Abstract
Radiation therapy is a widely used cancer treatment, but it causes side effects even when localized radiotherapy is used. Extensive apoptosis of microvascular endothelial cells of the lamina propria is the primary lesion initiating intestinal radiation damage after abdominal radiation therapy. Many in vitro studies suggest that angiopoietin-1 (Ang1) has potential therapeutic applications in enhancing endothelial cell survival. For in vivo use, we developed a soluble, stable, and potent Ang1 variant, COMP-Ang1. COMP-Ang1 is more potent than native Ang1 in phosphorylating the Tie2 receptor in lung endothelial cells in vivo. Interestingly, COMP-Ang1 administered i.v. was mainly localized to microvascular endothelial cells of the intestinal villi and lung but not to microvascular endothelial cells of the liver. In irradiated mice, i.v. COMP-Ang1 protected against radiation-induced apoptosis in microcapillary endothelial cells of the intestinal villi and prolonged survival. Thus, COMP-Ang1 could be used as a therapeutic protein for specific protection against endothelial cell injury.
Approximately 50% of cancer patients receive radiation therapy. Side effects due to radiotherapy are unavoidable, even with localized radiotherapy. Gastrointestinal tract damage during abdominal radiotherapy limits the dose that can be used during cancer treatment. According to a recent report (1), extensive apoptosis of microvascular endothelial cells of the lamina propria is the primary lesion initiating intestinal radiation damage. Radiation damage to vascular endothelial cells can be prevented by vascular endothelial growth factor (2) or basic fibroblast growth factor (1). However, vascular endothelial growth factor receptors are expressed in endothelial cells that are actively involved in vasculogenesis and angiogenesis, including tumor progression (3), and basic fibroblast growth factor receptors are expressed in both endothelial and nonendothelial cells, including cancer cells (1). Therefore, administration of vascular endothelial growth factor and basic fibroblast growth factor for protection of endothelial damage may enhance tumor progression, so their usefulness is limited.
Angiopoietin-1 (Ang1) has been identified as a secreted protein ligand of tyrosine kinase with Ig and epidermal growth factor homology domain 2 (Tie2) (4, 5). Tie2 is a member of the receptor tyrosine kinase family and is expressed predominantly on vascular endothelial cells, early hematopoietic cells, and their embryonic precursors (6, 7). In adults, Tie2 is expressed selectively in both its active and inactive form in the endothelial cells of blood vessels (8). In a series of in vitro experiments, we found that the Ang–Tie2 system in normal adult blood vessels was important in maintaining the integrity of nonproliferating endothelial cells by inducing strong endothelial cell survival against insult-mediated damage (9, 10). Ang1–Tie2-induced endothelial cell survival was mediated mainly by means of the intracellular phosphatidylinositol 3′-kinase–Akt signaling pathway (11, 12). Therefore, a controlled activation of Tie2 by precise administration of Ang1 protein could provide therapeutic benefit to microvascular endothelial cells of the lamina propria after intestinal radiation therapy.
Large-scale production of recombinant Ang1 is hindered by the aggregation and insolubility of the protein. The activity of the protein frequently varies after purification. These difficulties are due to its unique structural characteristics. In the accompanying article, we have shown the development of a soluble, stable, and potent Ang1 variant, COMP-Ang1. To create this protein, we replaced the N-terminal portion of Ang1 with the short coiled-coil domain of cartilage oligomeric matrix protein (COMP). COMP-Ang1 is more potent than native Ang1 in phosphorylating the Tie2 receptor and Akt in primary cultured endothelial cells. Thus, COMP-Ang1 is an effective alternative to native Ang1 for therapeutic application in vivo. In the present study, we found that, in irradiated mice, i.v. COMP-Ang1 protected against radiation-induced apoptosis in microcapillary endothelial cells of the intestinal villi and prolonged survival. Therefore, we conclude that COMP-Ang1 could be used as a therapeutic protein for specific protection against endothelial cell injury.
Methods
Animals. Male FVB mice (The Jackson Laboratory), 10–12 weeks old, were used for all experiments. Animal care and experimental procedures were performed with approval from the Animal Care Committees of Korea Advanced Institute of Science and Technology and Chonbuk National University. Tie2 expression and phosphorylation assays in mouse tissues were performed as described (12, 13).
Immunohistochemistry and Immunoblotting. After the mice were killed, the tissues were frozen in OCT in methyl-butane (Sigma–Aldrich) on dry ice or fixed in 10% neutral buffered formalin and embedded in paraffin. Frozen tissue blocks were sectioned at 10 μm, and the sections were incubated with anti-Tie2 Ab (C-20; Santa Cruz Biotechnology) or anti-PECAM-1 Ab (MEC13.3; BD Biosciences, PharMingen) at 4°C overnight. For immunohistochemical localization of administered COMP-Ang1 or native Ang1 after i.v. administrations, mice were given COMP-Ang1 (30 μg) or native Ang1 (60 μg) and killed after 10 min or at the indicated times. Organs were harvested, cryo-sectioned, fixed, and immunostained with anti-Ang1 polyclonal Ab (PA-hAng1FD-1). Signals were visualized with the Cell and Tissue Staining kit (R & D Systems) and counterstained with Meyer's hematoxylin. The PA-hAng1FD-1 Ab was produced by immunization of rabbit by using recombinant human Ang1 protein as an antigen. PA-hAng1FD-1 is specific to the fibrinogen domain of human and mouse Ang1 and does not crossreact with other Angs. It does work to detect exogenous COMP-Ang1 and endogenous Ang1 by immunoblotting, but it has relatively low sensitivity for detecting endogenous Ang1 in tissue by immunohistochemistry.
Irradiation, Treatment, and Survival Assay. Radiation was delivered with a 6-MeV (1 eV = 1.602 × 10–19 J) linear accelerator (NELac 1006X, NEC, Tokyo) at a dose of 12–15 Gy. For protein injection, mice were injected i.v. with 100 μg of COMP-Ang1 in 33-μg doses delivered 30 min before irradiation and 30 and 90 min after irradiation. For control mice, 100 μg of BSA was injected at the same time points. Survival was monitored every 12 h after irradiation. Actuarial survival was calculated by the product limit Kaplan–Meier method (14), and P values were evaluated by two-tailed Fisher's exact test.
Apoptosis Assay. To identify apoptotic endothelial cells, paraffin tissue blocks containing irradiated intestinal tissues were sectioned 6-μm thick and were stained by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling method according to the manufacturer's protocol (Intergen, Norcross, GA). Apoptotic nuclei were visualized by using Vector NovaRed chromogen (Vector Laboratories). The deoxynucleotidyl transferase-mediated dUTP nick-end labeling-stained sections were incubated with anti-PECAM-1 Ab for 2 h at room temperature and developed with biotinylated anti-rat Ab and avidin–biotin peroxidase complex. PECAM-1-positive cells were visualized by using SG chromogen (Vector Laboratories), which gives blue-gray staining. The microcapillary endothelial cells and apoptotic cells in the small intestinal villi were viewed, counted, and photographed with an Axioskop2 Plus microscope (Zeiss) equipped with a ProgResC14 color charge-coupled device camera (Jenoptik, Jena, Germany) and monitor. Two independent investigators who were unaware of the experimental conditions counted apoptotic endothelial cells in ≈1,400–1,600 villi from seven or eight different animals (≈200 villi per mouse) for each group. Interinvestigator variation was <5%.
Statistics. Data are expressed as mean ± SD. Statistical significance was tested by using one-way ANOVA followed by the Student–Newman–Keuls test. Statistical significance was set at P < 0.05.
Results
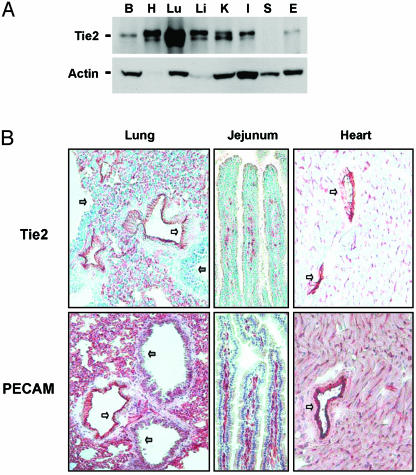
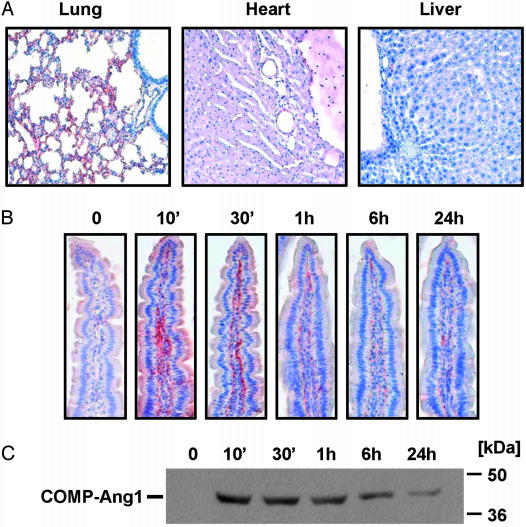
Distribution and Localization of COMP-Ang1 After I.V. Administration. Examination of the tissue expression of Tie2 in adult mouse revealed that the protein is most abundant in lung (Fig. 1A). Immunohistochemical analysis revealed that Tie2 is expressed in most endothelial cells of large vessels in most organs and also in the microcapillary endothelial cells in lung and intestinal villi, as evidenced by immunostaining to endothelial cell surface marker PECAM-1 (Fig. 1B). To determine the distribution and localization of native Ang1 and COMP-Ang1 in adult mouse tissues, we performed immunohistochemical localization after i.v. administration of native Ang1 and COMP-Ang1. To localize the proteins, we used Ab PA-hAng1FD-1, which recognizes human, but not rodent, Ang1. At 10 min, an abundant amount of COMP-Ang1 was located in microvascular and macrovascular endothelial cells but not in alveolar epithelial cells of lung (Fig. 2A). A moderate amount of COMP-Ang1 was located in the microvascular and macrovascular endothelial cells and in the pericardium of the heart, whereas almost no COMP-Ang1 was in the liver (Fig. 2 A). Interestingly, a notable amount of COMP-Ang1 was located in microvascular endothelial cells of the lamina propria endothelial cells of the small intestine (Fig. 2B). Similar findings were observed up to 24 h, although the amount of protein had deceased in the lung (data not shown) and intestinal villi (Fig. 2 B and C). This decrease was evident in both the immunohistochemistry and immunoblot analyses (Fig. 2 B and C). Thus, the distribution and localization of COMP-Ang1 is specific to the microvascular endothelial cells of certain organs. Its localization is well correlated with Tie2 distribution. In comparison, native Ang1 was mainly located in macrovascular endothelial cells of each organ at 10 min after i.v. administration, and its location was not correlated with Tie2 distribution up to 24 h (data not shown). Therefore, we conclude that i.v. administration of COMP-Ang1 demonstrated excellent pharmacokinetics compared with i.v. administration of native Ang1.
Fig. 1.
Distribution and localization of Tie2. (A) Distribution of Tie2 in several organs including brain (B), heart (H), lung (Lu), liver (Li), kidney (K), small intestine (I), spleen (S), and ear (E) of adult mice. Protein lysates from each organ were immunoprecipitated and immunoblotted with anti-Tie2 Ab (Upper). Each protein lysate was also immunoblotted with anti-actin Ab to verify equal amounts of total protein (Lower). (B) Immunohistochemical staining of Tie2 counterstained with methyl green and PECAM-1 counterstained with Meyer's hematoxylin. Most Tie2 staining corresponds to PECAM staining in alveolar capillary endothelial cells (red-brown). Unstained cells are alveolar epithelial cells. White arrows indicate large blood vessels with positive staining. Gray arrows indicate bronchioles with negative staining.
Fig. 2.
COMP-Ang1 injected i.v. localizes in microvascular endothelial cells of lung and intestinal villi. (A) Mice were given COMP-Ang1 (30 μg) i.v. and killed after 10 min. The indicated tissues were immunostained with PA-hAng1FD-1 Ab and counterstained with Meyer's hematoxylin. Reddish-brown indicates immunopositive signals. (B) Mice were given COMP-Ang1 (30 μg) i.v. and killed at the indicated times. Intestinal villi were stained as described in A.(C) Part of the jejunum was homogenized, 100 μg of protein homogenates was separated by SDS/PAGE, and the quantity of COMP-Ang1 was measured by immunoblotting with PA-hAng1FD-1 Ab. Results were similar from three independent experiments. Numbers and bars on the right indicate molecular sizes (kDa).
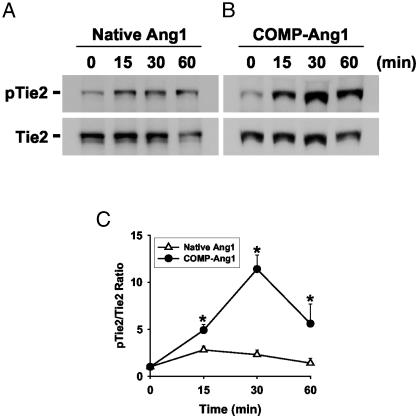
Acute Injection of COMP-Ang1 Induces Tie2 Phosphorylation in Lung. Based on the pharmacokinetic data, we examined the effect of acute administration of native Ang1 and COMP-Ang1 on Tie2 activation in vivo. We chose to use samples from lung because our current method could not detect Tie2 phosphorylations in intestinal villi. Tie2 phosphorylation induced by COMP-Ang1 was not only greater but also significantly more persistent than that of native Ang1 (Fig. 3). Consistent results were obtained also for the phosphorylation of Akt (data not shown). Thus, COMP-Ang1 is more potent than native Ang1 for activation of Tie2 in vivo. Moreover, these data imply that acute administration of COMP-Ang1 could activate Tie2 in most Tie2-expressing endothelial cells in vivo.
Fig. 3.
i.v. injection of COMP-Ang1 induces Tie2 phosphorylation in lung. In vivo phosphorylation of Tie2 stimulated by i.v. injection of native Ang1 (60 μg) (A) or COMP-Ang1 (30 μg) (B) assayed at the indicated times after injection. Tie2 was immunoprecipitated from lung protein lysate and immunoblotted with anti-phosphotyrosine to detect phosphorylated Tie2 (pTie2; Upper). The membrane was stripped and reprobed with anti-Tie2 Ab (Lower) to verify the equal loading of protein in each lane. (C) The relative ratio measured at time 0 is presented arbitrarily as 1. Datapoints represent the mean ± SD from five experiments. *, P < 0.05, compared with native Ang1.
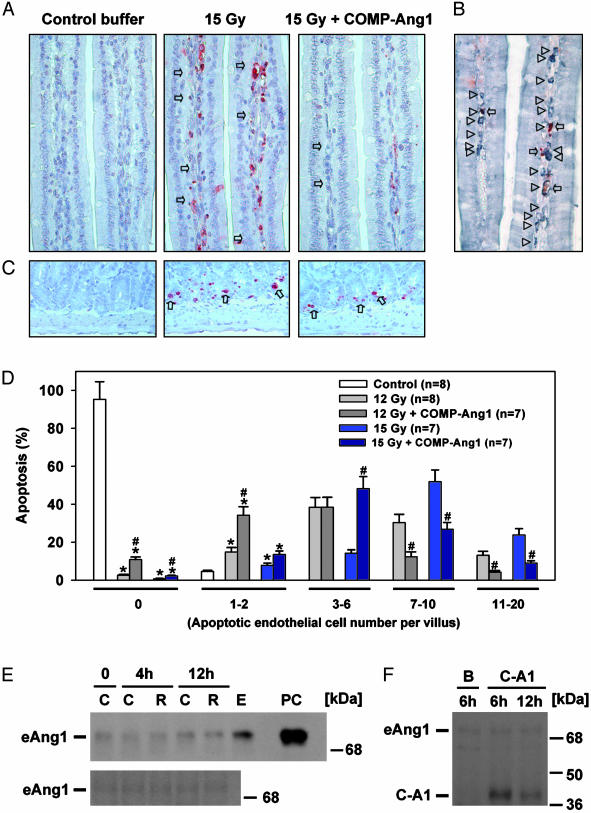
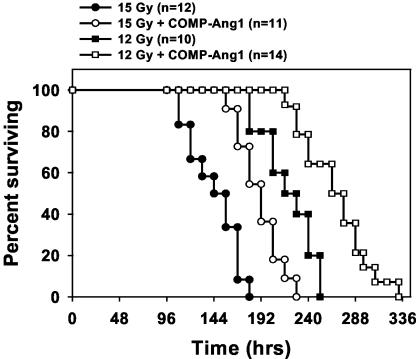
COMP-Ang1 Protects Against Radiation-Induced Apoptosis and Death. We examined the effect of i.v. treatment with COMP-Ang1 on microvascular endothelial survival in a murine whole-body irradiation model. The deoxynucleotidyl transferase-mediated dUTP nick-end labeling of intestinal specimens of FVB mice 4 h after irradiation (12 Gy and 15 Gy) showed maximal and extensive endothelial apoptosis in villi (Fig. 4), which is consistent with ref. 1. The i.v. injection of COMP-Ang1 into FVB mice immediately before and after irradiation with 12 or 15 Gy reduced extensive apoptotic endothelial damage dramatically (from 7–20 apoptotic cells per villus to 1–6 apoptotic cells per villus), but it did not affect apoptosis of intestinal epithelial cells and crypt cells (Fig. 4). Although we found extensive apoptosis in thymus, spleen, salivary gland cells, rectal cryptic cells, lymphoid tissues, and polymorphonuclear cells in blood after irradiation, the treatment with COMP-Ang1 had no significant effect on these nonendothelial cells (data not shown). Thus, COMP-Ang1 provides strong protection against irradiation damage specifically to Tie2-expressing endothelial cells. To exclude the possibility that endogenous Ang1 was protecting against endothelial apoptosis, we compared the levels of endogenous Ang1 in the intestine and lung by immunoblotting before and after whole-body irradiation (15 Gy). There were no notable changes in the levels of endogenous Ang1 at 4 h and 12 h after irradiation (Fig. 4E). Moreover, the levels of exogenous COMP-Ang1 were ≈8.2-fold and 4.3-fold higher than endogenous Ang1 in the intestine even at 6 h and 24 h after i.v. injection of COMP-Ang1 (100 μg) (Fig. 4F). Therefore, the observed protection against irradiation damage to endothelial cells is due to exogenous COMP-Ang1, not changes in endogenous Ang1. Accordingly, the actuarial survival of 15 Gy-irradiated mice increased from a mean of 144 ± 7 h (median, 144 h) in control mice to 190 ± 8 h (median, 192 h) in mice treated with i.v. COMP-Ang1 (Fig. 5). The survival of 12 Gy-irradiated mice after i.v. COMP-Ang1 treatment increased from a mean of 220 ± 9 h (median, 216 h) to 258 ± 11 h (median, 260 h). Thus, i.v. treatment with COMP-Ang1 could be very effective for suppressing side effects of radiation therapy in the gastrointestinal tract by protecting against microvascular endothelial apoptosis in intestinal villi.
Fig. 4.
COMP-Ang1 protects against radiation-induced microvascular apoptosis and death without changes of endogenous Ang1. (A) Mice were given whole-body irradiation with 15 Gy and killed after 4 h. Whereas small intestinal villi from control mice have almost no apoptosis, villi from irradiated mice reveal extensive apoptosis (arrows) in lamina propria cells. Injection of COMP-Ang1 markedly reduced radiation-induced apoptosis in lamina propria cells. (B) Costaining of PECAM-1 (gray-pastel; arrowheads), and deoxynucleotidyl transferase-mediated dUTP nick-end labeling (red-brown; arrows) allows for counting the apoptotic endothelial cells among the lamina propria cells of small-intestinal villi of the irradiated mice. (C) Whereas small intestinal crypts from control mice have almost no apoptosis, crypts from irradiated mice reveal extensive apoptosis (arrows) in the lamina propria. Injection of COMP-Ang1 did not reduce radiation-induced apoptosis in crypt cells. (D) Histograms of percentge of apoptotic endothelial cells in the lamina propria of ≈1,400–1,600 villi from each group (200 villi per mouse). Small-intestinal specimens were obtained at 4 h after 12 or 15 Gy irradiation with or without i.v. injection of COMP-Ang1. Apoptotic endothelial cells were counted in each villus, categorized (0, 1–2, 3–6, 7–10, or 11–20), and calculated as a percentage with 200 villi. Bars represent the mean ± SD. from each group. *, P < 0.05 versus control in 0 or 1–2.#, P < 0.05 versus 12 or 15 Gy irradiation only. (E) Mice were given whole-body irradiation with 15 Gy and killed at 4 h and 12 h. Part of the jejunum (Upper) and lung (Lower) was homogenized, 200 μg of protein homogenates was separated by SDS/PAGE, and the relative amount of endogenous Ang1 (eAng1) was determined by immunoblotting. Results were similar from three independent experiments. C, control; R, irradiation; E, 100 μg of protein homogenates from mouse embryonic day 12; PC, 20 ng of recombinant mouse native Ang1 as a positive control. Numbers and bars on the right indicate molecular masses (kDa). (F) Mice were given whole-body irradiation with 15 Gy and killed after 6 h or 24 h. The mice were injected i.v. with 100 μg of COMP-Ang1 (C-A1) in 33-μg doses delivered 30 min before, 30 min after, and 90 min after irradiation. For control mice, 100 μg of BSA (B) was injected at the same time points. Part of the jejunum was homogenized, 200 μg of protein homogenates were separated by SDS/PAGE, and the relative amount of COMP-Ang1 and endogenous Ang1 (eAng1) was determined by immunoblotting. Results were similar from three independent experiments. B, BSA at 6 h. Numbers and bars on the right indicate molecular masses (kDa).
Fig. 5.
Treatment with COMP-Ang1 prolongs survival in mice irradiated with 12 or 15 Gy. Survival was monitored every 12 h after irradiation. Numbers in parentheses indicate the number of animals per group. Two-tailed Fisher's exact test was performed to compare control and COMP-Ang1-treated mice (P = 0.002 for 12 Gy; P = 0.003 for 15 Gy).
Discussion
The integrity of the vascular endothelium in response to physical, biochemical, and immune-mediated damage is important to maintaining endothelial function and preventing vascular diseases (15). A recent report indicated that extensive apoptosis of microvascular endothelial cells of the lamina propria is the primary lesion initiating intestinal radiation damage (1). Thus, gastrointestinal tract damage during abdominal radiotherapy limits the dose that can be used during cancer treatment. The present study has shown that COMP-Ang1 treatment strongly protects against extensive radiation-induced endothelial apoptosis in villi but has no observed effect on nonendothelial cells. Considering that there were no notable changes in endogenous Ang1 levels in intestinal tissues after irradiation, and the levels of exogenous COMP-Ang1 were much higher than endogenous Ang1 in the irradiated intestines even at 6 h and 24 h after administration of COMP-Ang1, exogenous COMP-Ang1 per se provides a strong protection against irradiation damage to the endothelial cells. Given that the Tie2–phosphatidylinositol 3′-kinase–Akt system is mainly involved in endothelial cell survival (9–12), COMP-Ang1-induced Tie2–phosphatidylinositol 3′-kinase–Akt activation could be the main mechanism for protecting against the endothelial apoptosis in villi. In addition, COMP-Ang1 treatment prolongs survival after irradiation perhaps as a result of decreasing damage to the gastrointestinal tract. Studies have shown that radiation damage to vascular endothelial cells can be prevented by vascular endothelial growth factor (2) or basic fibroblast growth factor (1). However, these angiogenic factors may help tumor progression. Although the Ang1–Tie2 system is involved actively in angiogenesis during embryonic development and ischemic conditions (16–19), the role of Ang1 in tumor angiogenesis is still controversial. It has even been suggested that Ang1 may suppress tumor progression by means of the “stabilization” of tumor vessels (20–22). Therefore, the use of Ang1 for protection against radiation-induced endothelial cell damage in cancer patients could be beneficial.
In this study, we were able to show the distribution and localization of administered COMP-Ang1 and native Ang1 in several tissues by using a novel anti-Ang1 Ab (PA-hAng1FD-1). Notably, most administered COMP-Ang1 was distributed and localized to microvascular endothelial cells of the lung, heart, and intestinal villi but not to microvascular endothelial cells of the liver. Considering the tissue expression pattern of Tie2, it appears that administered COMP-Ang1 was able to actively access, bind, and activate Tie2 in Tie2-expressing tissues in vivo. In comparison, most administered native Ang1 was distributed and localized in macrovascular endothelial cells and did not reach the microvascular endothelial cells efficiently. Thus, in all experiments performed so far, the pharmacokinetics of COMP-Ang1 is better than that of native Ang1. In fact, Tie2 activation by COMP-Ang1 in vivo was more potent than by native Ang1. This result could be due not only to the fact that COMP-Ang1 is more potent than native Ang1 but also that COMP-Ang1 has better pharmacokinetics than native Ang1. Moreover, it should be noted that an appreciable amount of COMP-Ang1 still remained in the microendothelial cells of lung up to 24 h after single i.v. administration of COMP-Ang1. Thus, i.v. delivery of COMP-Ang1 could be pharmacologically effective with administration on a daily basis. Optimizing the dosage and route of administration of COMP-Ang1 could further improve endothelial cell survival after radiation-induced endothelial cell damage. In addition, further studies will be needed to assess the effect of COMP-Ang1 on tumors during radiation therapy.
In conclusion, COMP-Ang1 could be used as a therapeutic protein for specific protection against radiation-induced endothelial cell injury in vivo. It seems likely that COMP-Ang1 can be applied further to the prevention of vascular leakage (23, 24), protection against sepsis-induced endothelial cell injury, enhancement of reendothelialization after angioplasty, and in vitro amplification of Tie2 positive endothelial precursor stem cells (7).
Acknowledgments
We thank T. Suda, N. Gale, G. Thurston, and S. G. Kim for critical comments and helpful discussion; the members of Department of Radiation Oncology at Jeonju Presbyterian Medical Center for help in performing radiation to the mice; and Jennifer Macke for help in preparing the manuscript. R.A.K. is a Wellcome Trust Research Career Development Fellow. This work was supported by the Bio-Challenge Program (G.Y.K.) and National Research Laboratory Program Grant 2000-N-NL-01-C-228 of the Korean Ministry of Science and Technology and the Brain Korea 21 Project (to G.M.L.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ang, angiopoietin; COMP, cartilage oligomeric matrix protein; Tie2, tyrosine kinase with Ig and epidermal growth factor homology domain 2.
References
- 1.Paris, F., Fuks, Z., Kang, A., Capodieci, P., Juan, G., Ehleiter, D., Haimovitz-Friedman, A., Cordon-Cardo, C. & Kolesnick, R. (2001) Science 293, 293–297. [DOI] [PubMed] [Google Scholar]
- 2.Okunieff, P., Mester, M., Wang, J., Maddox, T., Gong, X., Tang, D., Coffee, M. & Ding, I. (1998) Radiat. Res. 150, 204–211. [PubMed] [Google Scholar]
- 3.Veikkola, T. & Alitalo, K. (1999) Semin. Cancer Biol. 9, 211–220. [DOI] [PubMed] [Google Scholar]
- 4.Davis, S., Aldrich, T. H., Jones, P. F., Acheson, A., Compton, D. L., Jain, V., Ryan, T. E., Bruno, J., Radziejewski, C., Maisonpierre, P. C., et al. (1996) Cell 87, 1161–1169. [DOI] [PubMed] [Google Scholar]
- 5.Yancopoulos, G. D., Davis, S., Gale, N. W., Rudge, J. S., Wiegand, S. J. & Holash, J. (2000) Nature 407, 242–248. [DOI] [PubMed] [Google Scholar]
- 6.Jones, N., Iljin, K., Dumont, D. J. & Alitalo, K. (2001) Nat. Rev. Mol. Cell. Biol. 2, 257–267. [DOI] [PubMed] [Google Scholar]
- 7.Takakura, N., Watanabe, T., Suenobu, S., Yamada, Y., Noda, T., Ito, Y., Satake, M. & Suda, T. (2000) Cell 102, 199–209. [DOI] [PubMed] [Google Scholar]
- 8.Wong, A. L., Haroon, Z. A., Werner, S., Dewhirst, M. W., Greenberg, C. S. & Peters, K. G. (1997) Circ. Res. 81, 567–574. [DOI] [PubMed] [Google Scholar]
- 9.Kwak, H. J., Lee, S. J., Lee, Y. H., Ryu, C. H., Koh, K. N., Choi, H. Y. & Koh, G. Y. (2000) Circulation 101, 2317–2324. [DOI] [PubMed] [Google Scholar]
- 10.Kim, I., Moon, S. O., Han, C. Y., Pak, Y. K., Moon, S. K., Kim, J. J. & Koh, G. Y. (2001) Cardiovasc. Res. 49, 872–881. [DOI] [PubMed] [Google Scholar]
- 11.Jones, N., Master, Z., Jones, J., Bouchard, D., Gunji, Y., Sasaki, H., Daly, R., Alitalo, K. & Dumont, D. J. (1999) J. Biol. Chem. 274, 30896–30905. [DOI] [PubMed] [Google Scholar]
- 12.Kim, I., Kim, H. G., So, J. N., Kim, J. H., Kwak, H. J. & Koh, G. Y. (2000) Circ. Res. 86, 24–29. [DOI] [PubMed] [Google Scholar]
- 13.Kim, I., Kim, H. G., Moon, S. O., Chae, S. W., So, J. N., Koh, K. N., Ahn, B. C. & Koh, G. Y. (2000) Circ. Res. 86, 952–959. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan, E. & Meier, P. (1958) J. Am. Stat. Assoc. 53, 457–816. [Google Scholar]
- 15.Cines, D. B., Pollak, E. S., Buck, C. A., Loscalzo, J., Zimmerman, G. A., McEver, R. P., Pober, J. S., Wick, T. M., Konkle, B. A., Schwartz, B. S., et al. (1998) Blood 91, 3527–3561. [PubMed] [Google Scholar]
- 16.Suri, C., Jones, P. F., Patan, S., Bartunkova, S., Maisonpierre, P. C., Davis, S., Sato, T. N. & Yancopoulos, G. D. (1996) Cell 87, 1171–1180. [DOI] [PubMed] [Google Scholar]
- 17.Sato, T. N., Qin, Y., Kozak, C. A. & Audus, K. L. (1993) Proc. Natl. Acad. Sci. USA 90, 9355–9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shyu, K. G., Manor, O., Magner, M., Yancopoulos, G. D. & Isner, J. M. (1998) Circulation 98, 2081–2087. [DOI] [PubMed] [Google Scholar]
- 19.Chae, J. K., Kim, I., Lim, S. T., Chung, M. J., Kim, W. H., Kim, H. G., Ko, J. K. & Koh, G. Y. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 2573–2578. [DOI] [PubMed] [Google Scholar]
- 20.Tian, S., Hayes, A. J., Metheny-Barlow, L. J. & Li, L. Y. (2002) Br. J. Cancer 286, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawighorst, T., Skobe, M., Streit, M., Hong, Y. K., Velasco, P., Brown, L. F., Riccardi, L., Lange-Asschenfeldt, B. & Detmar, M. (2002) Am. J. Pathol. 160, 1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoeltzing, O., Ahmad, S. A., Liu, W., McCarty, M. F., Wey, J. S., Parikh, A. A., Fan, F., Reinmuth, N., Kawaguchi, M., Bucana, C. D., et al. (2003) Cancer Res. 63, 3370–3377. [PubMed] [Google Scholar]
- 23.Thurston, G., Suri, C., Smith, K., McClain, J., Sato, T. N., Yancopoulos, G. D. & McDonald, D. M. (1999) Science 286, 2511–2514. [DOI] [PubMed] [Google Scholar]
- 24.Thurston, G., Rudge, J. S., Ioffe, E., Zhou, H., Ross, L., Croll, S. D., Glazer, N., Holash, J., McDonald, D. M. & Yancopoulos, G. D. (2000) Nat. Med. 6, 460–463. [DOI] [PubMed] [Google Scholar]