Abstract
S-nitrosothiols (SNOs) are endogenous signaling molecules with a broad spectrum of beneficial airway effects. SNOs are normally present in the airway, but levels tend to be low in cystic fibrosis (CF) patients. We and others have demonstrated that S-nitrosoglutathione (GSNO) increases the expression, maturation, and function of wild-type and mutant F508del cystic fibrosis transmembrane conductance regulator (CFTR) in human bronchial airway epithelial (HBAE) cells. We hypothesized that membrane permeable SNOs, such as S-nitrosoglutathione diethyl ester (GNODE) and S-nitroso-N-acetyl cysteine (SNOAC) may be more efficient in increasing the maturation of CFTR. HBAE cells expressing F508del CFTR were exposed to GNODE and SNOAC. The effects of these SNOs on the expression and maturation of F508del CFTR were determined by cell surface biotinylation and Western blot analysis. We also found for the first time that GNODE and SNOAC were effective at increasing CFTR maturation at the cell surface. Furthermore, we found that cells maintained at low temperature increased cell surface stability of F508del CFTR whereas the combination of low temperature and SNO treatment significantly extended the half-life of CFTR. Finally, we showed that SNO decreased the internalization rate of F508del CFTR in HBAE cells. We anticipate identifying the novel mechanisms, optimal SNOs, and lowest effective doses which could benefit cystic fibrosis patients.
Keywords: Cystic fibrosis, CFTR, S-nitrosothiol, S-nitrosylation, Chaperones, Molecular therapy
1. Introduction
Cystic fibrosis (CF) is the most common monogenetic disease caused by a mutation in the gene for CF transmembrane regulator (CFTR) protein, a cAMP activated chloride channel present mainly in epithelial cells [1–2]. More than 1500 mutations in the CFTR gene have been identified in CF patients. The most common mutation, found in 90% of CF patients, is F508del CFTR, which results from a deletion of three nucleotides in the gene sequence that codes the first nucleotide binding domain (NBD1). This deletion results in a loss of the amino acid phenylalanine (F) at the position 508 on the protein [1–2], which prevents the protein from folding efficiently. Therefore it accumulates in the rough endoplasmic reticulum (ER) where it is degraded [3–6].
Thus, like other integral membrane glycoproteins, CFTR and F508del CFTR biogenesis initiate with the formation in the rough ER as immature core-glycosylated (~130–140 KDa, known as band B). Properly folded, the immature form of CFTR (20–40%) travels through the Golgi complex, where it undergoes further glycosylation to the mature protein (~170–190 KDa, known as band C). Mature CFTR leaves the Golgi in vesicles that travel directly to the cell membrane [2]. Interestingly, F508del CFTR is synthesized and properly inserted into the membrane of rough ER, but fail to reach the native state and is thus recognized by the ER quality control system, polyubiquitinated, and rapidly degraded by proteasome. Therefore, this mutation affects the function and processing of the CFTR molecules [6].
Previous studies have shown that mutant F508del CFTR is functional [2]. Thus, clinically plausible strategies that increase the maturation of the mutant CFTR will be a potential benefit to majority of CF patients. Studies have revealed that inhibition of F508del CFTR ubiquitination and proteosomal degradation with chemical or pharmacological chaperones promotes its correct folding and channel function at the cell membrane [7–11]. Conditions that promote at least partial rescue of misfolded CFTR from proteosomal degradation include, low temperature [9,10], and introduction of an effective chemical chaperone such as glycerol. The butyrate class of compounds such as 4-phenylbutyrate, efficiently correct F508del CFTR processing, transport, and function in vitro [8]. Current literature suggests that other correctors were shown to be relatively specific for rescuing F508del CFTR [12]. For example, Corr-4, Corr2b, VX-809 and VX-532 promote maturation of F508del CFTR. In addition, multiple molecular chaperones assist in the productive folding of wild-type and mutant forms of CFTR, including heat shock protein 70 (Hsp70) and 90 (Hsp90), heat shock cognate 70 (Hsc70), cysteine string protein (Csp), and Hsp70/Hsp90 organizing protein (Hop) [12,13].
S-nitrosothiols (SNOs) are endogenous cell signaling molecules [14–16] and are present in the lungs; however at lower concentrations in CF patients [17]. SNOs inhibit the ubiquitin proteasome pathway, stabilizing the expression of post-translational degradation-regulated proteins such as hypoxia inducible factor 1 [18]. Because CFTR maturation is regulated in part by degradation, there has been interest in determining whether SNOs can augment CFTR maturation. Previous studies have shown that the endogenous SNO, S-nitrosoglutathione (GSNO) increases cellular expression, maturation, and function of CFTR in human airway epithelial monolayer cultures expressing wild-type and mutant F508del CFTR [13,19–26]. However, since GSNO requires transport into the cell, more membrane permeable SNOs, such as S-nitrosoglutathione diethyl ester (GNODE), and S-nitroso-N-acetyl cysteine (SNOAC) may be more efficient in increasing the expression, maturation, and function of F508del CFTR. Therefore, in the present study, we determined the effects of GNODE, SNOAC and GSNO on F508del CFTR maturation in the cell surface in human bronchial airway epithelial cells.
2. Materials and methods
2.1. Chemicals and reagents
The compounds used in the experiments were obtained from the following: Pepstatin A (Boehringer Mannheim Corp., Indianapolis, IN), Leupeptin and Aprotinin (Roche Diagnostics, Mannheim, Germany), Electrophoresis reagents were from Bio-Rad (Hercules, CA). All other chemicals were obtained from Sigma Chemical Company (St. Louis, MO) unless otherwise stated. GSNO was prepared as previously described [13].
2.2. Cell Culture
Human bronchial airway epithelial (HBAE) cell lines expressing wild-type and mutant F508del CFTR were provided by Dr. Eric Sorscher (University of Alabama). Primary human bronchial airway epithelial (PHBAE) cells expressing wild-type and mutant F508del CFTR were provided by Dr. Scott Randell (University of North Carolina). HBAE cells were grown in DMEM medium and PHBAE cells were grown in bronchial epithelial cell growth medium (BEGM) Bullet Kit (Lonza, Walkersville, MD). Cells were grown at 37 °C in a humidified atmosphere of 5% CO2 in air as described previously [13,19–21].
2.3. Western blotting
Western blot analysis was performed as previously described [13,19–21]. Briefly, whole cell extracts were prepared in 1% NP-40 lysis buffer and insoluble material was recovered and sheared by passage through a 25-gauge needle. Protein was quantitated by the Lowry assay by using protein assay kit (Sigma Chemical Co., St. Louis, MO). 100 μg of protein was fractionated on a 6% SDS polyacrylamide gel. The fractionated proteins were transferred to nitrocellulose membranes and blots were blocked in Tris buffered saline-Tween 20 containing 5% nonfat dried milk. Blots were probed with a 1:1000 dilution of anti-CFTR mAb 596 antibody (a kind gift from Dr. J. R. Riordan, University of North Carolina). Blots were washed and CFTR proteins was visualized by enhanced chemiluminescence (ECL, Amersham) using Hyperfilm (Amersham Pharmacia Biotech). Blots were stripped and probed with anti-α-tubulin antibodies (mouse monoclonal IgM, 1:5000; Biotech, Santa Cruz, CA) as a control for protein loading. Relative quantitation was performed by densitometric analysis of band intensity using Quantity One software (Bio-Rad).
2.4. Cell surface biotinylation
Cell surface biotinylation was performed as previously described [13]. Briefly, cells were treated for 4 h with or without different concentrations of SNOs. The cells were washed (×3) with ice-cold phosphate buffered saline (pH 7.4) containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBSCM) and then treated in the dark with PBSCM buffer containing 10 mM sodium periodate for 30 min at 20 °C The cells were washed (×3) with PBSCM and biotinylated by treating with sodium acetate buffer (100 mM sodium acetate buffer, pH 5.5; 0.1 mM CaCl2 and 1 mM MgCl2) containing 2 mM biotin-LC hydrazide (Pierce, Rockford, IL) for 30 min at 20 °C in the dark. The cells were then washed (×3) with sodium acetate buffer and solubilized with lysis buffer containing Triton X 100 and protease inhibitors. CFTR was immunoprecipitated as described previously [13,20] and subjected to SDS–PAGE on 6% gels. Biotinylated CFTR was detected with streptavidin-conjugated horseradish peroxidase.
2.5. Internalization assay
CFTR internalization assays were performed as described previously [10]. Briefly, HBAE cells were grown at 37 °C to 70% confluence, and then incubated for an additional 48 h at 27 °C in the absence or presence of GSNO (10 μM) for last 4 h. The cells were washed three times with ice-cold phosphate buffered saline (pH 7.4) containing 0.1 mM CaCl2 and 1 mM MgCl2. The glycosidic moieties of cell-surface membrane proteins were derivatized with sodium periodate and biotinylated using biotin-LC hydrazide (Pierce, Rockford, IL) for 30 min. Internalization of F508del CFTR, was conducted by including a 37 °C for 2.5 min incubation after sodium periodate oxidation but before biotinylation with biotin-LC hydrazide. The cells were then washed twice with sodium acetate buffer and solubilized with lysis buffer. CFTR was immunoprecipitated with monoclonal anti-CFTR mAb 596 antibody and subjected to SDS–PAGE on 6% gels. Biotinylated CFTR was detected with streptavidin-conjugated horseradish peroxidase. CFTR internalization was identified as the percentage CFTR remaining in the cell surface during the warm-up period compared with the control.
2.6. Statistics
We conducted two-way ANOVA for each experiment. In each model, we included the main effects of treatment and band, and their interaction. The statistical analyses were performed with SAS 9.1 (SAS Institute Inc., Cary, NC). Multiple comparisons were adjusted by the Dunnett's method. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. S-nitrosoglutathione diethyl ester and S-nitroso-N-acetyl cysteine increase F508del CFTR expression in the cell surface
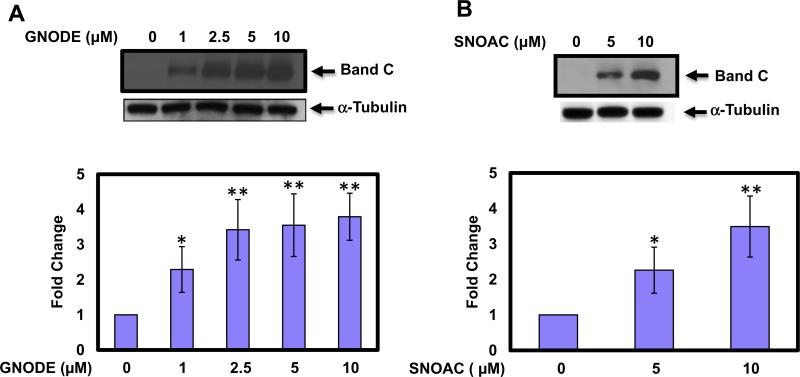
To confirm that mutant F508del CFTR is expressed on the cell surface following treatment with GNODE and SNOAC, we performed cell surface biotinylation and Western blot analysis. Human bronchial airway epithelial cells expressing mutant F508del CFTR treated in the presence or absence of increasing concentrations of GNODE (Fig. 1A) and SNOAC (Fig. 1B) for 4 h. These studies demonstrated that membrane permeable GNODE and SNOAC are also effectively increasing the F508del CFTR expression and maturation. GNODE began to significantly elevated expression of CFTR at low concentration as low concentration as 1 μM (2.7-fold, n = 3; Fig. 1A). However, the maximum increase in CFTR expression by GNODE (5.57-fold, n = 3) and SNOAC (3.1-fold, n = 3) occurred with 10 μM concentrations (Fig. 1A and B).
Fig. 1.
S-nitrosoglutathione diethyl ester and S-nitroso-N-acetyl cysteine increase F508del CFTR expression in human airway epithelial cell surface. HBAE cells were incubated in the absence or presence of GNODE and SNOAC for 4 h. To confirm that the fully glycosylated F508del CFTR was expressed at the cell surface, cells were surface biotinylated using biotin LC-hydrazide assay. CFTR protein expression was measured by immunoblot using anti CFTR mAb 596. The membrane was stripped and re-probed with a-tubulin to verify that equal amounts of protein were added. The optical densities of the bands were quantified by densitometry. Data are the mean ± SD, n = 3, *p < 0.05 **p < 0.001.
3.2. Low temperature and GSNO increase F508del CFTR expression and maturation in F508del CFTR HBAE cells
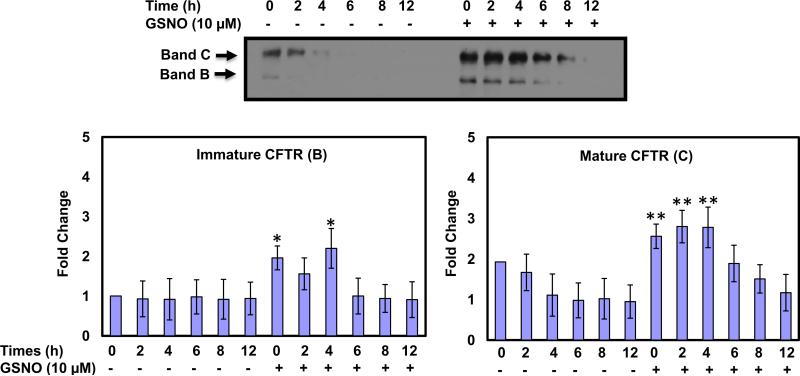
Here, we demonstrated that low temperature and GSNO affect the up-regulation of F508del CFTR expression by quantitative immunoblot analysis. HBAE cells expressing F508del CFTR were grown at 37 °C to 70% confluence, and then incubated for an additional 48 h at 27 °C in the absence or presence of 10 μM GSNO for the last 4 h. After 4 h of treatment, the old media were replaced with a new one without GSNO, and cells were returned to 37 °C incubator for 0, 2, 4, 6, 8, and 12 h. Our results show that the mature forms of F508del CFTR are stable without GSNO until 2 h after return to 37 °C and then expression starts to decline in a time dependent manner (Fig. 2). More importantly, our results show that after 4 h of treatment with 10 μM GSNO in the presence of low temperature (27 °C), both immature (band B) and mature (band C) expression of CFTR was significantly induced and started decline only after 8 h of incubation. At 0 h after treatment with GSNO for 4 h and 27 °C the immature CFTR (band B) induced almost 2-fold (n = 3) up to 4 h of incubation at 37 °C and then slowly started decline. However, mature CFTR (band C) induced almost 3-fold (n = 3) up to 4 h of incubation at 37 °C and then started to decline. These results indicate that surface expression of F508del CFTR can be markedly enhanced with SNO's treatment (Fig. 2).
Fig. 2.
Low temperature and S-nitrosoglutathione rescue and stabilize mutant F508del CFTR in HBAE cells. At each time point, cells were lysed in lysis buffer and total protein concentrations were measured and CFTR protein expression was measured by immunoblot using anti-CFTR mAb 596. Blots were scanned, and densitometry was performed for quantification. Data are the mean ± SD, n = 3, *p < 0.05 **p < 0.001.
3.3. Low temperature and GNODE increase the cell surface stability and extend the cell surface half-life of F508del CFTR
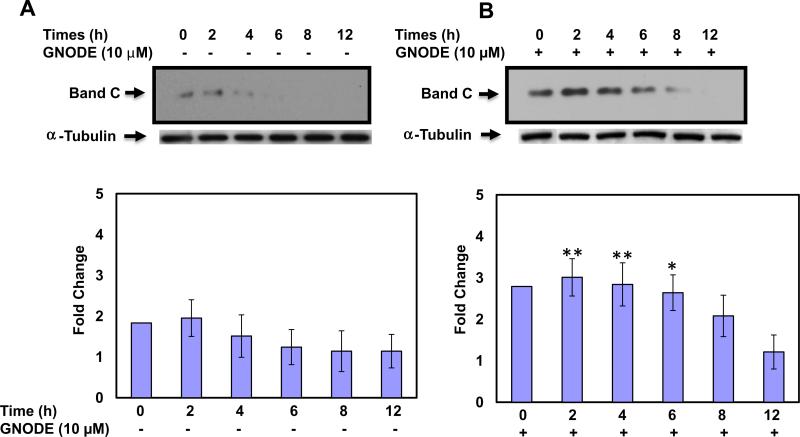
We monitored the effect of low temperature in the absence or presence of GNODE on the cell surface half-life of mutant primary human bronchial airway epithelial (PHBAE) cells by using cell surface biotinylation based assay. PHBAE cells expressing F508del CFTR were grown at 37 °C to 70% confluence, and then incubated for an additional 48 h at 27 °C in the absence or presence of GNODE (10 μM) for the last 4 h. After 4 h of treatment, the old media were replaced with a new media without GNODE, and cells were returned to 37 °C incubator for 0, 2, 4, 6, 8, and 12 h. The mature glycosylated forms of F508del CFTR is stable without GNODE until 2 h after return to 37 °C and after that expression started decline (Fig. 3A). However, F508del CFTR markedly induced almost 3-fold (n = 3) by combination treatment with GNODE and low temperature (27 °C), and stable up to 6 h and then slowly started decline (Fig. 3B). These results nicely demonstrated that GNODE also increases the cell surface stability, and extends the cell surface half-life of mutant F508del CFTR in PHBAE cells.
Fig. 3.
Low temperature and S-nitrosoglutathione diethyl ester increase the cell surface stability and extend the cell surface half-life of F508del CFTR in PHBAE cells. To confirm that the fully glycosylated F508del CFTR was expressed at the cell surface, cells were surface biotinylated using biotin-LC-hydrazide assay. CFTR was immunoprecipitated and subjected to SDS–PAGE on 6% gels; biotinylated CFTR was detected with streptavidin-conjugated horseradish peroxidase. The membrane was stripped and re-probed with a-tubulin to verify that equal amounts of protein were added. Blots were scanned, and densitometry was performed for quantification. Data are the mean ± SD, n = 3, *p < 0.05 **p < 0.001.
3.4. Internalization measurement
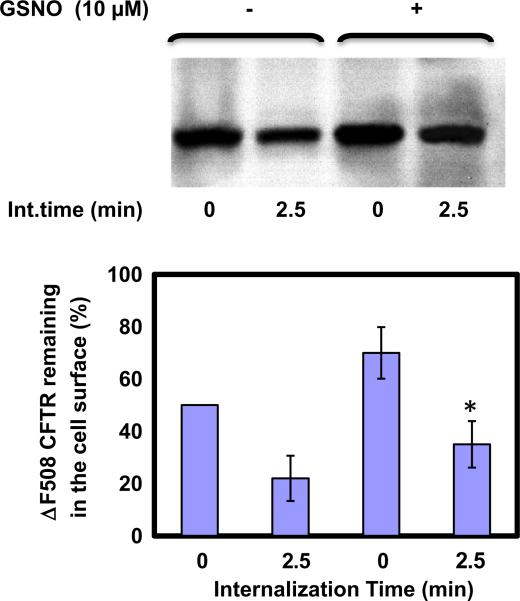
An internalization time of 2.5 min was selected for all assays conducted at 37 °C because, at this temperature, previous internalization times occur in different cell lines [10]. Biotin-LC-hydrazide is not membrane permeable; hence the only biotin-accessible CFTR is what remains on the cell surface during the warm-up period. Thus, alterations in the surface pool of CFTR after 2.5 min were reflected in a loss of biotinylated CFTR, and this loss corresponds to the CFTR that had been internalized from the cell surface (Fig. 4). After internalization, cells were lysed and biotinylated CFTR were analyzed by 6% SDS–PAGE with horseradish peroxidase-conjugated avidin. These results indicate that GSNO (10 μM) decreased the internalization rate about twofold within 2.5 min (Fig. 4).
Fig. 4.
S-nitrosoglutathione decreases the internalization rate of F508del CFTR. The measurement of internalization of glycoproteins in the cell surface studied by including a 37 °C incubations after sodium periodate oxidation treatment, but before biotynylation by biotin-LC hydrazide. Any glycoproteins present at the cell surface at the time of sodium periodate treatment, but then internalized during the 37°C incubation would not be available for biotynylation. After internalization, cells were lysed and proteins were analyzed by 6% SDS–PAGE. GSNO decreased the internalization rate within 2.5 min. Blots were scanned, and densitometry was performed for quantification. Data are the mean ± SD, n = 3, *p < 0.05.
4. Discussion
CF is a multi-organ system disease associated with mutations in the gene that codes for CFTR protein. The most prevalent mutation associated with CF, F508del CFTR, occurs in more than 90% of CF patients [1,2]. Therefore, most CF therapeutic efforts focus on correcting this mutant. The majority of wild-type and almost all F508del CFTR are degraded before reaching the cell surface. Most CFTR proteins are polyubiquitinated and rapidly degraded by the proteasome [3,4] and degradation of F508del CFTR is indistinguishable from the processes involved in the degradation of wild-type CFTR. Studies have shown that a number of enzymes required for ubiquitination activation, especially ubiquitin activating enzyme (E1) and ubiquitin conjugating enzymes (E2) contain reactive thiol residues [18]. Thus, the mechanisms that stress the biosynthesis, trafficking, and degradation of CFTR provide a unique opportunity to understand the pathogenesis of CF at the molecular levels. Therefore, there is a large interest in identifying compounds with a favorable pharmacological profile that could reverse the molecular defect and prevent CF disease progression in vivo. Several in vitro studies have shown that low temperature and chemical chaperones such as glycerol and 4-phenylbutyrate increase expression of F508del CFTR at the cell surface [8–11,13].
Using human airway epithelial monolayer culture, we and several other groups have found that GSNO increases the expression, and maturation of CFTR in F508del CFTR mutant homozygous CFPAC-1, F508del-transfected BHK cells, wild-type CFTR-transfected CFPAC-1 cells (CFPAC-1LJ6), BHK-wild-type transfected cells [13,19–21]. Additionally, GSNO increases the cell-surface expression and function of, F508del CFTR in mIMCD3 (mouse inner medullary collecting duct) cells infected with F508del-recombinant adenovirus [24] and F508del CFTR homozygous human airway epithelial cells [25]. Thus there is interest in these compounds as a novel class of corrector therapies for CF. We have reported that GSNO targets the CFTR co-chaperone, the Hsp70/Hsp90 organizing protein (Hop; or stress-induced phosphoprotein 1, Stip1) for S-nitrosylation and ubiquitination; and that this process is necessary and sufficient to explain the effect of GSNO to correct CFTR function in human airway epithelial cell monolayer culture [13]. In addition, we found that heat shock cognant (Hsc70) is associated with CFTR in the ER, and is S-nitrosylated by GSNO. In the presence of GSNO, S-nitrosylation of Hsc70 prevents CFTR degradation and allows for stabilization of CFTR as it leaves the ER and is transferred to the Golgi [13]. To date, the mechanisms influencing the abundance of S-nitrosylated Hop, and Hsc70 are not completely understood. Our preliminary data suggest that S-nitrosylation of Hop and Hsc70 are central target factors by which SNOs increase cellular expression and maturation of CFTR [13].
The data presented here provide the first evidence that membrane permeable SNOs, such as GNODE and SNOAC, more efficiently increase the expression of mutant F508del CFTR on the cell surface in a dose dependent manner of HBAE cells (Fig. 1). A number of studies have shown that cell culture at low temperature (27 °C) is the most effective method of rescue the trafficking of misfolded F508del CFTR protein to the cell surface [9–11]. Our present study demonstrated that when cells are kept at low temperature, the stability of F508del CFTR is enhanced, despite the fact that F508del CFTR is rapidly degraded once the temperature is raised to 37 °C. However, in the presence of GSNO, the up-regulation of immature and mature F508del CFTR expression significantly enhanced. The central aim of this experiment was to follow the cell surface fate of F508del CFTR at 27 °C and 37 °C and compared the results in the presence or absence of GSNO. This result showed us that the combination of both treatments (GSNO/low temperature) had a greater effect than low temperature alone on the up-regulation of CFTR expression in HBAE cells (Fig. 2).
Another critically important find from our study is that GSNO or GNODE treatment dramatically stabilized the surface pool of F508del CFTR. One explanation for this observation is that CFTR degradation slows down during hypothermia and S-nitrosylated Hop, which inhibit Hop from associating with CFTR, ultimately helps trafficking of CFTR to the cell surface. However, when cells were returned to 37 °C, the association of CFTR and co-chaperone Hop become stronger and CFTR reversed to a misfolded stage. In this misfolded stage, CFTR are likely to be accessible to ubiquitination and subsequent degradation. Further we monitored the effect of low temperature in the absence or presence of GNODE (10 μM) on the cell surface half-life of mutant F508del CFTR in primary human bronchial airway epithelial cells by using the cell surface biotinylation based assay. Interestingly, we found that cells maintained only at the low temperature (27 °C) minimally enhanced the cell surface stability. However, in the presence of GNODE (10 μM) significantly enhanced the cell surface stability and extend the cell surface half-life of F508del CFTR compared with untreated control (Fig. 3A and B). These results indicate that surface expression of F508del CFTR can be evidently boosted by carefully chosen combination agents.
Internalization rate decreased, but still occurred in rescued F508del CFTR in the presence of low temperature or GSNO (10 μM) (Fig. 4). Previous data suggest that low temperature block degradation of internalized proteins by inhibiting their transport to lysosomes [27]. However, it is not clear whether transport to the lysosome or the initial steps of ubiquitination-dependent internalization are still functional at low temperature. Our data illustrates that GSNO slows down the internalization rate of CFTR thus suggesting the possibility that GSNO acts by ubiquitin-dependent internalization. Note that the target of GSNO, Hop is important in cell surface CFTR recycling, and siRNA against this target helps to maintain cell surface expression [13,28]. We previously showed that the proteosomal inhibitor such as MG132 prevents the effect of GSNO on Hop degradation and further increases Hop-S-nitrosylation and ubiquitination [13].
The ability of SNOs to augment the maturation of the CFTR could be helpful on the treatment of CF. In contrast to glycerol and 4-phenylbutyrate; SNO is an endogenously produced and present at low concentration in the extracellular fluids of the human lung and brain. Thus, there is growing interest in these compounds as a novel class of corrector therapies for CF. Further, low doses GSNO inhalation increases oxygen saturation and is well tolerated in patients carrying a F508del CFTR mutation [22]. Taken together, these results suggest that specific SNOs treatment may supplemented by other corrector therapies to help re-establish mutant F508del CFTR function in CF patients.
Acknowledgments
We would like to thank Dr. Eric Sorscher and Dr. Scott Randell for providing HBAE and PHBAE cells. Also, we would like to thank Dr. John Riordan for providing the monoclonal anti-CFTR antibody. This research was supported by grants from the Cystic Fibrosis Foundation (Zaman 04GO) and from the National Institutes of Health 1PO1HL 101871-01A1 and HL096800 (FS).
References
- 1.Davis PB. Cystic fibrosis since 1938. Am. J. Respir. Crit. Care Med. 2006;173:475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 2.Riordan JR. CFTR function and prospects for therapy. Annu. Rev. Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 3.Kopito RR. Biosynthesis and degradation of CFTR. Physiol. Rev. 1999;79:S167–S172. doi: 10.1152/physrev.1999.79.1.S167. [DOI] [PubMed] [Google Scholar]
- 4.Skach WR. CFTR: new members join the fold. Cell. 2006;127:673–673. doi: 10.1016/j.cell.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Grove DE, Rosser MF, Ren HY, Naren AP, Cyr DM. Mechanisms for rescue of correctable folding defects in CFTR ΔF508. Mol. Biol. Cell. 2009;20:4059–4069. doi: 10.1091/mbc.E08-09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward CL, Kopito RR. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. In efficient processing and rapid degradation of wild-type and mutant proteins. J. Biol. Chem. 1994;269:25710–25718. [PubMed] [Google Scholar]
- 7.Sun F, Mi Z, Condliffe SB, Bertrand CA, Gong X, Lu X, Zhang R, Latoche JD, Pilewski JM, Robbins PD, Frizzell RA. Chaperone displacement from mutant cystic fibrosis transmembrane conductance regulator restores its function in human airway epithelia. FASEB J. 2008;22:3255–3263. doi: 10.1096/fj.07-105338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeitlin PL, Diener-West M, Rubenstein RC, Boyle MP, Lee C, Brass-Ernst I. Evidence of CFTR function in cystic fibrosis after systemic administration of 4-phenylbutyrate. Mol. Ther. 2002;6:119–126. doi: 10.1006/mthe.2002.0639. [DOI] [PubMed] [Google Scholar]
- 9.Heda GD, Marino CR. Surface expression of the cystic fibrosis transmembrane conductance regulator mutant deltaF508 is markedly upregulated by combination treatment with sodium butyrate and low temperature. Biochem. Biophys. Res. Commun. 2000;271:659–664. doi: 10.1006/bbrc.2000.2684. [DOI] [PubMed] [Google Scholar]
- 10.Varga K, Goldstein RF, Jurkuvena A, Chen L, Matalon S, Sorscher EJ, Bebok Z, Collawn JF. Enhanced cell-surface stability of rescued ΔF508 cystic fibrosis transmembrane conductance regulator (CFTR) by pharmacological chaperonce. Biochem. J. 2008;410:555–564. doi: 10.1042/BJ20071420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rennolds J, Boyaka P, Bellis S, Cormet-Boyaka E. Low temperature induces the delivery of mature and immature CFTR to the plasma membrane. Biochim. Biophys. Res. Commun. 2008;366:1025–1029. doi: 10.1016/j.bbrc.2007.12.065. [DOI] [PubMed] [Google Scholar]
- 12.Zaman K, Fraser-Butler M, Bennett D. Novel S-nitrosothiols have potential therapeutic uses for cystic fibrosis. Curr. Pharm. Des. 2013;19:3509–3520. doi: 10.2174/13816128113199990319. [DOI] [PubMed] [Google Scholar]
- 13.Marozkina N, Yemen S, Borowitz M, Liu L, Plapp M, Sun F, Islam R, Erdmann-Gilmore P, Townsend R, Lichti C, Manti S, Clapp P, Randell S, Gaston B, Zaman K. Hsp70/Hsp90 organizing protein as a nitrosylation target in cystic fibrosis. Proc. Natl. Acad. Sci. U.S.A. 2010;107:11393–11398. doi: 10.1073/pnas.0909128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaston B, Doctor A, Singel D, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am. J. Respir. Crit. Care Med. 2006;173:1186–1193. doi: 10.1164/rccm.200510-1584PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lancaster J, Gaston B. NO and nitrosothiols: spatial confinement and free diffusion. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:465–466. doi: 10.1152/ajplung.00151.2004. [DOI] [PubMed] [Google Scholar]
- 16.Gaston B. Nitric oxide and thiol groups. Biochim. Biophys. Acta. 1999;1411:323–333. doi: 10.1016/s0005-2728(99)00023-7. [DOI] [PubMed] [Google Scholar]
- 17.Grasemann H, Gaston B, Fang K, Paul K, Ratjen F. Decreased levels of nitrosothiols in the lower airways of patients with cystic fibrosis and normal pulmonary function. J. Pediatr. 1999;135:770–772. doi: 10.1016/s0022-3476(99)70101-0. [DOI] [PubMed] [Google Scholar]
- 18.Palmer LA, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 and activity: redox-dependent effect of nitrogen oxides. Mol. Pharmacol. 2000;58:1197–1203. doi: 10.1124/mol.58.6.1197. [DOI] [PubMed] [Google Scholar]
- 19.Zaman K, Palmer LA, Doctor A, Hunt J, Gaston B. Concentration-dependent effects of endogenous S-nitrosoglutathione on gene regulation by specificity proteins Sp3 and Sp1. Biochem. J. 2004;380:67–74. doi: 10.1042/BJ20031687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaman K, Carraro S, Doherty J, Henderson E, Lendermon E, Liu L, Verghese G, Ross M, Palmer LA, Doctor A, Stamler J, Gaston B. A novel class of compounds that increase CFTR expression and maturation in epithelial cells. Mol. Pharmacol. 2006;70:1435–1442. doi: 10.1124/mol.106.023242. [DOI] [PubMed] [Google Scholar]
- 21.Zaman K, McPherson M, Vaughan J, Hunt J, Mendes F, Gaston B, Palmer LA. S-nitrosoglutathione increases cystic fibrosis transmembrane regulator maturation. Biochem. Biophys. Res. Commun. 2001;284:65–70. doi: 10.1006/bbrc.2001.4935. [DOI] [PubMed] [Google Scholar]
- 22.Snyder A, McPherson M, Hunt JF, Stamler JS, Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002;165:1–5. doi: 10.1164/ajrccm.165.7.2105032. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Patel RP, Teng X, Bosworth CA, Lancaster JR, Matalon S. Mechanisms of cystic fibrosis transmembrane conductance regulator activation by S-nitrosoglutathione. J. Biol. Chem. 2006;281:9190–9199. doi: 10.1074/jbc.M513231200. [DOI] [PubMed] [Google Scholar]
- 24.Howard M, Fischer H, Roux J, Santos B, Gullans S, Yancey P, Welch W. Mammalian osmolytes and S-nitrosoglutathione promote F508 CFTR protein maturation and function. J. Biol. Chem. 2003;278:35159–35167. doi: 10.1074/jbc.M301924200. [DOI] [PubMed] [Google Scholar]
- 25.Andersson C, Gaston B, Roomans G. S-nitrosoglutathione induces functional F508 CFTR in cultured airway epithelial cells. Biochem. Biophys. Res. Commun. 2002;297:552–557. doi: 10.1016/s0006-291x(02)02245-3. [DOI] [PubMed] [Google Scholar]
- 26.Servetnyk Z, Krujkova J, Gaston B, Zaman K, Hjelte L, Roomans G, Dragomir A. Activation of delF508 CFTR in CF airway epithelial cell lines and CF nasal epithelial cells by S-nitrosoglutathione. Respir. Res. 2006;7:124–130. doi: 10.1186/1465-9921-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haylett I, Thilo L. Endosome–lysosome fusion at low temperature. J. Biol. Chem. 1991;266:8322–8327. [PubMed] [Google Scholar]
- 28.Okiyoneda T, Barriere H, Bagdany M, Rabeh WM, Du K, Hohfeld J, Young JC, Lukacs GL. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science. 2010;329:805–810. doi: 10.1126/science.1191542. [DOI] [PMC free article] [PubMed] [Google Scholar]