactness of white matter with relatively intact functional connectivity (Sahyoun and others 2010). Results from our laboratory have demonstrated that an increased volume of localized white matter connections within primary sensory-motor cortex robustly predicted motor impairment in children with autism. This was in sharp contrast to both typically developing children and children with ADHD, for whom increased white matter predicted better motor skill performance (Mostofsky and others 2007). The implication from this finding is that the white matter in the primary sensory-motor cortex may be disorganized. Corroborating this view, preliminary data from our laboratory have demonstrated, in 14 autistic subjects, a relationship between DTI-assessed white matter microstructure in the primary sensory-motor cortex and basic motor skill function as well as generalization on the upper extremity robot task (Crocetti and others 2010). In summary, there are significant structural connectivity data to suggest an alteration of both quantity of white matter and its microarchitecture. The data also suggest that microstructural abnormalities of white matter in the sensory-motor cortex may in part explain the abnormally increased reliance on proprioception in the creation of internal action models.
Although structural connectivity measures form a basis for our understanding of the role of altered connectivity in autistic phenomenology, it is the assessment of functional connectivity that more directly demonstrates this role. Most of the studies to date have been performed using functional connectivity MRI (fcMRI). fcMRI uses the same blood oxygen level–dependent (BOLD) signal used by fMRI. However, rather than simply looking at the magnitude of activation (i.e., magnitude of BOLD signal) from a single region, fcMRI involves examining correlation in the time course of the signal between different regions. Using fcMRI studies, several groups have found that individuals with autism show decreased functional connectivity between distant brain regions. The findings extend across a number of task conditions, including visual processing (Brieber and others 2010; Villalobos and others 2005), visual face processing specifically (Kleinhans and others 2008; Monk and others 2010), mentalizing (Kana and others 2006; Lombardo and others 2010), linguistic processing (Just and others 2004; Mason and others 2008), and executive function tasks (Just and others 2007; Koshino and others 2005; Lee PS and others 2009; Solomon and others 2009). Our group has looked specifically at functional connectivity during low-level motor tasks and has found diffusely decreased functional connectivity throughout the motor network (Mostofsky and others 2009). In addition, functional connectivity in the resting-state network (or default-mode network) has been examined, and again there is evidence for decreased functional connectivity in the relevant regions (Cherkassky and others 2006; Kennedy and Courchesne 2008a, 2008b; Monk and others 2009; Weng and others 2010). A more or less consistent theme in studies of functional connectivity under a range of tasks is that individuals with autism have decreased long-range functional connectivity, particularly decreased anterior-posterior, intrahemispheric connectivity.
There has been considerably less investigation of functional connectivity in autism using electrophysiologic methods, despite the fact that EEG-based techniques appear to offer a number of advantages. EEG directly records neural activity, with millisecond time resolution and a frequency range typically from 0.5 to 60 Hz. This contrasts with fcMRI, which records hemodynamic activity that is a downstream consequence of neural activity. The time resolution of fcMRI is on the order of a second, and the oscillations recorded are in the range of 0.01 to 0.1 Hz. The frequency range recorded by the EEG is in the band that has been associated extensively with cognitive processes (Basar and others 2001; Fries and others 2007; Singer 1999). EEG has the time resolution to record dynamic brain processes from step to step. Despite these benefits, the EEG-based functional connectivity literature on autism is as yet at an earlier stage than that of fcMRI research. Two data-driven studies have examined long-range connectivity using EEG-based techniques. One demonstrated decreased intrahemispheric coherence (similarity of two signals in the frequency domain) in slower (1-7 Hz) frequency bands (Coben and others 2008), whereas another found decreased coherence in 8- to 12-Hz activity between frontal and posterior regions (Murias and others 2007).
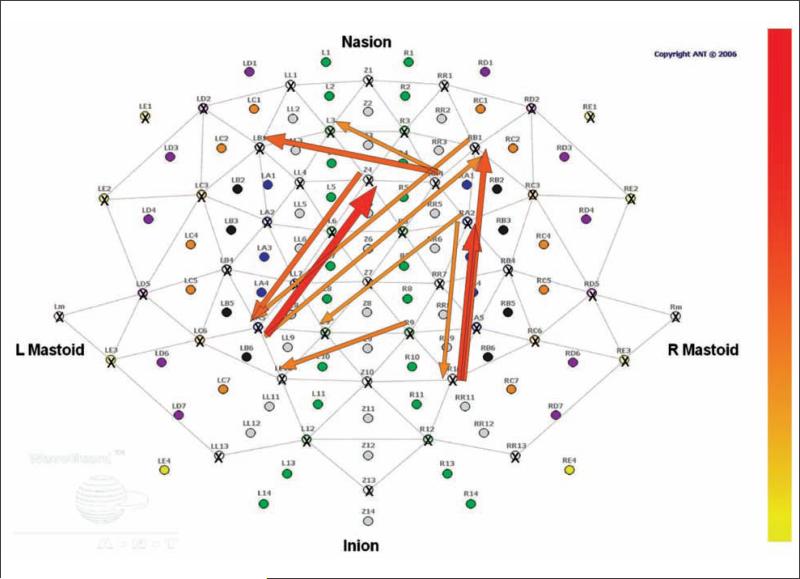
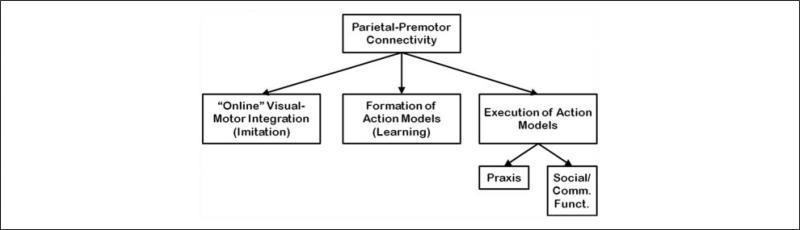
EEG may be an optimal technique for investigating alterations in connectivity associated with impaired formation and execution of internal action models. Although some aspects of the formation of internal action models are based on connectivity between the primary sensory cortex and primary motor cortex (such as in our studies of action model formation), the performance of praxis gestures relies on the function of and connectivity between inferior parietal and premotor regions. Given that parietal and premotor regions are not adjacent, the spatial limitations of EEG may not be a hindrance for the examination of connectivity in this system. The tuning of EEG to faster frequencies offers the ability to examine those oscillations that are likely most relevant to the communication between the regions. A series of papers from Wheaton, Hallett, and colleagues has characterized both the activation of parietal and premotor regions in association with praxis tasks (Wheaton and others 2009; Wheaton, Shibasaki, and others 2005). In addition, these investigators have successfully demonstrated functional connectivity of these regions during self-paced and cued praxis tasks by looking at the coherence between the signals from electrodes overlying the regions (Wheaton, Nolte, and others 2005). Preliminary analyses from our laboratory (Ewen JB, Korzeniewska A, Mostofsky SH, Franaszczuk PJ, Crone NE, unpublished data) have used novel EEG-based measures of functional connectivity (Korzeniewska and others 2008) to demonstrate directional information flow in adults between parietal and premotor regions during rehearsal and performance of praxis gestures (Figure 3). Ongoing studies in our laboratory are examining the magnitude of coherence and other connectivity measures in pediatric autistic subjects versus control subjects. Just as we are using a praxis task to examine the role of distant underconnectivity in the impaired execution of internal action models, we further plan to extend these investigations to studies of procedural learning of internal action models and to imitation (i.e., online visuomotor integration) (Figure 4).
Figure 3.
The event-related causality technique, which uses EEG data, can show information flow from one region to another associated with a cognitive task. Here, a single subject performing a praxis task demonstrates flow (red arrows) from left parietal regions to midline premotor regions and from right parietal regions to right lateral premotor regions.
Figure 4.
In our model, parietal-premotor connectivity is necessary for a number of related phenomena, including online visual-motor integration (i.e., motor imitation), formation of internal action models (i.e., procedural learning), and execution of internal action models, including motor action models (i.e., praxis) and social/communicative action models (i.e., normal social/communicative function).
Potential Therapies: Insights Gained from Examining Action Models and Connectivity
The understanding of the role of anomalous formation of internal action models in the autism phenotype offers a promising window into potential therapies. The findings generated from studies of motor learning not only provide crucial insight into the neural basis of impaired social and communicative development in autism. They also provide an important target for therapeutic intervention that can be used to develop methods for teaching skills to children with autism, as well as improve their ability to understand and interpret others’ actions. On one hand, we can “play to the strength” of children with autism by exploiting proprioceptive, rather than visual, feedback as a particularly effective means of improving skilled behavior in this population. Alternatively (or additionally), we can attempt to alter anomalous patterns of neural connectivity by using a noninvasive form of brain stimulation to normalize patterns of motor learning.
Ongoing studies in our laboratory are examining the approach of playing to the strength of children with autism by using augmented proprioceptive feedback to improve specific skill impairments. We are beginning by targeting a skill that children with autism often struggle with: handwriting. This is intended to lead to a longer term goal of investigating whether this approach can be used to address a range of motor, social, and communicative skill impairments in autism. Difficulty with handwriting has an impact on success in school for higher functioning children and for communicating with others, and this skill is important in building children's self-esteem (Feder and Majnemer 2007). Impaired handwriting can have a profound impact on a child's development; this is particularly true for children with autism, who otherwise face substantial challenges with academic and communicative tasks for which handwriting is necessary. Despite the commonality of observed handwriting impairment in autism and the known importance of handwriting to academic, social, and communicative functioning, there has been little to no systematic examination of handwriting in children with autism. We have begun to address this. In a recently published study (Fuentes and others 2009), we undertook a detailed examination of handwriting using the Minnesota Handwriting Assessment, finding that children with autism do indeed show impaired handwriting compared with age-, gender-, and intelligence-matched typically developing controls. Importantly, analyses of category scores revealed that handwriting impairment in children with autism is principally due to difficulty with forming the letters themselves. The results suggest that intervention targeting letter formation would be the best direction for improving handwriting in children with autism.
We are currently examining a novel approach for improving letter formation that relies on augmenting proprioceptive, rather than visual, feedback. Children typically learn to write letters by modeling the actions of others (e.g., by copying the teacher drawing on a chalk-board) (Feder and Majnemer 2007). The visual approach may not be well suited for children with autism, given their difficulty with visuomotor imitation (Williams and others 2004) and who show a bias toward reliance on proprioceptive, rather than visual, feedback during motor learning (Haswell and others 2009). Our approach involves using a robotic device that provides haptic (tactile, proprioceptive) input. We also expect that the anomalous pattern of motor learning seen in children with autism will be predictive of response to intervention. The results of these studies may be an archetype for other types of treatments that play to the strengths of autistic children, including interventions that address a range of social, communicative, and motor skill impairments. For instance, children with autism might be better able to learn sign language using a proprioceptive-based intervention in which their fingers are actually placed in the correct position (rather than asking them to learn by imitating the movements of others).
Augmenting proprioceptive feedback may prove to be a critical “way in,” increasing access to systems that children with autism typically rely on for skill-based learning. It is important, however, also to consider whether we can alter the way children with autism learn these skills, so that they are better able to form action models based on visual feedback from the external world, which is critical to acquiring social skills: One possible means of achieving this may be through the use of brain stimulatory techniques that affect neural activity and consequently enhance connectivity within particular functional networks. To this end, we are examining techniques that may be able to enhance visual-motor integration while decreasing proprioceptive-motor integration, thus “normalizing” the balance within autistic subjects. Specifically, it is conceivable that down-regulating excitability in the sensory-motor cortex to decrease the overly strong proprioceptive-motor connections, while up-regulating excitability in posterior parietal-premotor networks to increase visual-motor effective connections, would rebalance patterns of motor leaning in children with autism. TMS is one method of inducing small electrical currents in the cortex by creating a strong, transient magnetic field at the scalp. This approach is being used experimentally to alter neural connectivity following stroke (Kirton and others 2010; Perez and Cohen 2009). Another technique, transcranial direct current stimulation (tDCS), provides a prolonged, low-voltage current through two electrodes at the scalp. This technique has been used experimentally to enhance motor learning (Reis and others 2008). The polarity of the electrode at a particular site can increase (anodal) or decrease (cathodal) cortical excitability by modulating Na- and Ca-dependent channel activity (Liebetanz and others 2003) and NMDA function via modulation of BDNF and TrK receptors (Fritsch and others 2010). Indeed, our published findings show that anodal tDCS over the primary sensory-motor cortex in healthy adults can up-regulate learning processes when applied during motor practice (Reis and others 2009). Interestingly, other investigators have applied cathodal tDCS to decrease excitability of the nonperforming motor cortex, resulting in motor behavior improvement, likely through up-regulation of the practicing motor cortex (Boggio and others 2007). Thus, it is possible that we can up-regulate the association between motor commands and visual feedback in autism by enhancing the excitability of visual-motor association areas in the parietal cortex and/or decreasing the excitability of the primary sensory-motor cortex. This intervention may thereby prove effective for helping children with autism better rely on external visual cues and thereby potentially improve their ability to acquire skills important to social development and interpret others’ actions.
In conclusion, the concept of procedural learning of internal models of action arises from the study of normal brain function but represents a perspective that, when applied to the study of autism, coalesces a wide range of observations that previously have been viewed through separate lenses. Theory of mind, the mirror neuron system, motor dyspraxia, and abnormal sensory responses can all be understood through anomalous patterns of internal action model formation in autism. It appears that the underlying neurobiological pathogenesis of this abnormality is altered patterns of cerebral connectivity that have been demonstrated substantially in autism, and research is ongoing to evaluate this hypothesis. This line of research has the potential to offer novel therapies to reduce the impact of autism.
Acknowledgments
The authors thank Reza Shadmehr, PhD, for his helpful suggestions, Deana Crocetti for her rendering of Figure 2, and Balaji Lakshmanan, MS, for his rendering of Figure 3.
Financial Disclosure/Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: The research conducted in our laboratory was supported by grants to SHM from the National Institutes of Health (NIH; R01 NS048527) and Autism Speaks. The research was also supported by grant funding to JBE from NIH (K12 NS001696; principal investigator: Harvey Singer) and institutional funding to the Kennedy Krieger Institute (Intellectual and Developmental Disabilities Research Center: P30 HD024061; Johns Hopkins Institute for Clinical and Translational Research, Neurobehavioral Research Unit: M01 RR00052).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 2000. Text rev. [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39(2–3):241–8. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Beilin H, Fireman G. The foundation of Piaget's theories: mental and physical action. Adv Child Dev Behav. 1999;27:221–46. doi: 10.1016/s0065-2407(08)60140-8. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24(42):9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25(2):123–9. [PubMed] [Google Scholar]
- Boria S, Fabbri-Destro M, Cattaneo L, Sparaci L, Sinigaglia C, Santelli E. Intention understanding in autism. PLoS One. 2009;4(5):e5596. doi: 10.1371/journal.pone.0005596. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieber S, Herpertz-Dahlmann B, Fink GR, Kamp-Becker I, Remschmidt H, Konrad K. Coherent motion processing in autism spectrum disorder (ASD): an fMRI study. Neuropsychologia. 2010;48(6):1644–51. doi: 10.1016/j.neuropsychologia.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR. 2009;58(SS–10):1–20. [PubMed] [Google Scholar]
- Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage. 2010;50(3):873–82. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17(16):1687–90. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Coben R, Clarke AR, Hudspeth W, Barry RJ. EEG power and coherence in autistic spectrum disorder. Clin Neurophysiol. 2008;119(5):1002–9. doi: 10.1016/j.clinph.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brain development in autism: early over-growth followed by premature arrest of growth. Ment Retard Dev Disabil Res Rev. 2004;10(2):106–11. doi: 10.1002/mrdd.20020. [DOI] [PubMed] [Google Scholar]
- Crocetti D, Srinivasan P, Izawa J, Shadmehr R, Mostofsky S. Sensorimotor DTI abnormality is associated with anomalous motor learning in autism.. Paper presented at: Ninth International Meeting for Autism Research; Philadelphia, PA. 2010. [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9(1):28–30. doi: 10.1038/nn1611. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMeyer MK, Alpern GD, Barton S, DeMyer WE, Churchill DW, Hingtgen JN. Imitation in autistic, early schizophrenic, and non-psychotic subnormal children. J Autism Child Schizophr. 1972;2(3):264–87. doi: 10.1007/BF01537618. others. [DOI] [PubMed] [Google Scholar]
- Dewey D, Cantell M, Crawford SG. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 2007;13(2):246–56. doi: 10.1017/S1355617707070270. [DOI] [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, Mostofsky SH. Associations of postural knowledge and basic motor skill with dyspraxia in autism: implication for abnormalities in distributed connectivity and motor learning. Neuropsychology. 2009;23(5):563–70. doi: 10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: association with motor, social, and communicative deficits. Dev Med Child Neurol. 2007;49(10):734–9. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Feder KP, Majnemer A. Handwriting development, competency, and intervention. Dev Med Child Neurol. 2007;49(4):312–7. doi: 10.1111/j.1469-8749.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage. 2010;51(3):1117–25. doi: 10.1016/j.neuroimage.2010.01.083. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Hardan AY. A meta-analysis of the corpus callosum in autism. Biol Psychiatry. 2009;66(10):935–41. doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30(7):309–16. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66(2):198–204. doi: 10.1016/j.neuron.2010.03.035. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes CT, Mostofsky SH, Bastian AJ. Children with autism show specific handwriting impairments. Neurology. 2009;73(19):1532–7. doi: 10.1212/WNL.0b013e3181c0d48c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V. Embodied simulation: from mirror neuron systems to interpersonal relations. Novartis Found Symp. 2007;278:3–12. discussion 12–9, 89–96, 216–21. [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man: I. Brain. 1965;88(2):237–94. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M, Butler E. Clumsiness in autism and Asperger syndrome: a further report. J Intellect Disabil Res. 1998;42(Pt 1):43–8. doi: 10.1046/j.1365-2788.1998.00065.x. [DOI] [PubMed] [Google Scholar]
- Gidley Larson J, Mostofsky S. Motor deficits in autism. In: Tuchman R, Rapin I, editors. Autism: a neurological disorder of early brain development. Mac Keith Press for the International Review of Child Neurology Series; London: 2006. [Google Scholar]
- Grafton ST. Embodied cognition and the simulation of action to understand others. Ann N Y Acad Sci. 2009;1156:97–117. doi: 10.1111/j.1749-6632.2009.04425.x. [DOI] [PubMed] [Google Scholar]
- Ham H, Bartolo A, Corley M, Rajendran G, Szabo A, Swanson S. Exploring the relationship between gestural recognition and imitation: evidence of dyspraxia in autism spectrum disorders. J Autism Dev Disord. 2010 Apr 21; doi: 10.1007/s10803-010-1011-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ham HS, Bartolo A, Corley M, Swanson S, Rajendran G. Case report: selective deficit in the production of intransitive gestures in an individual with autism. Cortex. 2010;46(3):407–9. doi: 10.1016/j.cortex.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Haswell CC, Izawa J, Dowell LR, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. Nat Neurosci. 2009;12(8):970–2. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. Clinical neuropsychology. Oxford University Press; Oxford, UK: 2003. [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55(4):530–40. doi: 10.1002/ana.20032. others. [DOI] [PubMed] [Google Scholar]
- Hofvander B, Delorme R, Chaste P, Nyden A, Wentz E, Stahl-berg O. Psychiatric and psychosocial problems in adults with normal-intelligence autism spectrum disorders. BMC Psychiatry. 2009;9:35. doi: 10.1186/1471-244X-9-35. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Mazziotta JC. Mirror neuron system: basic findings and clinical applications. Ann Neurol. 2007;62(3):213–8. doi: 10.1002/ana.21198. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. 2005;3(3):e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger's syndrome from controls. J Autism Dev Disord. 2006;36(5):613–21. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17(4):951–61. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(Pt 8):1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(Pt 9):2484–93. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective conduct. Nervous Child. 1943;2:217–250. [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc Cogn Affect Neurosci. 2008a;3(2):177–90. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008b;39(4):1877–85. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kirton A, Deveber G, Gunraj C, Chen R. Cortical excitability and interhemispheric inhibition after subcortical pediatric stroke: plastic organization and effects of rTMS. Clin Neurophysiol. 2010;121(11):1922–9. doi: 10.1016/j.clinph.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(Pt 4):1000–12. doi: 10.1093/brain/awm334. others. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F. The enactive mind, or from actions to cognition: lessons from autism. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):345–60. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124(5):1395–403. doi: 10.1542/peds.2009-1522. others. [DOI] [PubMed] [Google Scholar]
- Korzeniewska A, Crainiceanu CM, Kus R, Franaszczuk PJ, Crone NE. Dynamics of event-related causality in brain electrical activity. Hum Brain Mapp. 2008;29(10):1170–92. doi: 10.1002/hbm.20458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24(3):810–21. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007;424(2):127–32. doi: 10.1016/j.neulet.2007.07.042. others. [DOI] [PubMed] [Google Scholar]
- Lee PS, Yerys BE, Della Rosa A, Foss-Feig J, Barnes KA, James JD. Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: a fcMRI study of response inhibition. Cereb Cortex. 2009;19(8):1787–94. doi: 10.1093/cercor/bhn209. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Paulus W. Pharmacology of transcranial direct current stimulation: missing effect of riluzole. Suppl Clin Neurophysiol. 2003;56:282–7. doi: 10.1016/s1567-424x(09)70232-6. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ. Atypical neural self-representation in autism. Brain. 2010;133(Pt 2):611–24. doi: 10.1093/brain/awp306. others. [DOI] [PubMed] [Google Scholar]
- Martineau J, Cochin S, Magne R, Barthelemy C. Impaired cortical activation in autistic children: is the mirror neuron system involved? Int J Psychophysiol. 2008;68(1):35–40. doi: 10.1016/j.ijpsycho.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46(1):269–80. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47(2):764–72. doi: 10.1016/j.neuroimage.2009.04.069. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Weng SJ, Wiggins JL, Kurapati N, Louro HM, Carrasco M. Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatry Neurosci. 2010;35(2):105–14. doi: 10.1503/jpn.090085. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalbano R, Roccella M. The quality of life of children with pervasive developmental disorders. Minerva Pediatr. 2009;61(4):361–70. [PubMed] [Google Scholar]
- Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852–73. doi: 10.1111/j.1365-2788.2009.01197.x. [DOI] [PubMed] [Google Scholar]
- Mostofsky S, Izawa J, Marko M, Pekny S, Dowell L, Shadmehr R. Evidence for specificity of anomalous motor learning in autism.. Paper presented at: Ninth International Meeting for Autism Research; Philadelphia, PA. 2010. [Google Scholar]
- Mostofsky SH, Burgess MP, Gidley Larson JC. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130(Pt 8):2117–22. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. J Int Neuropsychol Soc. 2006;12(3):314–26. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132(Pt 9):2413–25. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry. 2007;62(3):270–3. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noterdaeme M, Mildenberger K, Minow F, Amorosa H. Evaluation of neuromotor deficits in children with autism and children with a specific speech and language disorder. Eur Child Adolesc Psychiatry. 2002;11(5):219–25. doi: 10.1007/s00787-002-0285-z. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. The corticospinal system and transcranial magnetic stimulation in stroke. Top Stroke Rehabil. 2009;16(4):254–69. doi: 10.1310/tsr1604-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Robertson E, Krakauer JW, Rothwell J, Marshall L, Gerloff C. Consensus: “Can tDCS and TMS enhance motor learning and memory formation?”. Brain Stimul. 2008;1(4):363–9. doi: 10.1016/j.brs.2008.08.001. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106(5):1590–5. doi: 10.1073/pnas.0805413106. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart NJ, Bellgrove MA, Tonge BJ, Brereton AV, Howells- Rankin D, Bradshaw JL. An examination of movement kinematics in young people with high-functioning autism and Asperger's disorder: further evidence for a motor planning deficit. J Autism Dev Disord. 2006;36(6):757–67. doi: 10.1007/s10803-006-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart NJ, Tonge BJ, Iansek R, McGinley J, Brereton AV, Enticott PG. Gait function in newly diagnosed children with autism: cerebellar and basal ganglia related motor disorder. Dev Med Child Neurol. 2006;48(10):819–24. doi: 10.1017/S0012162206001769. others. [DOI] [PubMed] [Google Scholar]
- Rogers S, Pennington B. A theoretical approach to the deficits in infantile autism. Dev Psychopathol. 1991;3:137–62. [Google Scholar]
- Sahyoun CP, Belliveau JW, Soulieres I, Schwartz S, Mody M. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48(1):86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R. Generalization as a behavioral window to the neural mechanisms of learning internal models. Hum Mov Sci. 2004;23(5):543–68. doi: 10.1016/j.humov.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185(3):359–81. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14(5 Pt 2):3208–24. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24(1):49–65. 111–25. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47(12):2515–26. doi: 10.1016/j.neuropsychologia.2009.04.019. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25(3):916–25. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–14. doi: 10.1016/j.brainres.2009.11.057. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton L, Fridman E, Bohlhalter S, Vorbach S, Hallett M. Left parietal activation related to planning, executing and suppressing praxis hand movements. Clin Neurophysiol. 2009;120(5):980–6. doi: 10.1016/j.clinph.2009.02.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton LA, Hallett M. Ideomotor apraxia: a review. J Neurol Sci. 2007;260(1–2):1–10. doi: 10.1016/j.jns.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Nolte G, Bohlhalter S, Fridman E, Hallett M. Synchronization of parietal and premotor areas during preparation and execution of praxis hand movements. Clin Neurophysiol. 2005;116(6):1382–90. doi: 10.1016/j.clinph.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Shibasaki H, Hallett M. Temporal activation pattern of parietal and premotor areas related to praxis movements. Clin Neurophysiol. 2005;116(5):1201–12. doi: 10.1016/j.clinph.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Williams JH. Self-other relations in social development and autism: multiple roles for mirror neurons and other brain bases. Autism Res. 2008;1(2):73–90. doi: 10.1002/aur.15. [DOI] [PubMed] [Google Scholar]
- Williams JH, Whiten A, Singh T. A systematic review of action imitation in autistic spectrum disorder. J Autism Dev Disord. 2004;34(3):285–99. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]