Abstract
Objective
To evaluate the chemopreventive potential of the black tea polyphenols Polyphenon-B and BTF-35 during the preinitiation phase of 7,12-dimethylbenz[a]anthracene (DMBA)-induced hamster buccal pouch (HBP) carcinogenesis.
Design
Hamsters were divided into 6 groups. Groups 2 and 3 animals received diet containing Polyphenon-B and BTF-35 respectively, four weeks before carcinogen administration when they were 6 weeks of age and continued until the final exposure to carcinogen. At 10 weeks of age, animals in groups 1, 2 and 3 were painted with 0.5% DMBA 3 times a week for 14 weeks. Groups 4 and 5 animals were given Polyphenon-B and BTF-35 alone respectively as in groups 2 and 3. Animals in group 6 served as control. All the animals were sacrificed after an experimental period of 18 weeks. Phase I and phase II xenobiotic-metabolizing enzymes and 8-hydroxy deoxyguanosine (8-OH dG) in the buccal pouch and liver were used as biomarkers of chemoprevention.
Results
Hamsters painted with DMBA showed increased expression of 8-OH-dG and enhanced activities of phase I (CYP450; total as well as CYP1A1, 1A2 and 2B isoforms and cytochrome b5) and phase II (GST and quinone reductase) xenobiotic-metabolizing enzymes with increased immunohistochemical expression of CYP1A1, and CYP1B1 isoforms in the buccal pouch. This was accompanied by increased phase I and decreased phase II enzyme activities in the liver. Administration of Polyphenon-B and BTF-35 significantly decreased tumour incidence, oxidative DNA damage, phase I enzyme activities as well as expression of CYP1A1 and CYP1B1 isoforms, while enhancing phase II enzyme activities in the buccal pouch and liver.
Conclusion
Our results provide a mechanistic basis for the chemopreventive potential of black tea polyphenols. Furthermore, the greater efficacy of BTF-35 in chemoprevention of HBP carcinomas via inhibition of oxidative DNA damage and modulation of xenobiotic-metabolizing enzymes may have a major impact in human oral cancer prevention.
Keywords: Black tea polyphenols, chemoprevention, DMBA, hamster buccal pouch carcinogenesis, xenobiotic-metabolizing enzymes, 8-OHdG
Introduction
Cancer, the second most common cause of death worldwide, results as a consequence of mutations that arise spontaneously or induced by the environment (1). A large proportion of environmental carcinogens require metabolic activation by phase I and phase II xenobiotic-metabolizing enzymes (XME) to exert their carcinogenic effects. Cytochrome P450s, a family of phase I hemoproteins, catalyse the functional activation of various procarcinogens to highly reactive proximate carcinogens. The proximate carcinogen can interact directly with cellular DNA to form adducts resulting in chromosomal abnormalities that eventually culminate in neoplastic transformation. Phase II enzymes that detoxify pro- and proximate forms of chemical carcinogens decrease DNA damage (2). The co-ordinated expression and regulation of XME may be an important contributing factor in determining susceptibility to cancer (3). Recent efforts to control the incidence of environmentally induced cancers have therefore focused on identifying chemopreventive agents that modulate XME (4).
Chemoprevention by dietary agents including fruits, vegetables and beverages has received growing attention in recent years as a promising strategy for cancer prevention (5). Tea made from the leaves of Camellia sinensis is one of the most widely consumed beverages next to water. About 3 billion kilograms of tea are produced and consumed annually, of which black tea accounts for nearly 80 per cent. The predominant consumption of black tea has stimulated interest in its possible beneficial effects. Many health benefits of black tea are mainly attributed to the dimeric flavanols and polymeric polyphenols such as theaflavins and thearubigins. Black tea polyphenols are known to exert a wide range of pharmacological effects including antioxidative, antimutagenic and antiproliferative activities (6). Since epidemiological studies on black tea consumption and cancer prevention have not been conclusive, current research efforts have focused on establishing the chemopreventive potential of black tea polyphenols in experimental animal models of carcinogenesis. The tumour inhibitory effects of black tea polyphenols have been demonstrated in a wide range of animal tumour models including lung, colon, and skin (7). However, there is paucity of information on the mechanism of the chemopreventive efficacy of black tea polyphenols in experimental oral carcinogenesis especially with reference to DNA damage and XME.
The hamster buccal pouch (HBP) carcinogenesis model is an excellent animal system for investigating the efficacy of chemopreventive agents on oral carcinogenesis. HBP carcinomas induced by the application of 7,12-dimethyl-benz[a]anthracene (DMBA) to the cheek pouch of Syrian hamsters closely mimic oral squamous cell carcinomas at the morphological, biochemical, cytogenetic and molecular levels (8,9) We have used this model extensively to document the chemopreventive efficacies of several functional foods and diet-derived agents (10,11). Recently, we demonstrated the modulatory effects of black tea polyphenols on reactive oxygen species (ROS)-induced lipid and protein oxidation as well as the status of antioxidants during DMBA-induced HBP carcinogenesis (12,13).
The present study was designed to evaluate the chemopreventive potential of dietary administration of black tea polyphenols, Polyphenon-B (a mixture of black tea polyphenols) and BTF-35 (a well-characterized black tea extract enriched with theaflavins and catechins) during the preinitiation phase of DMBA-induced HBP carcinogenesis using the status of phase I (cytochrome P450; total as well as CYP1A1, 1A2 and 2B isoforms and cytochrome b5) and phase II (glutathione S-transferase [GST] and quinone reductase) biotransformation enzymes in the buccal pouch and liver as biomarkers. In addition, the expression of CYP1A1 and CYP1B1 as well as 8-hydroxy deoxyguanosine (8-OHdG), a sensitive and useful marker of oxidative DNA damage was also analysed by immunohistochemical localization.
Materials and methods
Chemicals
Bovine serum albumin, cytochrome C, 1-chloro-2,4-dinitrobenzene (CDNB), 2,6-dichlorophenolindophenol (DCPIP), DMBA, 3,3′-diaminobenzidine (DAB), 7-ethoxyresorufin, methoxyresorufin, pentoxyresorufin, reduced glutathione (GSH), reduced nicotinamide adenine dinucleotide (NADH), reduced nicotinamide adenine dinucleotide phosphate (NADPH), resorufin and sodium dithionite were purchased from Sigma Chemical Company (St. Louis, USA). 8-OH-dG, mouse monoclonal antibody was purchased from JaICA, Shizuoka, Japan. CYP1A1, mouse monoclonal antibody was generously provided by Dr. Stegeman, Woods Hole Oceanographic Institute (WHOI), USA and Dr Gelboin, National Cancer Institute (NCI), USA. CYP1B1 antibody was purchased from Santa Cruz Biotechnology, CA, USA. Polyphenon-B and BTF-35 were kindly provided by Mitsui Norin Co., Ltd., Tokyo, Japan. The composition of Polyphenon-B and BTF-35 are given in table I. All other reagents used were of analytical grade.
Table I.
Composition of Polyphenon-B and BTF-35
| Polyphenolic constituents | Polyphenon-B (%w/w) | BTF-35 (%w/w) |
|---|---|---|
|
| ||
| Epigallocatechin (EGC) | - | 0.1 |
| Epicatechin (EC) | 0.4 | 0.2 |
| Epigallocatechin gallate (EGCG) | 1.4 | 2.6 |
| Epicatechin gallate (ECG) | 0.1 | 2.1 |
| Gallocatechin gallate (GCG) | 0.2 | 0.3 |
| Catechin gallate (CG) | - | 0.1 |
| Catechin (C) | - | 0.1 |
| Free theaflavin | 0.32 | 7.1 |
| Theaflavinmonogallate-A | 0.14 | 8.3 |
| Theaflavinmonogallate-B | 0.15 | 2.6 |
| Theaflavindigallate | 0.24 | 9.8 |
| Tannin | 35.6 | - |
| Caffeine | 4.9 | 0.5 |
The composition of Polyphenon-B and BTF-35 was kindly provided by Mitsui Norin Co., Ltd., Japan.
Animals and diet
The experiment was carried out with male Syrian hamsters aged 6–10 weeks weighing between 90–110g obtained from the Central Animal House, Annamalai University, India. Animals housed six to a polypropylene cage were provided food and water ad libitum and maintained under controlled conditions of temperature and humidity with an alternating 12 hours light/dark cycle. The animals were maintained in accordance with the guidelines of the Indian Council of Medical Research, and approved by the ethical committee, Annamalai University. The experimental diet was prepared every day by mixing black tea polyphenols to preweighed standard pellet diet (Mysore Snack Feed, Mysore, India) at a concentration of 0.05%. The standard pellet diet contains crude protein (22.12%), crude oil (4.12%), crude fibre (3.18%), ash (5.17%) and sand silica (1.13%) with energy value of 3625 kcal/kg. The diet was replenished every day and the food consumption was recorded.
Treatment schedule
The hamsters were randomized into experimental and control groups and divided into 6 groups of 6 animals each. Animals in groups 2 and 3 received diet containing 0.05% of Polyphenon-B and BTF-35 respectively four weeks before carcinogen administration when they were 6 weeks of age and continued until the final exposure to carcinogen. At 10 weeks of age, the hamsters in groups 1 to 3 were painted with a 0.5% solution of DMBA in liquid paraffin on the right buccal pouches using a number 4 brush three times a week for 14 weeks. Each application leaves approximately 0.4mg DMBA (8). Hamsters in group 1 received no further treatment. Animals in groups 4 and 5 received Polyphenon-B and BTF-35 alone respectively as in groups 2 and 3. Group 6 animals received basal diet and served as control. The dose for black tea polyphenols used in the present study corresponds to the daily intake of four cups of tea (30–40mg of tea polyphenols per kilogram body weight by humans) (14). The experiment was terminated at the end of 18 weeks and all animals were sacrificed by cervical dislocation after an overnight fast. Before an animal was killed, the right buccal pouch was grossly inspected to evaluate premalignant lesions or tumour development and photographed. The tumour burden was calculated by multiplying the mean tumour volume (4/3 πr3) (r= ½ tumour diameter in mm) with the mean number of tumours. The buccal pouch tissues were subdivided and variously processed for distribution to each experiment.
Immunohistochemistry
The tissue sections were deparaffinised by heat at 60°C for 10 minutes, followed by three washes in xylene. After gradual hydration through graded alcohol, the slides were incubated in citrate buffer (pH 6.0) for two cycles of 5 minutes in a microwave oven for antigen retrieval. The sections were allowed to cool for 20 minutes and then rinsed with Tris-buffered saline (TBS). The sections were treated for 15 minutes with 3% H2O2 in distilled water to inhibit endogenous peroxidase activity. Non-specific antibody binding was reduced by incubating the sections with normal goat serum for 25 minutes. The sections were then incubated with 8-OH-dG, CYP1A1 (mouse monoclonal antibody) and CYP1B1 (rabbit polyclonal antibody) at room temperature for one hour. The slides were washed with TBS and then incubated with anti-mouse and anti-rabbit biotin-labelled secondary antibodies followed by streptavidin-biotin-peroxidase for 30 minutes each at room temperature. The immunoprecipitate was visualized by treating with DAB and counterstaining with haematoxylin. For negative controls, the primary antibody was replaced with TBS. Positive controls were also processed simultaneously. The immunohistochemical data for CYP1A1, CYP1B1 and 8-OHdG were expressed as the number of cells with positive staining per 100 counted cells.
Preparation of tissue homogenate
Fresh tissues were used for biochemical estimations. The buccal pouch and liver tissues after weighing were homogenized in an all glass homogenizer with Teflon pestle and stored in ice until use.
S9 fraction preparation
The S9 fraction has been extensively used to investigate the metabolic conversion of various compounds (15–17) because it requires only small amounts of sample, and is obtained during the early stages of fractionation. Furthermore, it provides more metabolic information than the microsomal fraction because it contains both microsomal and cytosolic enzymes.
All the steps for the preparation of S9 fraction were carried out at 4°C as described by Ames et al (17). The buccal pouch and liver tissues after weighing were washed with cold 0.15M KCl and homogenized in 3 volumes of 0.15M KCl in an all glass homogenizer with Teflon pestle. The homogenate was centrifuged for 10 minutes at 9000 x g and the supernatant so collected is the S9 mix fraction. The biochemical analyses were carried out immediately.
Biochemical assays
Cytochrome P450 and cytochrome b5 content were assayed by the method of Omura and Sato (23). Cytochrome P450 was determined by using the carbon monoxide difference spectra. Reduced cytochrome P450 combines with carbon monoxide to yield a pigment with an absorbance maximum at 450nm. Cytochrome b5 was measured from the difference spectrum between reduced and oxidized cytochrome b5. The ethoxyresorufin-O-deethylase (EROD), methoxyresorufin-O-demethylase (MROD) and pentoxyresorufin-O-depentylase (PROD) activities were assayed as described by Baer-Dubowska et al (19) and Burke et al (20). The activity of GST was determined as described by Habig et al (21) by following the increase in absorbance at 340nm using CDNB as the substrate. The activity of quinone reductase was assayed as described by Ernster (22). This method involves measurement of reduction at 550nm using NADPH as the electron donor and 2,6-dichlorophenolindophenol as electron acceptor. The protein content was estimated by the method of Lowry et al (23).
Statistical analysis
The data are expressed as mean ± standard deviation. Tumour incidence and tumour burden were analysed by χ2-test and Student’s t test respectively. Statistical analyses on the data for body weights, biochemical assays and immunohistochemical staining of 8-OH-dG, CYP1A1 and 1B1 isoforms were analysed using analysis of variance (ANOVA) and the group means were compared by the Tukey’s test. The results were considered statistically significant if the p value was < 0.05.
Results
Table II shows the mean body weight, tumour incidence, tumour burden and SCCs in the buccal pouch of experimental and control animals. Hamsters in group 1 showed a tendency to be lower in body weight during the experiment and the mean final weights were decreased significantly (p<0.05) compared to control (group 6). At the end of the experimental period, the tumour incidence in group 1 was 100 per cent with a mean tumour burden of 127mm3. Administration of Polyphenon-B and BTF-35 effectively suppressed the development of HBP carcinomas. In group 2 animals Polyphenon-B effectively decreased the incidence of SCC to 17 per cent with a mean tumour burden of 8.3 mm3 compared to group 1. No tumours were observed in animals treated with DMBA and BTF-35 (group 3). In groups 4–6, the epithelium was normal, intact and continuous.
Table II.
Body weight, tumour incidence, tumour burden and SCC incidence in different groups (mean ± SD; n=6)
| Group | Treatment | Final weight (g) | Tumour incidence | Tumour burdena mm3 | Squamous cell carcinoma |
|---|---|---|---|---|---|
| 1. | DMBA | 118 ±16.4b | 6/6 | 127.21 ± 87.42 | + (100%) |
| 2. | DMBA+ Polyphenon-B | 132 ± 12.9 | 1/6c | 8.31 ± 7.92 d | + (16.6%)c |
| 3. | DMBA+ BTF-35 | 134 ± 12.4 | - | - | - |
| 4. | Polyphenon-B | 139 ± 14.7 | - | - | - |
| 5. | BTF-35 | 140 ± 14.1 | - | - | - |
| 6. | Control | 143 ± 12.3 | - | - | - |
+ - well differentiated; parantheses, percentage of lesions.
Mean tumour burden was calculated by multiplying the mean tumour volume with the mean number of tumours (tumour volume was calculated using 4/3 πr3, where r = ½ tumour diameter in mm)
Significantly different from group 6 (p<0.05) ANOVA followed by Tukey’s test
Significantly different from group 1 by χ2-test (p<0.001)
Significantly different from group 1 by Student’s t test (p<0.001)
Immunohistochemical findings
Figures 1 and 2 show the expression of CYP1A1, CYP1B1 and 8-OH-dG in the buccal pouch and liver of control and experimental animals. In DMBA painted hamsters, the expression of CYP1A1, CYP1B1 and 8-OH-dG in the pouch and liver tissues was significantly higher than in control animals (group 6). Although administration of Polyphenon-B (group 2) and BTF-35 (group 3) significantly decreased protein expression in both the tissues as compared to group 1, BTF-35 was more effective. No significant changes in the expression of CYP isoforms and 8-OH-dG were observed in animals administered Polyphenon-B and BTF-35 alone (groups 4 and 5) as compared to control.
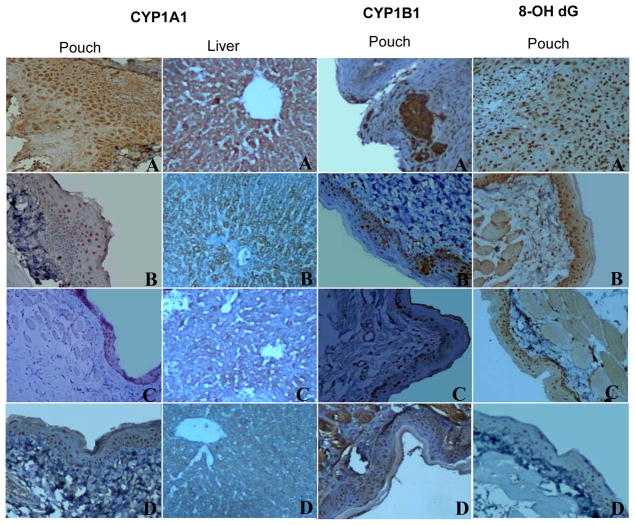
Figure 1. Photomicrographs of immunohistochemical staining of CYP1A1, 1B1 and 8-OH-dG expression in the buccal pouch and liver of control and experimental animals (20X).
A Overexpression of CYP1A1, 1B1 and 8-OH-dG in the pouch and liver tissues of DMBA treated animals (group 1)
B, C Downregulation of CYP1A1, B1 and 8-OH-dG in the pouch and liver tissues of DMBA+P-B and DMBA+BTF-35 treated animals (groups 2 and 3 respectively)
D Normal expression of CYP1A1, B1 and 8-OH-dG in the pouch and liver tissues of animals administered P-B and BTF-35 alone and control animals (Groups 4, 5 and 6 respectively)
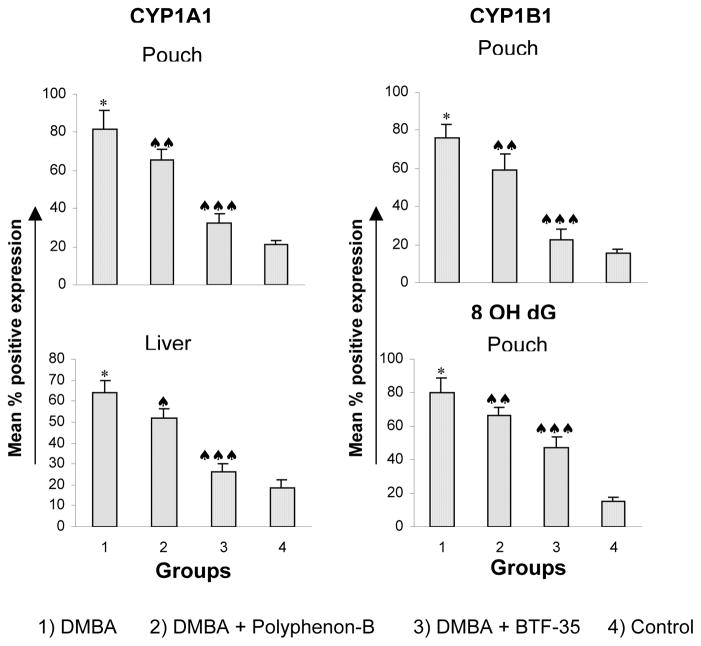
Figure 2. The expression of CYP1A1, CYP1B1 and 8-OH-dG in the buccal pouch and liver of control and experimental animals.
* Significantly different from group 6 (p<0.001) ANOVA followed by Tukey’s test
♠ Significantly different from group 1 (p<0.05) ANOVA followed by Tukey’s test
♠♠ Significantly different from group 1 (p<0.01)
♠♠♠ Significantly different from group 1 (p<0.001)
Biochemical findings
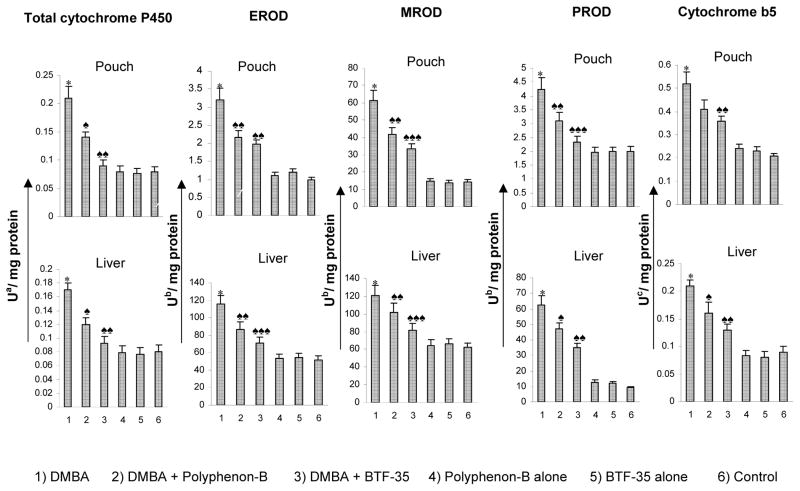
The activities of total cytochrome P450, CYP1A1, CYP1B1 and cytochrome b5 in the S9 fraction of buccal pouch and liver of experimental and control animals are presented in figure 2. Total cytochrome P450 and its isoforms CYP1A1, 1A2 and 2B as well as cytochrome b5 were significantly increased in the pouch and liver of DMBA-painted animals (group 1) compared to control (group 6). Dietary administration of black tea polyphenols significantly decreased phase I enzyme activities compared to group 1 with a greater decrease in hamsters administered BTF-35 (group 3) compared to those administered Polyphenon-B (group 2). Treatment with black tea polyphenols alone (groups 4 and 5) did not produce any significant changes in phase I enzyme activities in the pouch and liver.
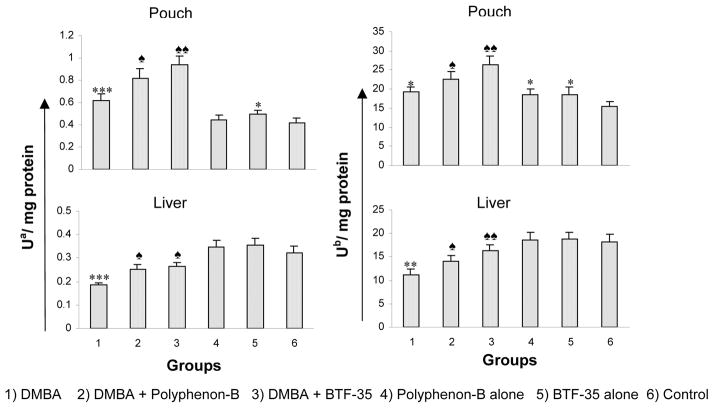
The effect of pretreatment with Polyphenon-B and BTF-35 on the activities of GST and quinone reductase in the S9 fraction of buccal pouch and liver are shown in figure 3. In DMBA treated animals (group 1), the activities of phase II enzymes were significantly increased in the buccal pouch and significantly decreased in the liver compared to control (group 6). Dietary administration of BTF-35 (group 3) increased phase II enzyme activities more significantly than Polyphenon-B (group 2) as compared to group 1. Administration of Polyphenon-B and BTF-35 alone (groups 3 and 4) significantly enhanced enzyme activities compared to those of group 6.
Figure 3.
Activities of phase I enzymes in the S9 fractions of the buccal pouch and liver of experimental and control animals. (mean ± SD; n=6)
* Significantly different from group 6 (p<0.001) ANOVA followed by Tukey’s test
♠ Significantly different from group 1 (p<0.05) ANOVA followed by Tukey’s test
♠♠ Significantly different from group 1 (p<0.01)
♠♠♠ Significantly different from group 1 (p<0.001)
a - nmoles of cytochrome
b - pmoles resorufin/min
c - nmoles of cytochrome
Discussion
DMBA, a pluripotent polycyclic aromatic hydrocarbon (PAH), has been extensively used to induce a wide range of epithelial tumours. Humans are exposed to PAHs like DMBA through tobacco smoke reported to contain 3800 chemicals, of which PAHs are one of the most biologically active compounds responsible for oral cancer development (24). Studies relating DNA adduct profiles in cancer tissues as well as genetic polymorphisms of XME to smoking history have revealed that individual variations in carcinogen metabolism and DNA repair play a major role in cancer susceptibility (25,26).
In the present study, topical application of DMBA to the hamster buccal pouch for 14 weeks resulted in well-differentiated squamous cell carcinomas (SCCs) associated with an increase in XME activities as well as 8-OHdG, an oxidative DNA base. PAHs such as DMBA are bifunctional inducers that induce both phase I and phase II detoxifying enzymes via the arylhydrocarbon receptor that binds to xenobiotic response elements of phase I and phase II enzyme genes, transactivating these genes (2,27,28). DMBA, a procarcinogen is metabolized by the consecutive actions of cytochrome P450 and epoxide hydrolase to the ultimate carcinogen 7,12-DMBA 3,4-diol 1,2-epoxide (29). Cytochrome b5, a ubiquitous electron transport protein, enhances the catalytic efficiency of cytochrome P450 (30). Increased expression of CYP1A1 observed in tumour bearing hamsters in the present study underscores the importance of this enzyme in the metabolic activation of DMBA in the well-oxygenated buccal pouch. Although the major function of CYP1A1 is to detoxify hydrophobic PAHs, high CYP1A1 activity could result in the accumulation of toxic PAH intermediates. Previously CYP1A1 was considered as the sole enzyme responsible for metabolic activation of most of the PAHs. Subsequent experiments on CYP isoforms established that CYP1B1 also activates PAHs at rates similar to or even higher than CYP1A1 in experimental animals (31). Furthermore, studies with CYP1B1-knockout mice have shown the critical role of CYP1B1 in DMBA-induced tumorigenesis (32).
GSTs, a multigene family of phase II detoxifying enzymes, together with quinone reductase, a ubiquitous flavoprotein, favour the elimination of functionalised P450 metabolites (33,34). Phase II enzymes are generally induced concomitantly with phase I enzymes. Although DMBA is detoxified by the combined action of phase I and phase II enzymes, some of the diol epoxide derivatives of DMBA that have escaped detoxification can bind to DNA causing mutations in growth sensitive oncogenes (35). 8-OH-dG that arises due to oxidative DNA damage can cause G to T and A to C mutations and is thus likely to be involved in the transformation of normal cells to a malignant phenotype (36). A higher frequency of G to T transversion has been documented in animals exposed to PAH carcinogens (37). The enhanced activities of phase I and II enzymes as well as increased expression of 8-OH-dG seen in HBP tumours in the present study corroborate overexpression of these enzymes and oxidative DNA damage reported in cell lines and malignant tumours (26,38,39).
In contrast to the buccal pouch, in the liver of tumour bearing animals, the increase in phase I enzymes was accompanied by a decrease in phase II enzymes. The increase in hepatic cytochrome P450 (total as well as isoforms CYP1A1, CYP1A2 and CYP2B), and cytochrome b5 activity may be attributed to metabolic activation of DMBA in the liver in addition to the buccal pouch. Hepatic metabolism of PAHs is known to produce short-lived electrophiles that can cause DNA damage in extrahepatic tissues. Proximate mutagens produced in the liver are suggested to be transported to the target tissue for final metabolic activation to form ultimate DNA-reactive metabolites (40). Deficient DNA repair and detoxification systems in the target tissue can cause permanent DNA damage and increase cancer risk. Thus enhanced hepatic CYP enzyme expression with compromised phase II detoxification system seen in the present study may play a critical role in extrahepatic carcinogenesis.
Dietary administration of Polyphenon-B and BTF-35 starting from 4 weeks before initiation of DMBA treatment significantly suppressed tumour incidence in the HBP. These results are consistent with the chemopreventive effects of black tea reported by other workers. Dietary intake of black tea extract is known to protect against azoxymethane (AOM)-induced colon carcinogenesis (14). Oral administration of black tea preparations has been documented to inhibit N-nitrosomethylbenzylamine-induced esophageal tumorigenesis (41). Cao et al (42) reported the chemopreventive efficacy of black and green tea on diethylnitrosamine induced pulmonary and hepatic carcinogenesis. Several other studies have revealed that black tea is effective at initiation, promotion and progression stages of carcinogenesis (6,43).
The modulatory effects of Polyphenon-B and BTF-35 on XME and inhibition of DNA damage observed in the present study may be a key determinant in inhibiting DMBA-induced cancer initiation in the HBP. Polyphenon-B and BTF-35 function as dual-acting agents by inhibiting phase I enzymes while simultaneously enhancing phase II detoxification enzyme activities in the buccal pouch as well as in the liver. Dual-acting agents are effective as chemopreventive agents because they inhibit metabolic activation of carcinogens and promote detoxification and excretion (4). In particular, inducers of phase II detoxification enzymes are currently under active investigation as cancer chemopreventive agents. Several in vitro studies have shown the modulatory effects of black tea on XME. Krishnan and Maru (44) documented the inhibitory effect of polymeric black tea fractions on cytochrome P450 isoenzymes in rat liver microsomes. Black tea infusion was found to induce activities of GST and quinone reductase in cell lines and animal tumour models (45,46). Recently, we demonstrated the potent radical scavenging effects of Polyphenon-B and BTF-35 both in vitro and in vivo [47]. We speculate that Polyphenon-B and BTF-35 prevent oxidative DNA damage as reflected by reduced formation of 8-OHdG adducts in the buccal pouch by scavenging ROS generated during metabolic activation of DMBA.
The protective effect of Polyphenon-B and BTF-35 against DMBA-induced HBP carcinogenesis may be attributed to the polyphenolic constituents such as tannins, catechins and theaflavins. Tannin has been reported to inhibit CYP2E1, GST and QR in mouse liver, while increasing GST activity in the kidney (19,50). The catechin, EGCG, was found to inhibit the activities of CYP4501A1 and 2B1, during 4-(methyl nitrosamine)-1-(3 pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice (48). Theaflavin, a dimeric black tea polyphenol, was demonstrated to inhibit CYP450-dependent bioactivation of carcinogens, enhance phase II metabolizing enzymes and exert antimutagenic effects by suppressing aryl hydrocarbon receptor (AhR) transformation (49–52). Phase II clinical trials with EGCG have shown diminished oxidative DNA damage in individuals at high risk for liver cancer (53). Taken together, these findings provide evidence for the anticarcinogenic effect of black tea polyphenols.
Although both PB and BTF-35 exerted inhibitory effects against HBP carcinogenesis, BTF-35 was more effective than Polyphenon-B in reducing tumour burden, modulating phase I and II biotransformation enzymes and inhibiting DNA damage. The mechanisms underlying the higher efficacy of BTF-35 compared to Polyphenon-B remain unclear. We suggest that the greater inhibitory effect of BTF-35 may be due to the higher content of theaflavins and catechins such as EGCG and ECG compared to Polyphenon-B. However, further studies on the protective effects on different animal tumour models and signal transduction pathways are warranted for validating the chemopreventive potential of BTF-35.
Figure 4.
Activities of phase II enzymes in the S9 fractions of the buccal pouch and liver of experimental and control animals. (Mean ± SD; n=6)
* Significantly different from group 6 (p<0.05) ANOVA followed by Tukey’s test
** Significantly different from group 6 (p<0.01)
*** Significantly different from group 6 (p<0.001)
♠ Significantly different from group 1 (p<0.05) ANOVA followed by Tukey’s test
♠♠ Significantly different from group 1 (p<0.01)
a - μmoles of CDNB conjugated with reduced glutathione per min
b - μmoles of DCPIP reduced per min
Acknowledgments
This work was funded by a grant from the Mitsui Norin Co., Ltd., Tokyo, Japan.
References
- 1.Luch A. Nature and nurture – Lessons from chemical carcinogenesis. Nature Rev. 2005;5:113–125. doi: 10.1038/nrc1546. [DOI] [PubMed] [Google Scholar]
- 2.Shimada T. Xenobiotic-metabolising enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet. 2006;21:257–276. doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- 3.Chacko P, Joseph T, Mathew BS, Rajan B, Pillai MR. Role of xenobiotic metabolizing gene polymorphisms in breast cancer susceptibility and treatment outcome. Mutat Res. 2005;581:153–163. doi: 10.1016/j.mrgentox.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Moon YJ, Wang X, Morris ME. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 5.Smith-Warner S, Genkinger J, Giovannucci E. Fruit and vegetable consumption and cancer. Nutr Oncol. 2006:97–173. [Google Scholar]
- 6.Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katiyar SK, Mukhtar H. Tea in chemoprevention of cancer: epidemiological and experimental studies (review) Int J Oncol. 1996;8:221–238. doi: 10.3892/ijo.8.2.221. [DOI] [PubMed] [Google Scholar]
- 8.Shklar G. Development of experimental oral carcinogenesis and its impact on current oral cancer research. J Dent Res. 1999;78:1768–1772. doi: 10.1177/00220345990780120101. [DOI] [PubMed] [Google Scholar]
- 9.Balasenthil S, Saroja M, Ramachandran CR, Nagini S. Of humans and hamsters: A comparative analysis of lipid peroxidation, glutathione and glutathione dependent enzymes during oral carcinogenesis. Br J Oral Maxillofacial Surg. 2000;38:267–270. doi: 10.1054/bjom.1999.0445. [DOI] [PubMed] [Google Scholar]
- 10.Bhuvaneswari V, Abraham SK, Nagini S. Combinatorial antigenotoxic and anticarcinogenic effects of tomato and garlic through modulation of xenobiotic-metabolizing enzymes during hamster buccal pouch carcinogenesis. Nutr. 2005;21:726–731. doi: 10.1016/j.nut.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Chandra Mohan KVP, Devaraj H, Prathiba D, Hara Y, Nagini S. Antiproliferative and apoptosis inducing effect of lactoferrin and black tea polyphenol combination on hamster buccal pouch carcinogenesis. Biochim Biophys Acta. 2006;1760:1536–1544. doi: 10.1016/j.bbagen.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Vidjaya Letchoumy P, Chandra Mohan KVP, Kumaraguruparan R, Hara Y, Nagini S. Black tea polyphenols protect against 7,12-dimethylbenz[a]anthracene induced hamster buccal pouch carcinogenesis. Oncol Res. 2006;16:167–178. doi: 10.3727/000000006783981116. [DOI] [PubMed] [Google Scholar]
- 13.Vidjaya Letchoumy P, Subapriya R, Abraham SK, Nagini S. Protective effect of black tea polyphenols against 7,12-dimethylbenz[a]anthracene-induced genotoxicity and oxidative stress during hamster buccal pouch carcinogenesis. Toxicol Mech Methods. 2007;17:93–100. doi: 10.1080/15376510600860193. [DOI] [PubMed] [Google Scholar]
- 14.Caderni G, Filippo CD, Luceri C, Salvadori M, Giannini A, Biggeri A, Remy S, Cheynier V, Dolara P. Effects of black tea, green tea and wine extracts on intestinal carcinogenesis induced by azoxymethane in F344 rats. Carcinogenesis. 2000;21:1965–1969. doi: 10.1093/carcin/21.11.1965. [DOI] [PubMed] [Google Scholar]
- 15.Ryu EK, Choe YS, Kim DH, Ko B-H, Choi Y, Lee K-H, Kim B-T. In vitro metabolism studies of 18F-labeled 1-phenylpiperazine using mouse liver S9 fraction. Nuclear Med Biol. 2006;33:165–172. doi: 10.1016/j.nucmedbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Yoshihara S, Makishima M, Suzuki N, Ohta S. Metabolic activation of bisphenol A by rat liver S9 fraction. Toxicol Sci. 2000;62:221–227. doi: 10.1093/toxsci/62.2.221. [DOI] [PubMed] [Google Scholar]
- 17.Ames BN, Durston WE, Yamasaki E, Lee FD. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci USA. 1973;70:2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omura T, Sato R. The carbon monoxide binding pigment of liver. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 19.Baer-Dubowska W, Szaefer H, Krajka-Kuzniak V. Inhibition of murine hepatic cytochrome P450 activities by natural and synthetic phenolic compounds. Xenobiotica. 1998;28:735–743. doi: 10.1080/004982598239155. [DOI] [PubMed] [Google Scholar]
- 20.Burke MD, Thompson S, Elcombe CR, Halpert J, Haparata T, Mayer RT. Ethoxy-, pentoxy- and benzyloxy phenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P450. Biochem Pharmacol. 1985;34:3337–3345. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- 21.Habig WH, Pabst M, Jakoby WB. Glutathione S-transferases, the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:130–139. [PubMed] [Google Scholar]
- 22.Ernster L. Methods Enzymol. Vol. 10. Academic Press; New York: 1967. DT-diaphorase; pp. p309–317. [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Nishikawa A, Mori Y, Lee IS, Tanaka T, Hirose M. Cigarette smoking, metabolic activation and carcinogenesis. Curr Drug Metab. 2004;5:363–373. doi: 10.2174/1389200043335441. [DOI] [PubMed] [Google Scholar]
- 25.Rojas M, Alexandrov K, Cascorbi I, Brockmoller J, Likhachev A, Pozharisski K, Bouvier G, Auburtin G, Mayer L, Kopp-Schneider A, Roots I, Bartsch H. High benzo[a]pyrene diol-epoxide DNA adduct levels in lung and blood cells from individuals with combined CYP1A1 MspI/Msp–GSTM1*0/*0 genotypes. Pharmacogenetics. 1998;8:109–118. [PubMed] [Google Scholar]
- 26.Li D, Firozi PF, Zhang W, Shen J, DiGiovanni J, Laud S, Evans D, Friess H, Hassan M, Abbruzzese JL. DNA adducts, genetic polymorphisms, and K-ras mutation in human pancreatic cancer. Mutat Res. 2002;513:37–48. doi: 10.1016/s1383-5718(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 27.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 28.Ahlgren-Beckendorf JA, Reising AM, Schander MA, Herdler JW, Johnson JA. Coordinate regulation of NAD(P)H:quinone oxidoreductase and glutathione-S-transferases in primary cultures of rat neurons and glia: role of the antioxidant/electrophile responsive element. Glia. 1999;25:131–42. [PubMed] [Google Scholar]
- 29.Rajapaksa KR, Sipes IG, Hoyer PB. Involvement of microsomal epoxide hydrolase enzyme in ovotoxicity caused by 7,12-dimethylbenz[a]anthracene. Toxicol Sci. 2007;96:327–334. doi: 10.1093/toxsci/kfl202. [DOI] [PubMed] [Google Scholar]
- 30.Duarte MP, Palma BB, Gilep AA, Laires A, Olivera JS, Usanov SA, Rueff J, Kranendonk M. The stimulatory role of human cytochrome b5 in the bioactivation of human CYP1A2, 2A6 and 2E1: a new cell expression system to study cytochrome P450-mediated biotransformation. Mutagenesis. 2007;22:75–81. doi: 10.1093/mutage/gel054. [DOI] [PubMed] [Google Scholar]
- 31.Buters JTM, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, Doehmer J, Ward JM, Jefcoate CR, Gonzalez FJ. Cytochrome P450 CYP1B1 determines susceptibility to 7,12-dimethylbenz[a]anthracene-induced lymphomas. Proc Natl Acad Sci USA. 1999;96:1977–1982. doi: 10.1073/pnas.96.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez FJ. The use of gene knock out mice to unravel the mechanisms of toxicity and chemical carcinogenesis. Toxicol Lett. 2001;120:199–208. doi: 10.1016/s0378-4274(01)00296-x. [DOI] [PubMed] [Google Scholar]
- 33.Fravo C. Glutathione transferases in the genomic era: new insights and perspectives. Biomol Eng. 2006;23:149–169. doi: 10.1016/j.bioeng.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Vasiliou V, Ross D, Neber DW. Update of the NAD(P)H:quinone oxidoreductase (NQO) gene family. Hum Genomics. 2006;2:329–335. doi: 10.1186/1479-7364-2-5-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin H. Synergistic mechanism in carcinogenesis by polycyclic aromatic hydrocarbons and by tobacco smoke: a bio-historical perspective with updates. Carcinogenesis. 2001;22:1903–1930. doi: 10.1093/carcin/22.12.1903. [DOI] [PubMed] [Google Scholar]
- 36.Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G–T and A–C substitutions. J Biol Chem. 1992;267:166–172. [PubMed] [Google Scholar]
- 37.Nakae D, Andoh N, Mizumoto Y, Endoh T, Shimoji N, Horiguchi K, Shiraiwa K, Tamura K, Denda A, Konishi Y. Selective 8-hydroxyguanine formation in pancreatic DNA due to a single intravenous administration of 4-hydroxyaminoquinoline 1-oxide in rats. Cancer Lett. 1994;83:97–103. doi: 10.1016/0304-3835(94)90304-2. [DOI] [PubMed] [Google Scholar]
- 38.Begleiter A, Fourie J. Induction of NQO1 in cancer cells. Methods Enzymol. 2004;382:320–351. doi: 10.1016/S0076-6879(04)82018-4. [DOI] [PubMed] [Google Scholar]
- 39.Ali S, El-Rayes BF, Heilbrun LK, Sarkar FH, Ensley JF, Kucuk O, Philip PA. Cytochrome P450 and glutathione transferase expression in squamous cell cancer. Clin Cancer Res. 2004;10:4412–4416. doi: 10.1158/1078-0432.CCR-04-0145. [DOI] [PubMed] [Google Scholar]
- 40.Williams JA, Phillips DH. Mammary expression of xenobiotic metabolizing enzymes and their potential role in breast cancer. Cancer Res. 2000;60:4667–4677. [PubMed] [Google Scholar]
- 41.Wang ZY, Wang LD, Lee MJ, Ho CT, Huang MT, Conney AH, Yang CS. Inhibition of N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in rats by green and black tea. Carcinogenesis. 1995;16:2143–2148. doi: 10.1093/carcin/16.9.2143. [DOI] [PubMed] [Google Scholar]
- 42.Cao J, Xu Y, Chen J, Klaunig JE. Chemopreventive effects of green and black tea on pulmonary and hepatic carcinogenesis. Fundam Appl Toxicol. 1996;29:244–250. doi: 10.1006/faat.1996.0028. [DOI] [PubMed] [Google Scholar]
- 43.De Flora S, Izzotti A, D’Agostini F, Balansky RM, Noonan D, Albini A. Multiple points of intervention in the prevention of cancer and other mutation-related diseases. Mutat Res. 2001;480–481:9–22. doi: 10.1016/s0027-5107(01)00165-8. [DOI] [PubMed] [Google Scholar]
- 44.Krishnan R, Maru GB. Inhibitory effect(s) of polymeric black tea polyphenols on the formation of B(a)P-derived DNA adducts in mouse skin. J Environ Pathol Toxicol Oncol. 2005;24:79–90. doi: 10.1615/jenvpathtoxoncol.v24.i2.20. [DOI] [PubMed] [Google Scholar]
- 45.Steele VE, Kelloff GJ, Balentine D, Boone CW, Mehta R, Bagheri D, Sigman CC, Zhu S, Sharma S. Comparative chemopreventive mechanisms of green tea, black tea and selected polyphenol extracts measured by in vitro bioassays. Carcinogenesis. 2000;21:61–67. doi: 10.1093/carcin/21.1.63. [DOI] [PubMed] [Google Scholar]
- 46.Sengupta A, Ghosh S, Das S. Tea can protect against aberrant crypt foci formation during azoxymethane induced rat colon carcinogenesis. J Exp Clin Cancer Res. 2003;22:185–191. [PubMed] [Google Scholar]
- 47.Vidjaya Letchoumy P, Chandra Mohan KVP, Nagini S. Antioxidative potential of black tea polyphenols in vitro and protective effects in vivo on mitochondrial redox status during experimental oral carcinogenesis. Arch Med Sci. 2007 (In press) [Google Scholar]
- 48.Krajka-Kuzniak V, Baer-Dubowska W. The effects of tannic acid on cytochrome P450 and phase II enzymes in mouse liver and kidney. Toxicol Lett. 2003;143:209–216. doi: 10.1016/s0378-4274(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 49.Shi T, Wang ZY, Theresa JS, Hong JY, Chen WF, Ho CT, Yang CS. Effect of green and black tea on 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone bioactivation, DNA methylation and lung tumorigenesis in A/J mice. Cancer Res. 1994;54:4641–4647. [PubMed] [Google Scholar]
- 50.Catteral F, Copeland E, Clifford MN, Loannides C. Contribution of theaflavins to the antimutagenicity of black tea: their mechanism of action. Mutagenesis. 1998;13:631–638. doi: 10.1093/mutage/13.6.631. [DOI] [PubMed] [Google Scholar]
- 51.Saha P, Das S. Elimination of deleterious effects of free radicals in murine skin carcinogenesis by black tea infusion, theaflavins and epigallocatechin gallate. Asian Pac J Cancer Prev. 2002;3:225–230. [PubMed] [Google Scholar]
- 52.Fukuda I, Sakane I, Yabushita Y, Sawamura S, Kanazawa K, Ashida H. Black tea theaflavins suppress dioxin-induced transformation of the aryl hydrocarbon receptor. Biosci Biotechnol Biochem. 2005;69:883–890. doi: 10.1271/bbb.69.883. [DOI] [PubMed] [Google Scholar]
- 53.Luo H, Tang L, Tang M, Billam M, Huang T, Yu J, Wei Z, Liang Y, Wang K, Zhang ZQ, Wang JS. Phase II a chemoprevention trial of green tea polyphenols in high risk individual of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27:262–26. doi: 10.1093/carcin/bgi147. [DOI] [PubMed] [Google Scholar]