Abstract
Objective
The present study was designed to evaluate the in vitro antioxidant potential of bovine lactoferrin (bLF) and black tea polyphenol (Polyphenon-B; P-B) as well as in vivo inhibitory effects on the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced hamster buccal pouch (HBP) carcinomas.
Design
Antioxidant activity was screened using a panel of assays including 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2'-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS), hydroxyl radical anion (OH•), superoxide anion (O2•−), and nitric oxide (NO) radical scavenging assays as well as assay for reducing power. The chemopreventive potential of bLF and P-B was assessed in the HBP model based on the modulatory effects on DMBA-induced oxidative DNA damage as well as the expression of proteins associated with carcinogen activation (CYP1A1, CYP1B1), cell proliferation (cyclin D1, proliferating cell nuclear antigen; PCNA, glutathione S-transferase pi; GST-P), angiogenesis (vascular endothelial growth factor; VEGF, VEGF receptor 1; VEGFR1), and invasion and metastasis (matrix metalloproteinase-9; MMP-9 and tissue inhibitors of MMP-2; TIMP-2).
Results
Both bLF and P-B showed high radical scavenging activity and reductive potential. Although administration of bLF and P-B alone suppressed DMBA-induced HBP tumors, combined administration of bLF and P-B was more effective in inhibiting HBP carcinogenesis by inhibiting oxidative DNA damage, carcinogen activation, cell proliferation, invasion and angiogenesis.
Conclusion
Our study suggests that the antioxidative property of bLF and P-B may be responsible for chemoprevention of HBP carcinogenesis by modulating multiple molecular targets.
Keywords: Anitoxidants, chemoprevention, carcinogen activation, cell proliferation, angiogenesis
Introduction
Combination chemoprevention is a promising strategy to control the incidence of cancer because it involves multifocal signal modulation, has higher efficacy and minimizes adverse effects. In particular, combination regimens that use tea polyphenols as one of the constituents are potentially effective in chemoprevention and chemotherapy trials (1, 2).
In most parts of the world, tea is consumed together with milk. Milk and tea exhibit a wide range of pharmacological effects including antioxidant, immunomodulatory, antimetastatic, and anticarcinogenic activities. The major health benefits of milk and tea have been attributed to the presence of bioactive compounds and nutraceuticals with radical scavenging properties. In particular, bovine milk lactoferrin and polyphenols in black tea provide substantial health benefits beyond basic nutrition when administered as single agents (3–5). Furthermore, research over the last decade has shown that bLF and black tea suppress free radical mediated tumorigenesis by their potent antioxidant property (4,6). Previously, we reported that a combination of black tea polyphenols, (Polyphenon-B: P-B) and bovine lactoferrin (bLF) exerts synergistic effect and acts as a suppressing agent by upregulating the antioxidant status, modulating xenobiotic metabolizing enzymes, inhibiting cell proliferation, and inducing apoptosis during HBP carcinogenesis (7,8). The present study was designed to evaluate the relative antioxidant potential of bLF and P-B in vitro and inhibitory effects on 7,12-dimethylbenz[a]anthracene (DMBA) induced HBP carcinogenesis in vivo. The extent of oxidative DNA damage and expression of proteins associated with carcinogen activation (CYP1A1, CYP1B1), cell proliferation (cyclin D1, PCNA, GST-P), angiogenesis (VEGF, VEGFR1) and invasion and metastasis (MMP-9 and TIMP-2), were used to biomonitor chemoprevention.
Materials and Methods
Chemicals
Ascorbic acid, 2,2'-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS), bovine serum albumin, DMBA, 2,4-dinitrophenylhydrazine, 1,1-diphenyl-2-picryl-hydrazyl (DPPH), 5,5'-dithiobis (2-nitrobenzoic acid) (DTNB) and 2-thiobarbituric acid (TBA) were purchased from Sigma Chemical Company, St, Louis, MO, USA. bLF (lot No. 020119) of purity ≥96.2% was obtained from Morinaga Milk Industry Co., Ltd, Tokyo, Japan. P-B was kindly provided by Mitsui Norin Co., Ltd., Tokyo, Japan. The composition of P-B is the same as described previously (7,8). It is a mixture of epicatechin (0.4%), epigallocatechin-3-gallate (1.4%), epicatechin-3-gallate (0.1%), gallocatechin-3-gallate (0.2%), free theaflavins (0.32%), theaflavinmonogallate-A (0.14%), theaflavinmonogallate-B (0.15%), theaflavindigallate (0.21%), tannin (35.6%) and caffeine (4.9%). All other reagents used were of analytical grade.
In vitro free radical scavenging assays
1,1-Diphenyl-2-picryl-hydrazyl (DPPH) assay
The free radical scavenging capacities of bLF and P-B was evaluated by the DPPH assay following the methodology described by Blois (9). In its radical form, DPPH absorbs at 517 nm, but upon reduction by an antioxidant or a radical species, the absorption decreases. Briefly, 0.25 mM solution of DPPH• in methanol was prepared and 1 mL of this solution was added to 1 mL of bLF and P-B solution in methanol at different concentrations (1–30 μg/mL). After 20 minutes, the absorbance was measured at 517 nm. Ascorbic acid was used as a positive control. The percentage DPPH decolorisation of the sample was calculated by the equation, % DPPH scavenging = [(Acontrol -Aextract)/Acontrol] × 100, where A is the absorbance.
2,2'-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) assay
The total antioxidant potential was measured by ABTS assay that measures the relative ability of antioxidant substances to scavenge the ABTS•+ cation radical generated in the aqueous phase (10). The 3.5 mL reaction mixture contained 0.17mM ABTS, 1–10μg bLF and P-B, and phosphate buffer (pH 7.4). The absorbance was measured at 734 nm.
Hydroxyl radical scavenging assay
The hydroxyl radical scavenging activity was determined by the method of Halliwell et al (11) based on the ability to compete with deoxyribose for hydroxyl radicals. Hydroxyl radicals produced by the reduction of H2O2 by iron, in presence of ascorbic acid degrade deoxyribose to form products, which on heating with TBA form a pink colored chromogen. Briefly, the reaction mixture, in a final volume of 1.0 mL, containing 0.4 mL of 20 mM sodium phosphate buffer (pH 7.4), 0.1 mL of 1–10 μg/mL of bLF/P-B, 0.1 mL of 60 mM deoxyribose, 0.1 mL of 10 mM H2O2, 0.1 mL of 1 mM ferric chloride, 0.1 mL of 1 mM EDTA and 0.1 mL of 2 mM ascorbic acid, was incubated at 37°C for 1 h. The reaction was terminated by addition of 1ml of 17 mM TBA and 1ml of 17 mM trichloroacetic acid (TCA). The mixture was boiled for 15 min, cooled in ice, and the absorbance measured at 532 nm. Ascorbic acid was used as a positive control. Distilled water in place of bLF and P-B or ascorbic acid was used as control and the sample solution without deoxyribose as sample blank.
Superoxide anion scavenging activity
The superoxide anion scavenging activity was determined by the method of Nishimiki et al (12). Superoxide anion derived from dissolved oxygen by a PMS/NADH coupling reaction reduces nitroblue tetrazolium (NBT), which forms a violet coloured complex. A decrease in colour after addition of the antioxidant is a measure of its superoxide scavenging activity. To the reaction mixture containing phosphate buffer (100 mM, pH 7.4), NBT (1 mM) solution, NADH (1 mM) and of bLF/P-B (1–10 μg/mL) in methanol, 1 mL of 1 mM PMS was added. After incubation at 25°C for 5 min., the absorbance was measured at 560 nm against a blank. Ascorbic acid was used as a positive control.
Nitric oxide radical inhibition assay
The nitric oxide radical inhibition activity was measured by the method of Garrat (13) using Griess reagent. Briefly, sodium nitroprusside (10 mM) in phosphate buffered saline was mixed with different concentrations of bLF/P-B dissolved in methanol and incubated at room temperature for 150 min followed by addition of 0.5 mL of Griess reagent (1% sulfanilamide, 2% H3PO4 and 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride). The absorbance of the chromophore formed was read at 546 nm.
Reducing power
The reductive potential was determined according to the method of Oyaizu (14) based on the chemical reaction of Fe(III) to Fe(II). To 1–10 μg/mL bLF and P-B and ascorbic acid standard in 1 mL of methanol, 2.5 ml each of phosphate buffer (0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (1% w/v) was added and the mixture incubated at 50°C for 20 min, followed by addition of 2.5 mL TCA (10% w/v). The mixture was centrifuged for 10 min at 1000g, the upper layer (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1% w/v), and the absorbance was measured at 700 nm.
Animals
The experiment was carried out with male Syrian hamsters aged 6–10 weeks weighing between 90–110 g obtained from the Central Animal House, Annamalai University, India. The animals were housed five to a polypropylene cage and provided food and water ad libitum. The animals were maintained in a controlled environment under standard conditions of temperature and humidity with an alternating 12-hours light/dark cycle. The animals were maintained in accordance with the guidelines of the Indian Council of Medical Research and approved by the ethical committee, Annamalai University. Experimental diet was prepared everyday by mixing chemopreventive agents to preweighed standard pellet diet (Mysore Snack Feed, Mysore, India). The diet was replenished everyday and the food consumption was recorded.
Treatment schedule
The animals were randomized into experimental and control groups and divided into 8 groups of 20 animals each. In group 1, the right buccal pouches of hamsters were painted three times per week with a 0.5% solution of DMBA in liquid paraffin with a number 4 brush. Each application leaves 0.4 mg (15). Hamsters in group 1 received no further treatment. In groups 2–4, the right buccal pouches painted with DMBA, as in group 1, received in addition, basal diet containing 0.2% bLF, 0.05% P-B and a diet containing combination of 0.2% bLF and 0.05% P-B respectively (8) Animals in groups 5 through 7 were administered bLF, P-B alone and in combination respectively. Group 8 animals served as control. The dose for bLF used in the present study is that recommended by Tsuda et al. (4) and corresponds to the intake of 9 g by a person weighing around 60 kg, and the dose for black tea polyphenols (P-B) corresponds to the daily dietary intake of four cups of tea (30–40 mg of tea polyphenols per kilogram body weight by humans) (16). The experiment was terminated at 14 weeks and all animals were killed by cervical dislocation after an overnight fast. Before an animal was killed, the right pouch was grossly inspected to evaluate premalignant lesions or tumour development and photographed. The tumour burden was calculated by multiplying the mean tumour volume (4/3πr3) (r=1/2 tumour diameter in mm) with the mean number of tumours. The buccal pouch tissues were subdivided and variously processed for distribution to each experiment. A portion of the tissue was immediately frozen in liquid nitrogen for subsequent RNA extraction and another portion was processed using lysis buffer for Western blot analysis. The remaining tissues were fixed in 10% formalin, embedded in paraffin, sectioned and mounted on polylysine-coated glass slides. One section from each specimen was stained with haematoxylin and eosin. The other sections were used for immunohistochemical staining.
Immunohistochemistry
The tissue sections on glass slides were deparaffinised by heat at 60°C 30 minutes, followed by three washes in xylene. After gradual hydration through graded alcohol, the slides were incubated in citrate buffer (pH 6.0) for two cycles of 5 minutes in a microwave oven for antigen retrieval. The sections were allowed to cool for 20 minutes, rinsed with Tris-buffered saline (TBS) and treated with 3% H2O2 in distilled water for 15 minutes to inhibit endogenous peroxidase activity. Nonspecific antibody binding was reduced by incubating the sections with normal goat serum for 25 minutes. The sections were then incubated with mouse monoclonal antibodies 8-OH-dG (JaICA, Shizuoka, Japan), CYP1A1 (generously provided by Dr. Stegeman, Woods Hole Oceanographic Institute (WHOI), USA and Dr Gelboin, National Cancer Institute (NCI), USA.) and rabbit polyclonal antibody CYP1B1 (Santa Cruz Biotechnology, CA, USA.) at room temperature for one hour. The slides were rinsed in TBS and incubated with anti-mouse and anti-rabbit biotin-labelled secondary antibody (DAKO) followed by streptavidin-biotin-peroxidase for 30 minutes each at room temperature. The immunoprecipitate was visualized by treating with 3,3'-diaminobenzidine (DAKO) and counterstaining with haematoxylin. Positive controls were also processed simultaneously. The immunohistochemical data for CYP1A1, CYP1B1 and 8-OH-dG were expressed as the number of cells with positive staining per 100 counted cells.
Extraction of RNA
Total RNA from the hamster buccal pouch was extracted using Trizol reagent (Sigma) (17). The RNA concentration was determined from the optical density at a wavelength of 260nm (by using an OD260 unit equivalent to 40μg/ml of RNA). In brief, 50 mg pouch tissue was homogenized using (1 ml) trizol reagent. The homogenate was then treated with 0.2 ml of chloroform and shook vigorously. The mixture was then centrifuged at 12,000 g for 15 min at 4°C. To the aqueous phase, 0.5 ml of isopropanol was added, and centrifuged at 12,000 g for 8 minutes at 4°C. The supernatant was discarded gently and the precipitated RNA was rinsed twice with 1 ml of 75% ethanol and dried in air. The RNA was resuspended in 100μl of diethylpyrocarbonate (Sigma) treated water at a final concentration of 1 μg/μl and stored at −80°C until further use.
Reverse Transcriptase (RT) reaction: (cDNA synthesis)
Isolated total RNA (1μg) was reverse-transcribed to cDNA in a reaction mixture containing 4 μl of 5X reaction buffer, 2 μl of dNTPs mixture (10mM), 20 units of RNase inhibitor, 200 units of avian-myeloblastosis virus (AMV) reverse transcriptase and 0.5 μg of oligo(dT) primer (Promega, WI, USA) in a total volume of 20 μl. The reaction mixture was incubated at 42°C for 60 minutes and the reaction terminated by heating at 70°C for 10 minutes. The resultant cDNA was stored at −80°C until further use.
PCR amplification
All oligonucleotide primers were purchased from Sigma Genosys, India. Details about the primers used for PCR reactions are given in table 2. The primers for VEGF amplified two of four different molecular species produced by alternative splicing of mRNA-VEGFI21 and VEGFI65 of sizes 444 bp and 576 bp, respectively.
Table 2.
Oligonucleotide primers used for RT-PCR of cyclin D1, GST-P, VEGF, VEGFR1, MMP-9, TIMP-2 with β-actin as an internal control
| Gene Product | Primers | Oligonucleotide sequences | Fragment size |
|---|---|---|---|
|
| |||
| Cyclin D1 | Sense | 5'-CGGAGGACAACAAACAGATC-3' | 331 bp |
| Antisense | 5'-GGGTGTGCAAGCCAGGTCCA-3' | ||
|
| |||
| GST-P | Sense | 5'-TCATCTACACCAACTATGAG-3' | 226 bp |
| Antisense | 5'-GCCACATAGGCAG AGAGCAG-3' | ||
|
| |||
| VEGF | Sense | 5'-ATGAACTTTCTGCTGTCTTGG-3' | 444 bp and 576 bp* |
| Antisense | 5'-TCACCGCCTCGGCTTGTCACA-3' | ||
|
| |||
| VEGFR1 | Sense | 5'-AGGAGAGGACCTGAAACTGTCTT-3' | 230bp |
| Antisense | 5'-ATTCCTGGGCTCTGCAGGCATAG-3' | ||
|
| |||
| MMP-9 | Sense | 5'-AGTTTGGTGTCGCGGAGCAC-3' | 753bp |
| Antisense | 5'-TACATGAGCGCTTCCGGCAC-3' | ||
|
| |||
| TIMP-2 | Sense | 5'-GTTTTGCAATGCAGACGTAG-3' | 539bp |
| Antisense | 5'-ATGTCAAGAAACTCCTGCTT-3' | ||
|
| |||
| β-actin | Sense | 5'-AACCGCGAGAAGATGACCCAGATCATGTTT-3' | 350 bp |
| Antisense | 5'-AGCAGCCGTGGCCATC TCTTGCTCGAAGTC-3' | ||
The primers for VEGF detect two of four different molecular species produced by alternative splicing of mRNA-VEGFI21 and VEGFI65, with expected fragment sizes of 444 bp and 576 bp, respectively.
The PCR amplification reaction mixture (in a final volume of 25μl) contained 1 μl of cDNA, 0.5μl of forward primer, 0.5μl of reverse primer and 10 μl of Hot Master Mix (2.5X) (Eppendorf, Hamburg, Germany). The PCR was carried out in a thermal cycler (Eppendorf). Thermocycling conditions included initial denaturation at 94°C for 5 minutes/10 minutes for VEGF (one cycle), then denaturation at 95°C for 1 minute/30 seconds for VEGF and VEGFR1, annealing at 55°C (1 minute) for cyclin D1 and GST-P, 51°C (30 seconds) for VEGF, 60°C (30 seconds) for VEGFR1, (1 min 20 seconds) for MMP-9 and TIMP-2 and (1 minute) for β-actin, and extension at 72°C (1 minute) for 30 cycles (40 cycles for VEGF and 34 cycles for MMP-9 and TIMP-2) and a final extension at 72°C for 7 minutes. Negative controls without cDNA were also performed. Amplification products were analysed by electrophoresis in a 2% agarose gel containing ethidium bromide with 100bp DNA ladder. The PCR products were visualized as bands with a UV-transilluminator and photographs were taken using gel documentation system (GelDocMega™, United Kingdom).
SDS-PAGE and Western blot analysis
Approximately, 50 mg of each tissue sample was subjected to lysis in a sample buffer containing 62.5mM Tris (pH 6.8), 2% SDS, 5% 2-mercaptoethanol, 10% glycerol and bromophenol blue. The protein concentration of lysates was determined by Bradford method (18). SDS-PAGE was performed using equivalent protein extracts (55 μg) from each sample according to Laemmli (19). The resolved proteins were electrophoretically transferred to polyvinylidene difluoride membranes (Sartorius, Germany). The membranes were incubated in 1X PBS containing 5% non-fat dry milk for 2 h to block non-specific binding sites. The blots were incubated with 1:400 dilution of anti-PCNA (Santa Cruz Biotechnology) and 1:200 dilution of anti-CYP1A1 and anti-CYP1B1 overnight at 4°C. The blots were extensively washed with TBS containing 0.1% Tween-20 (TBS-T) and then incubated with 1:1000 dilution of horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) for 30–45 min at room temperature. After extensive washes in TBS-T, the proteins were visualized using enhanced chemiluminescence (ECL) detection reagents (Sigma). Densitometry was performed on IISP flat bed scanner and quantitated with Total Lab 1.11 software.
Statistical analysis
The data are expressed as mean ± SD. Tumour incidence and grading of 8-OH-dG, CYP1A1 and 1B1 isoforms were statistically compared using χ2-test. Statistical analysis on the data for tumour multiplicity, tumour burden was carried out using Analysis of variance (ANOVA) followed by Tukey-Kramer test. The data for densitometric analysis was analysed using ANOVA followed by Least Significant Difference (LSD). The results were considered statistically significant if the p value was <0.05. IC50 values were determined by plotting dose response curves of radical scavenging activities vs concentration of bLF and P-B using GraphPad Prism version 4.00 for Windows, (GraphPadTM Software Inc., San Diego, California, USA). The reduction potential was statistically compared by Student's t test.
Results
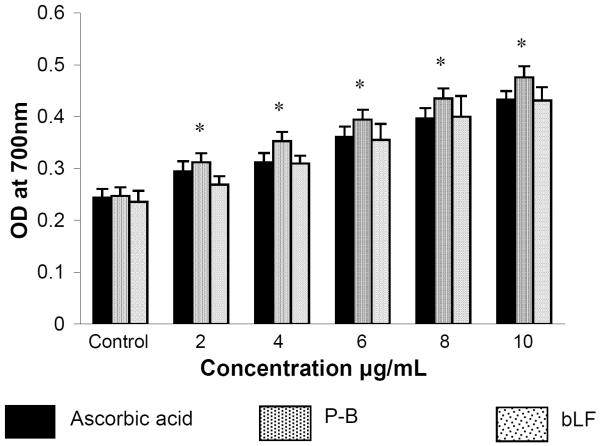
Figure 1 and table 1 show the free radical scavenging effect of ascorbate, P-B and bLF and their IC50 values. Both P-B and bLF showed concentration dependent antiradical activity resulting from reduction of DPPH•, ABTS•+, superoxide (O2•−), hydroxyl (OH•) and nitric oxide (NO) radicals to non-radical form. Ascorbic acid, the positive control was more effective in scavenging DPPH, ABTS, O2•−, OH•, and NO radicals compared to P-B and bLF. The order of radical scavenging effect was ascorbic acid >P-B>bLF. The reducing power of ascorbic acid, P-B and bLF increased gradually with increasing concentration (Figure 2) and the order of reduction potential was PB>ascorbic acid>bLF. Of the two agents analysed, P-B, enriched in polyphenolic content showed more radical scavenging activity as well as reductive potential than bLF.
Figure 1.
Free radical scavenging effects of bLF and P-B in vitro.
A. DPPH assay B. ABTS radicals scavenging assay C. hydroxy radical scavenging assay D. superoxide radical scavenging assay E. nitric oxide scavenging assay. ◻ Ascorbate, ◯ bLF, ▾ P-B.
Data are represented as mean ± SD of two independent experiments each performed in triplicate.
Table 1.
IC50 values of ascorbate, bLF and P-B against various free radicals
| Antioxidant activity | IC50 values | ||
|---|---|---|---|
| Ascorbate | P-B | bLF | |
| DPPH scavenging (μg/ml) | 3.2 | 4.6 | 4.9 |
| ABTS scavenging (μg/ml) | 3.4 | 4.9 | 5.32 |
| OH• scavenging (μg/ml) | 2.4 | 4.4 | 7.64 |
| O2•− scavenging (μg/ml) | 30.17 | 41.25 | 44.16 |
| NO scavenging (μg/ml) | 5.32 | 16.09 | 21.98 |
IC50 values were determined by plotting dose response curves of radical scavenging activities vs concentration of bLF and P-B using GraphPad Prism version 4.00 for Windows, (GraphPadTM Software Inc., San Diego, California, USA).
Figure 2.
Reducing potential of bLF and P-B in vitro.
*significantly different from ascorbate acid and bLF (p<0.05) by Student's t test
Table 3 shows the tumour incidence, tumour multiplicity and mean tumour burden in experimental animals. The tumour incidence in group 1 was 100 per cent with a multiplicity of 1.55 tumours per hamster. These tumours were exophytic and well defined with a mean tumour burden of 172.97 mm3. Administration of bLF and P-B alone and in combination (groups 2–4) significantly reduced the tumour incidence, tumour multiplicity and tumour burden. Among the groups 2–4, the combination of bLF and P-B (group 4) more significantly reduced these changes. No tumours were observed in groups 5–8, and the epithelium was normal, intact and continuous.
Table 3.
Tumour incidence, tumour multiplicity, tumour burden and histopathological lesions of hamsters in different groups (mean ± SD; n=20)
| Group | Treatment | Tumour incidence | Tumour multiplicity | Tumour burden | sec |
|---|---|---|---|---|---|
| 1. | DMBA | 20/20 | 1.55±1.57 | 172.97±124.01 | 100% |
| 2. | DMBA + bLF | 13/20a | 0.70±0.57d | 55.73±119.96e | 65% |
| 3. | DMBA + P-B | 9/20b | 0.55±0.75e | 24.00±84.66f | 45% |
| 4. | DMBA + bLF +P-B | 4/20c♣ | 0.20±0.41f | 0.96±3.23f | 20% |
| 5. | bLF | - | - | - | - |
| 6. | P-B | - | - | - | - |
| 7. | bLF+P-B | - | - | - | - |
| 8. | Control | - | - | - | - |
Significantly different from group 1 by χ2 –test combined with Yates' correction (p<0.04)
Significantly different from group 1 by χ2 –test combined with Yates' correction (p<0.001)
Significantly different from group 1 by χ2 –test combined with Yates' correction (p<0.0001)
Significantly different from group 1 (p<0.05) ANOVA followed by Tukey-Kramer test
Significantly different from group 1 (p<0.01) ANOVA followed by Tukey-Kramer test
Significantly different from group 1 (p<0.001) ANOVA followed by Tukey-Kramer test
Synergistic effect
Figure 3 depicts the immunohistochemical analysis of CYP1A1, CYP1B1 and 8-OH-dG in the hamster buccal pouch of control and experimental animals. Topical application of DMBA (group 1) significantly increased the expression of CYP1A1, CYP1B1 and 8-OH-dG as compared to control (group 8). Dietary administration of P-B and bLF alone (groups 2 and 3 respectively) significantly decreased the expression of CYP1A1, CYP1B1 and 8-OH-dG, the combination of bLF and P-B (group 4) more significantly reduced 8-OH-dG adducts and protein expression compared to group 1 (p<0.0001). Administration of chemopreventive agents alone (groups 5–7) did not significantly influence the expression of CYP isoforms and 8-OH-dG compared to untreated control (group 8).
Figure 3.
(I) Photomicrographs of immunohistochemical staining of CYP1A1, 1B1 and 8-OH-dG expression in the buccal pouch of control and experimental animals (20X)
A Overexpression of CYP1A1, 1B1 and 8-OH-dG in the pouch and liver tissues of DMBA treated animals (group 1)
B,C,D Downregulation of CYP1A1, B1 and 8-OH-dG in animals administered DMBA+bLF, DMBA+P-B and DMBA+bLF+P-B treated animals (groups 2, 3 and 4 respectively)
E Normal expression of CYP1A1, B1 and 8-OH-dG in animals groups 5 through 8.
(II) The expression of CYP1A1, 1B1 and 8-OH-dG in the buccal pouch of control and experimental animals (Mean ± SD; n=20)
♠♠ Significantly different from group 8 animals (p< 0.0001)
* Significantly different from group 1 animals (p<0.05)
** Significantly different from group 1 animals (p< 0.001)
*** Significantly different from group 1 animals (p< 0.0001)
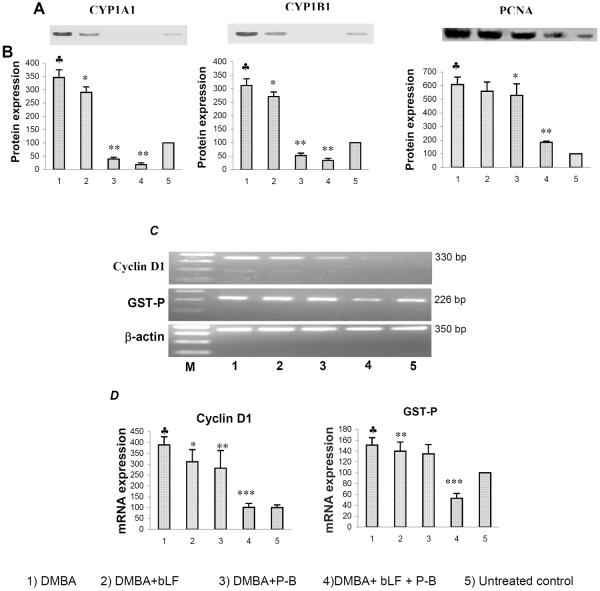
Figure 4 shows the representative western blot and RT-PCR data for CYP1A1, 1B1, PCNA, cyclin D1 and GST-P in the buccal pouch of control and experimental animals. Quantification of each band by densitometric scanning shows significant increase in the expression of CYP1A1, 1B1, PCNA, cyclin D1 and GST-P in DMBA painted hamsters (group 1) compared to untreated control. Although dietary administration of bLF alone (group 2) significantly decreased the expression of CYP isoforms, PCNA, cyclin D1, and GST-P, P-B alone and the combination of bLF and P-B (groups 3 and 4) more significantly decreased the expression of proteins compared to group 1 animals (P<0.0001). No significant changes in the expression of CYP1A1, 1B1, PCNA, cyclin D1 and GST-P were observed in animals treated with chemopreventive agents alone as compared to control.
Figure 4.
The effect of bLF and P-B combination on the expression of CYP1A1, 1B1, PCNA, GST-P, and cyclin D1 in the buccal pouch of control and experimental animals (Mean ± SD; n=10).
A) Representative immunoblot of CYP1A1, 1B1 and PCNA
B) Densitometric analysis.
♣ Significantly different from untreated control (p<0.001)
* Significantly different from group 1 animals (p< 0.001)
** Significantly different from group 1 animals (p< 0.0001)
C) RT-PCR analyses of cyclin D1 and GST-P. β-actin inserted as a control
D) Densitometric analysis.
♣ Significantly different from untreated control (p<0.001)
* Significantly different from group 1 animals (p<0.01)
** Significantly different from group 1 animals (p< 0.001)
*** Significantly different from group 1 animals (p< 0.0001)
Figure 5 shows the representative RT-PCR data for VEGF, VEGFR1, MMP-9 and TIMP-2 in the buccal pouch of control and experimental animals. Quantification of each band by densitometric scanning shows significant increase in the expression of VEGF, VEGFR1, MMP-9 with decreased expression of TIMP-2 in DMBA painted group 1 hamsters compared to untreated control. Combined administration of bLF and P-B decreased the expression of VEGF, VEGFR1, MMP-9 and increased TIMP-2 expression more significantly than either agent alone. In animals administered chemopreventive agents alone (groups 5–7) the mRNA expression of VEGF, VEGFR1, MMP-9 and TIMP-2 was not significantly different from that in controls.
Figure 5.
The effect of bLF and P-B combination on the mRNA expression of VEGF, VEGFR1, MMP-9 and TIMP-2 in the buccal pouch of control and experimental animals. (Mean ± SD; n=10).
(A) RT-PCR analyses of VEGF, VEGFR1, MMP-9 and TIMP-2. (B) Densitometric analysis.
♣ Significantly different from untreated control (p<0.0001)
* Significantly different from group 1 animals (p< 0.01)
** Significantly different from group 1 animals (p< 0.001)
*** Significantly different from group 1 animals (p< 0.0001)
Discussion
DMBA, an indirect carcinogen undergoes epoxidation by the CYP isoforms CYP1A1 and CYP1B1 to form DMBA-3,4-dihydrodiol-1,2-epoxide, an electrophilic ultimate carcinogen capable of forming DNA adducts (20). 8-OH-dG, one of the major oxidative adducts used as a fingerprint of radical attack on DNA is a promutagen that causes mutations in growth-sensitive oncogenes. Furthermore, proliferation of cells that have undergone oxidative DNA damage leads to accumulation of further mutations and neoplastic transformation (21, 22). Constitutive expression of cyclin D1, PCNA and GST-P that play key roles in cell cycle progression confers a proliferative advantage to transformed cells and may limit the repair of genomic DNA damage induced by DMBA exposure. Overexpression of these proteins has been documented to increase during progression of normal epithelium to SCC through premalignant stages (23–25). Thus enhanced expression of CYP isoforms, 8-OH-dG and cell cycle associated proteins indicates increased proliferation of oxidatively damaged cells that could facilitate the development of HBP carcinomas.
Uncontrolled cell proliferation in tumours creates hypoxia, a potent stimulus of angiogenesis. Among the numerous proangiogenic mediators, VEGF, is the best characterized cytokine implicated in the regulation of angiogenesis. Activation of VEGF/VEGFR axis triggers a signaling network that results in the formation of new blood vessels, a prerequisite for metastasis (26). VEGFR1 acts both as a negative regulator of angiogensis by sequestering VEGF itself, and as a positive regulator by signaling migration/proliferation of endothelial cells (27). Thus VEGFR1 is considered as an important target for the suppression of tumour angiogenesis and metastasis. Metastatic spread of tumours requires efficient degradation of the extracellular matrix (ECM) by matrix metalloproteinases (MMPs). The functional activities of MMPs are regulated by the endogenous tissue inhibitors of matrix metalloproteinases (TIMPs). Deregulation of MMPs and TIMPs has been found in different types of cancer and is correlated with tumour aggressiveness (28). Overexpression of VEGF, VEGFR1, MMP-9 and downregulation of TIMP-2 in HBP carcinomas seen in the present study may confer a survival advantage by acquisition of an invasive and angiogenic phenotype.
Combined administration of bLF and P-B was more effective than either agent alone in suppressing the development of DMBA-induced HBP carcinomas as revealed by decreased tumour incidence and tumour burden. The inhibitory effects of bLF and P-B combination on CYP isoforms, DNA damage, cell proliferation, invasion and angiogenesis observed in the present study may be a key determinant in inhibiting DMBA-induced tumorigenesis in the HBP. Fujita et al (29) have demonstrated inhibition of CYP1A2 by bLF during experimental liver and colon carcinogenesis. The inhibitory effect of polymeric black tea fractions on benzo[a]pyrene-induced CYP1A1 and CYP1A2 expression in mouse liver and lungs has been documented (30). In addition, phase II clinical trials with the tea polyphenol EGCG have shown diminished oxidative DNA damage in individuals at high risk for liver cancer (31). Furthermore, downregulation of cell cycle, tumour invasion and angiogenesis related proteins by bLF and P-B combination seen in the present study is in line with the anticarcinogenic properties of bLF and tea polyphenols documented by us and other workers (8,32–35).
The chemopreventive potential of bLF and PB combination seen in the present study may be attributed to its antioxidant properties. The in vitro data provide evidence for the potent antioxidative potential of bLF and PB. Although the exact signal transduction pathways behind tumour suppression remain unclear, the in vitro data suggests that the antioxidative potential of bLF and P-B may block HBP carcinogenesis by multiple mechanisms including prevention of procarcinogen activation, inhibition of cell proliferation, invasion and angiogenesis.
References
- 1.Ohigashi H, Murakami A. Cancer prevention with food factors: alone and in combination. BioFactors. 2004;22:49–55. doi: 10.1002/biof.5520220109. [DOI] [PubMed] [Google Scholar]
- 2.Sakamoto K. Synergistic effects of thearubigin and genistein on human prostate tumor cell (PC-3) growth via cell cycle arrest. Cancer Lett. 2004;151:103–109. doi: 10.1016/s0304-3835(99)00423-1. [DOI] [PubMed] [Google Scholar]
- 3.Yang CS, Lambert JD, Ju J, Lu G, Sand S. Tea and cancer prevention: Molecular mechanisms and human relevance. Toxicol. Appl. Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuda H, Sekine K, Fujita K, Ligo M. Cancer prevention by bovine lactoferrin and underlying mechanisms: a review of experimental and clinical studies. Biochem. Cell Biol. 2002;80:131–136. doi: 10.1139/o01-239. [DOI] [PubMed] [Google Scholar]
- 5.Moyers SB, Kumar NB. Green tea polyphenols and cancer chemoprevention: multiple mechanisms and endpoints for phase II trials. Nutr. Rev. 2004;62:204–211. doi: 10.1111/j.1753-4887.2004.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Tu Y, Xia H, Jie G, Chen X, He P. Suppression of free-radicals and protection against H2O2-induced oxidative damage in HPF-1 cell by oxidized phenolic compounds present in black tea. Food Chem. 2007;105:1349–1356. [Google Scholar]
- 7.Chandra Mohan KVP, Letchoumy PV, Hara Y, Abraham SK, Nagini S. Combination chemoprevention of hamster buccal pouch carcinogenesis by bovine milk lactoferrin and black tea polyphenols, Cancer Invest. 2008;26:193–201. doi: 10.1080/07357900701511961. [DOI] [PubMed] [Google Scholar]
- 8.Chandra Mohan KVP, Devaraj H, Prathiba D, Hara Y, Nagini S. Antiproliferative and apoptosis inducing effect of lactoferrin and black tea polyphenol combination on hamster buccal pouch carcinogenesis. Biochim. Biophys. Acta. 2006;1760:1536–1544. doi: 10.1016/j.bbagen.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. [Google Scholar]
- 10.Miller NJ, Castelluccio C, Tijburg L, Rice-Evans C. The antioxidant properties of theaflavins and their gallate esters radical scavengers or metal chelators? FEBS Lett. 1996;392:40–44. doi: 10.1016/0014-5793(96)00780-6. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B, Gutteridge JMC, Aruoma OI. The deoxyribose method: a simple test tube assay for determination of rate constants for reactions of hydroxy radicals. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 12.Nishimiki M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–853. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 13.Garratt CJ. Effect of iodination on the biological activity of insulin. Nature. 1964;28:1324–1325. doi: 10.1038/2011324a0. [DOI] [PubMed] [Google Scholar]
- 14.Oyaizu M. studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986;44:307–315. [Google Scholar]
- 15.Shklar G. Development of experimental oral carcinogenesis and its impact on current oral cancer research. J. Dent. Res. 1999;78:1768–1772. doi: 10.1177/00220345990780120101. [DOI] [PubMed] [Google Scholar]
- 16.Caderni G, Filippo CD, Luceri C, et al. Effects of black tea, green tea and wine extracts on intestinal carcinogenesis induced by azoxymethane in F344 rats. Carcinogenesis. 2000;21:1965–1969. doi: 10.1093/carcin/21.11.1965. [DOI] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musarrat J, Areziana-Wilson J, Wani AA. Prognostic and aetiological relevance of 8-hydroxyguanosine in human breast carcinogenesis. Eur. J. Cancer. 1996;32A:1209–1214. doi: 10.1016/0959-8049(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 22.Floyd RA. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990;11:1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- 23.Kohno Y, Patel V, Kim Y, Tsuji T, Chin B-R, Sun M, Donoff RB, Kent R, Wong D, Todd R. Apoptosis, proliferation and p12doc-1 profiles in normal, dysplastic and malignant squamous epithelium of the Syrian hamster cheek pouch model. Oral Oncol. 2002;38:274–280. doi: 10.1016/s1368-8375(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 24.Kotelnikov VM, Coon JS, Mundle S, Kelanic S, LaFollette S, Taylor S. Cyclin D1 expression in squamous cell carcinomas of the head and neck and in oral mucosa in relation to proliferation and apoptosis. Clinical Cancer Res. 1997;3:95–101. [PubMed] [Google Scholar]
- 25.Chen YK, Lin LM, Hsu SS, Lin DT. The mRNA expression of placental glutathione S-transferase isoenzyme in hamster buccal pouch carcinomas using reverse transcription-polymerase chain reaction. Oral Oncol. 2002;38:158–162. doi: 10.1016/s1368-8375(01)00039-2. [DOI] [PubMed] [Google Scholar]
- 26.Lee TH, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, Avraham S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4(6):e186. doi: 10.1371/journal.pmed.0040186. doi:10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis. 2006;9:225–230. doi: 10.1007/s10456-006-9055-8. DOI 10.1007/s10456-006-9055-8. [DOI] [PubMed] [Google Scholar]
- 28.Baker EA, Leaper DJ, Hayter JP, Dickenson AJ. The matrix metalloproteinase system in oral squamous cell carcinoma. Brit. J. Oral Maxillofac. Surg. 2006;44:482–486. doi: 10.1016/j.bjoms.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Fujita KI, Ohnishi T, Sekine K, Ligo M, Tsuda H. Down-regulation of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx)-induced CYP1A2 expression is associated with bovine lactoferrin inhibition of MeIQx-induced liver and colon carcinogenesis in rats. Jpn. J. Cancer Res. 2002;93:616–625. doi: 10.1111/j.1349-7006.2002.tb01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan R, Raghunathan R, Maru GB. Effect of polymeric black tea polyphenols on benzo[a]pyrene [B(a)P]-induced cytochrome P4501A1 and 1A2 in mice. Xenobiotica. 2005;35:671–682. doi: 10.1080/00498250500202155. [DOI] [PubMed] [Google Scholar]
- 31.Luo H, Tang L, Tang M, Billam M, Huang T, Yu J, Wei Z, Liang Y, Wang K, Zhang ZQ, Wang JS. Phase II a chemoprevention trial of green tea polyphenols in high risk individual of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27:262–26. doi: 10.1093/carcin/bgi147. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta A, Ghosh S, Das S. Tea can protect against aberrant crypt foci formation during azoxymethane induced rat colon carcinogenesis. Exp. Clin. Cancer Res. 2001;22:185–191. [PubMed] [Google Scholar]
- 33.Liberto M, Cobrinik D. Growth factor-dependent induction of p21CIP1 by the green tea polyphenol, epigallocatechin gallate. Cancer Lett. 2000;154:151–161. doi: 10.1016/s0304-3835(00)00378-5. [DOI] [PubMed] [Google Scholar]
- 34.Mader JS, Smyth D, Marshall J, Hoskin DW. Bovine lactoferricin inhibits basic fibroblast growth factor- and vascular endothelial growth factor165 induced angiogenesis by competing for heparin like binding sites on endothelial cells. Am J Pathol. 2006;169:1753–1766. doi: 10.2353/ajpath.2006.051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho Y-C, Yang S-F, Peng C-Y, Chou M-Y, Chang Y-C. Epigallocatechin-3-gallate inhibits the invasion of human oral cancer cells and decreases the productions of matrix metalloproteinases and urokinase-plasminogen activator. J. Oral Pathol. Med. 2007;36:588–93. doi: 10.1111/j.1600-0714.2007.00588.x. [DOI] [PubMed] [Google Scholar]