Abstract
Rationale
A well-developed coronary collateral circulation improves the morbidity and mortality of patients following an acute coronary occlusion. Although regenerative medicine has great potential in stimulating vascular growth in the heart, to date there have been mixed results, and the ideal cell type for this therapy has not been resolved.
Objective
To generate induced vascular progenitor cell(s) (iVPC(s)) from endothelial cells, which can differentiate into vascular smooth muscle cells (VSMC(s)) or endothelial cells (EC(s)), and test their capability to stimulate coronary collateral growth.
Methods and Results
We reprogrammed rat ECs with the transcription factors Oct4, Klf4, Sox2 and c-Myc. A population of reprogrammed cells was derived which expressed pluripotent markers Oct4, SSEA-1, Rex1 and AP and hemangioblast markers CD133, Flk1, and c-kit. These cells were designated iVPCs because they remained committed to vascular lineage and could differentiate into vascular ECs and VSMCs in vitro. The iVPCs demonstrated better in vitro angiogenic potential (tube network on 2D culture, tube formation in growth factor reduced Matrigel) than native ECs. The risk of teratoma formation in iVPCs is also reduced compared to fully reprogrammed induced pluripotent stem cell(s) (iPSC(s)). When iVPCs were implanted into myocardium, they engrafted into blood vessels and increased coronary collateral flow (microspheres) and improved cardiac function (echocardiography) better than iPSCs, mesenchymal stem cells, native ECs and sham treatments.
Conclusions
We conclude that iVPCs, generated by partially reprogramming ECs, are an ideal cell type for cell-based therapy designed to stimulate coronary collateral growth.
Keywords: vascular progenitor cells; coronary collateral growth; coronary circulation, induced pluripotent cells
Introduction
Coronary heart disease (CHD) is the leading cause of mortality and morbidity in the United States. Although there have been numerous advances in the treatment of CHD over the last many years (drug eluting stents, statins), the realization of therapeutic angiogenesis for stimulation of coronary collateral growth remains an elusive goal. Cell-based therapies for cardiovascular diseases offer a new paradigm for treatment of CHD. The outcomes of cell-based therapies in the improvement of left ventricular function and reduction of myocardial ischemia are controversial.1, 2,3-5 The challenge of growing new myocardium is enormous, involving essentials such as cardiomyocytes, conductive tissue and a complete circulation. However, the challenge of stimulating coronary collateral growth is far less involved, and is a strategy more likely to produce an immediate benefit in the treatment of ischemic heart disease.6, 7 “Ideal” stem/iPS/progenitor cell population for optimal coronary collateral growth (also termed arteriogenesis and collaterogenesis) in ischemic myocardium has not been identified. 6, 8 Many cell types, such as endothelial progenitor cells from blood or bone marrow, cardiac progenitor cells from the heart, mesenchymal stem cells from bone marrow and others, are currently being examined as cell sources for cardiovascular regenerative cell therapy. Unfortunately the benefits are modest.9, 10 The goal of this study is to generate induced vascular progenitor cell(s) (iVPC(s)) that are capable of becoming both smooth muscle and endothelium, and stimulating the growth of coronary collateral vessels.
Induced pluripotent stem cell(s) (iPSC(s)) are somatic cells reprogrammed to pluripotency by introducing a combination of four transcription factors out of Oct4, Klf4, Sox2, c-Myc, Nanog, and Lin28.11, 12 So far, iPSCs are the strongest example of the plasticity of cells in response to a disruption in the stoichiometry of their transcriptional regulators.13 iPSCs potentially can avoid the ethical and legal controversy and practical difficulty associated with using human embryos. Importantly, the autologous source of iPSCs also avoids issues with immuno-incompatibility. iPSCs are becoming one of the more promising candidates for regenerative medicine, but one drawback of iPSCs is the risk of tumor formation.14-17 Somatic stem cells such as hematopoietic stem cells and mesenchymal stem cells have multipotency, but do not form teratomas. Accordingly, the goal of our study was to reprogram somatic cells, not to full pluripotency, but rather to a progenitor-type cell that remained committed to a specific lineage, which would greatly reduce the risk of tumor formation. Our goal was to partially reprogram endothelial cell into a putative iVPC that hopefully could differentiate into endothelial and vascular smooth muscle cells, but not other cell types.
Since we have an established rat model for coronary collateral growth,17 we reprogrammed rat cells. The reason why we elected to reprogram vascular ECs instead of other cells such as fibroblasts is based on recent studies, which suggest that an “epigenetic memory” of their origins of somatic tissue in early passage of iPSCs favors a commitment to a cell lineage related to the donor cell while restricting alternative cell fate.18, 19 Our hypothesis is that implantation of iVPCs would more likely result in more robust vascular growth in the heart than iPSCs, because the former cell type would remain committed to a vascular lineage which serves as “building blocks” for blood vessels; whereas the latter cell type could differentiate into multiple cell types not necessarily to be involved in vascular growth. Our results show that iVPCs can be generated by reprogramming rat vascular ECs, demonstrate distinct DNA methylation profiles of the promoters of Nanog and Oct4, and eNOS compared to native ECs and iPSCs, have low risk of teratoma formation compared to iPSCs, and better stimulate coronary collateral growth and improve myocardial function than iPSCs, mesenchymal stem cells (MSCs), or native ECs in a rat model of repetitive ischemia.
Methods
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Viral Transduction of ECs and Doxycycline-Induced Reprogramming
Lentiviral vectors expressing mouse transcription factors Oct4 (O), Klf4 (K), Sox2 (S) and c-Myc (M) were used to reprogram rat ECs. Doxycyline (2 μg/mL) was added at day 3 for induction of the reprogramming and withdrawn at day 15. Colonies were picked at day 21 to 23.
Immunocytochemical and Immunohistochemical Analyses
Samples were fixed with 4% (w/v) paraformaldehyde, incubated with the primary antibody overnight at 4°C, and then were incubated with a fluorescence-conjugated secondary antibody for 1 hour at room temperature. Gold anti-fade with DAPI was added for imaging.
iVPC Differentiation, Matrigel Assay and Responses to Shear Stress
For differentiation, iVPCs were cultured on collagen IV coated plates either in EC culture medium with VEGF, or VSMC culture medium with PDGF. For the Matrigel assay, 5×104 cells were added on Matrigel in 24 well plate and incubated at 37°C for 16 to 24 hours. For the shear stress experiment, differentiated iVPCs were exposed to a uni-directional laminar shear stress.
Teratoma Formation
Cells were injected into kidney or testis capsule of SCID mice for teratoma formation analysis.
Fluorescence-Activated Cell Sorting (FACS) Analysis
106 cells were used for staining. Cells were incubated with 5% BSA for 30 minutes, then stained with the primary antibody for one hour at 4°C, washed, stained with the fluorescence-conjugated secondary antibody 30 minutes, and then fixed in 1% PFA for FACS.
Quantitative RT-PCR
RNA was isolated from the cells and real time RT-PCR was performed as described previously.20
Bisulfite Genomic Sequencing
Genomic DNA was extracted from cells and bisulfite treatment was performed. Methylated DNA was purified for PCR and PCR products were cloned in TA vector. At least eight clones were sent for sequencing and sequences were analyzed by the software BiQ analyzer.
Karyotyping
Cells were spun down and hypotonic KCl solution was added. Cells were fixed and spread on glass slides. Glass slides were stained with Giemsa's stain and chromosomes were counted.
tdTomato Labeling of iVPCs
tdTomato lentivirus was generated from ptdTomato-N1 Vector and transduced into iVPCs as described above.
Isolectin-B4 Infusion
Rats were injected intravenously via the femoral vein with 250μg isolectin GS-IB4. The heart was fixed and tissue samples were taken from the collateral dependent and normal zones of the left ventricle.
Rat Model of Collateral Growth/Repetitive Ischemia (RI) and Cell Transplantation
SD rats were used for chronic implantation of a pneumatic occluder over the left anterior descending coronary artery (LAD), as described by Toyota et al.17 Briefly, the rats were anesthetized and intubated, and the left chest was opened between 3rd-5th intercostal spaces. An occluder was attached to the left ventricle over the LAD, and one million cells were injected in 2-3 sites in the myocardium around the balloon. The chest was closed and rats were allowed to rest for 2 days before undergoing in the RI protocol for 10 days.
Microsphere Measurements of Collateral Flow
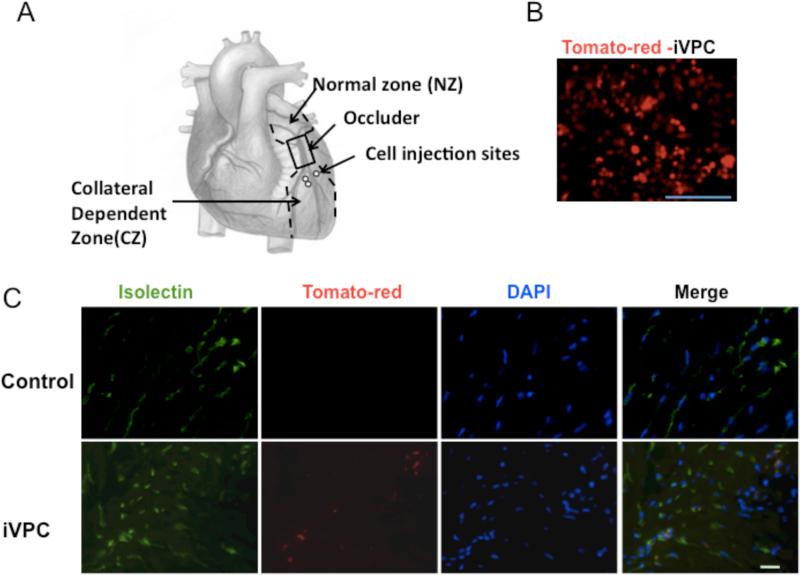
Coronary collateral growth was evaluated from blood flow to the collateral-dependent region by using neutron-activated microspheres injected into the left ventricle (LV) lumen. Collateral flow was calculated as a ratio between activity (dpm/g) of the tissues from the collateral-dependent zone and normal zone (CZ/NZ), as shown in the diagram in Figure 5A.
Figure 5.
iVPCs co-localized with Isolectin B4 after implantation into rat ischemic myocardium. A diagram for surgical procedure was shown in A. The iVPCs were labeled with Td-tomato by lentiviral vector before injection (B). Fluorescent images show rat myocardium in control group and iVPC-implanted group after 10 days of the RI protocol (C). iVPCs were labeled with tdTomato, the native endothelium was labeled with FITC-isolectin B4, and DAPI reveals nuclei. Note the absence of tdTomato in the control images, but the presence of this fluorochrome and its overlap with FITC in the iVPCs implantation images. Scale bar equals 100μm in B and 10μm in C.
Echocardiographic Analysis of Cardiac Function
In vivo heart function was evaluated by echocardiography. All measurements were performed before and after inflation of occluder at day 0 and day 10. Percentage changes of LV EF%, FS% and LVESV were calculated as the difference between the values obtained before and after the occluder inflation at day 0 and day 10.
Statistics
Unpaired two-tailed t-tests with Welch's correction were used for comparison of 2 or more groups.
Results
Generation of iVPCs
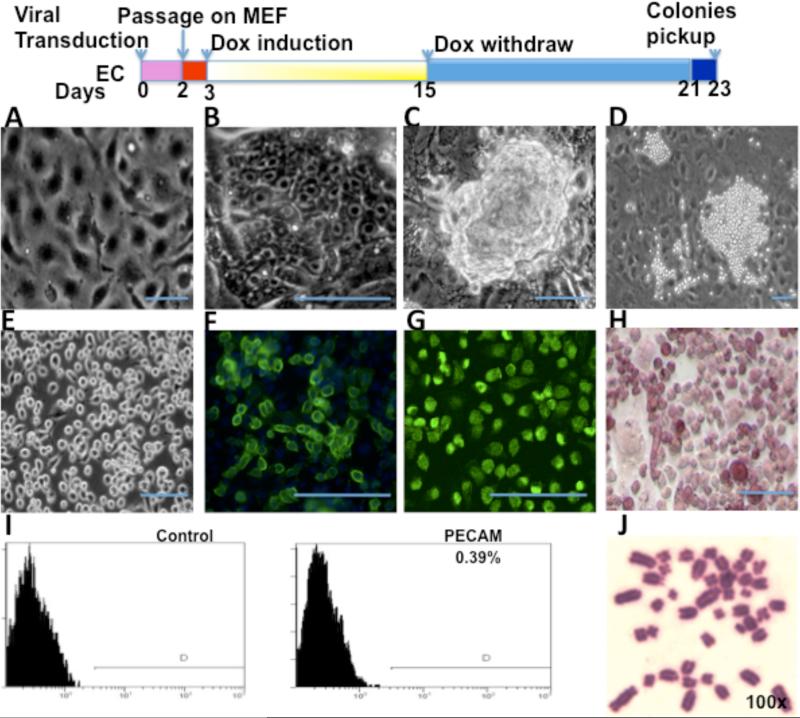
The protocol for reprogramming ECs with transcription factors Oct4, Klf4, Sox2 and c-Myc is shown in the top panel of Figure 1. Before transduction of ECs, confluent cultures showed typical cobblestone morphology (Figure 1A). At 3–5 days after transduction, the cells showed typical ES-like morphology (e.g. compact colonies, high nucleus-to-cytoplasm ratio, and prominent nuclei, Figure 1B). These reprogrammed ECs maintained ES-like morphology until day 12 to 16 (Figure 1C). Then their morphology changed to intense ball round colonies at day 20 to 21 (Figure 1D). After expansion, most of the colonies retained the same morphology (Figure 1E). Only one colony (#9) out of 36 colonies had some ES-like cells appearing in the round cell population during late passages (Figure 8A). The transduced ECs expressed ES cell marker Specific Stage Embryonic Antigen-1 (SSEA-1) (Figure 1F), Oct4 (Figure 1G) and were positive for Alkaline Phosphatase (AP) staining (Figure 1H). However, these reprogrammed ECs did not express (or expressed very little) native EC marker PECAM (0.39%), as shown by FACS analysis (Figure 1I). During the passage, these cells kept a normal number of chromosomes. Figure 1J shows iVPCs at passage 16 with 42 chromosomes. The morphology changes in transduced ECs indicated that they went through the early events of reprogramming, but they did not commit to faithful reprogramming to become typical iPSCs at the end. Immunostaining and FACS analysis suggested that a somatic lineage specific marker (e.g. PECAM) was down-regulated and some pluripotency markers (e.g. SSEA-1, Oct4 and AP) were up-regulated in the transduced ECs. Consistent with morphology changes, these data suggested that the transduced ECs were only partially reprogrammed.
Figure 1.
Generation of iVPCs from rat ECs transduced with transcription factors OKSM. Top panel is the schematic diagram of the protocol for reprogramming. (A) Confluent rat EC morphology before reprogramming. After transduction ECs showed ES-like morphology at day 4 (B), formed ES-like colonies at day 14 and retained the morphology until 16 days (C), and changed to ball-like iVPCs at day 21 (D). Most iVPCs retained stable morphology during expansion (E). iVPCs expressed ES cell marker SSEA-1 (F),Oct4 (G), Alkaline Phosphatase (H) by immunostaining. iVPCs showed only 0.39% EC marker PECAM by FACS analysis (I). iVPCs at passage 16 showed a normal 42xy Karyotype (J). Scale bar equals 100μm.
Figure 8.
Less Risk of Teratoma Formation from iVPCs than from iPSCs. A. Morphology of iPSC, iVPC and clone #9 (named as iVPC/iPSC because ES-cell-like fully reprogrammed cells appeared in the iVPCs). B. When seeded on low attachment dishes with differentiation medium, iPSC grew in suspension, aggregated and formed embryo bodies. In contrast, iVPCs attached to the plates and spread, and did not from embryo bodies. C. Kidneys and testis harvested after injection with iPSC, iVPCs and iVPCs/iPSCs. iVPCs did not form teratomas while iPSCs and iVPCs formed teratomas. D shows the probabilities of teratoma formation from iVPCs, iPSCs and iVPC/iPSC. 1 refers the teratoma formed and 0 refers no teratoma formed. The “N” refers to injection times. So for iPSCs, the probability of teratoma formation was 100% (4 out of 4). For iVPC, the probability of teratoma formation is 0% (0 out of 15). For iVPC/iPSC, the probability of teratoma formation is 100% (3 out of 3). Even if including iVPC/iPSC (clone#9), the probability of teratoma formation of iVPCs is 0.46% (3 out of 18 injections in the context of one clone out of 36 clones became iVPC/iPSC). Scale bar equals 100μm.
Gene Expression Profile of iVPCs
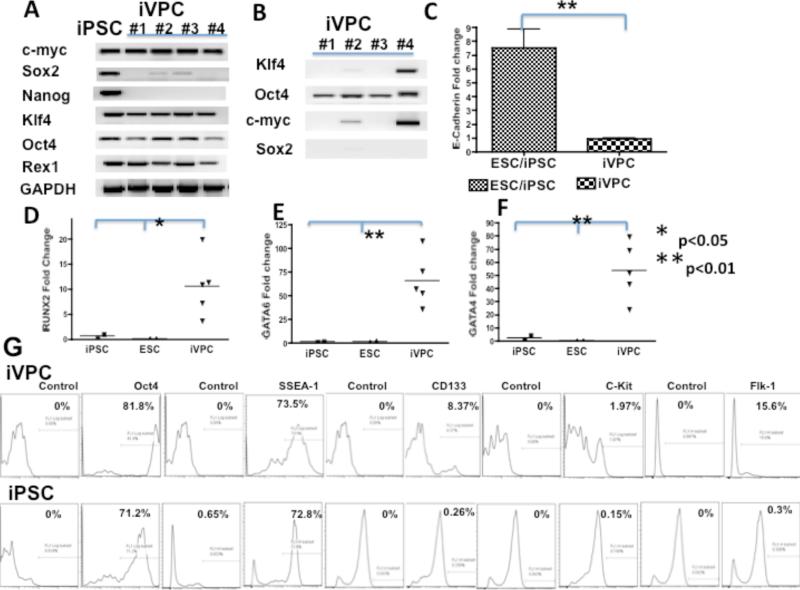
To further characterize the iVPCs, four colonies were randomly picked and RT-PCR was performed to compare their endogenous and exogenous gene expression with mouse ES cells and fully reprogrammed rat iPSCs derived from fibroblasts and bone marrow cells in the Xiao lab.21 iPSCs endogenously expressed c-Myc and pluripotent genes such as Sox2, Nanog, Klf4, Oct4, Rex1 (also known as Zfp42) (Figure 2A). Similarly, colonies of iVPCs expressed c-myc, Klf4, Rex1, and Oct4, but not all iVPCs expressed Sox2 and none expressed Nanog. Figure 2B shows that exogenous expression of transgenes Oct4, Klf4, Sox2, c-myc in iVPCs were not fully inactivated. These results were consistent with reports that in partially reprogrammed iPSCs, exogenous transgenes were not fully silenced.22, 23
Figure 2.
Gene expression profile of iVPC. RT–PCR analysis of endogenous gene expression in iVPCs and iPSCs (A) and exogenous expression of transcription factors Oct4, Klf4, Sox2, c-Myc in iVPCs (B). q RT-PCR analysis of E-cadherin (C) and cell lineage markers RUNX2 (D), GATA4 (E), and GATA6 (F) in iVPCs, ES cells and iPSCs. FACS analysis of iVPCs and iPSCs of pluripotent cell markers Oct4 and SSEA-1; hemangioblast progenitor markers CD133, Flk1, and c-kit (G).
Since E-cadherin is crucial for embryonic stem cell pluripotency and fully reprogrammed cells cannot be obtained in the absence of E-cadherin,24 we performed RT-PCR to examine the expression of E-cadherin in iVPCs. Figure 2C showed that mouse ES cells and iPSCs had greater expression of E-cadherin than iVPCs.
To elucidate the cell lineage of iVPCs, qRT-PCR was performed to detect the expression of three germ layers’ lineage markers. Compared to iPSCs, there were significant differences in the expression level of three transcription factors RUNX2 (Figure 2D), GATA6 (Figure 2E) and GATA4 (Figure 2F) between iVPCs and iPSCs. In undifferentiated ES cells and iPSCs, these genes had lower levels of expression than in iVPCs.
FACS analysis was used to further characterize the iVPCs. As Figure 2G illustrates, the majority of iVPCs expressed pluripotent cell markers: 81.8% for Oct4 and 73.5% for SSEA-1. Only a minority of iVPCs expressed hemangioblast markers: 8.37% for CD133, 15.6% for Flk1 and 1.97% for c-kit. Interestingly, iPSCs did not express hemangioblast marker: CD133, Flk1 and c-kit. The majority of iPSCs also expressed Oct4 (71.2%) and SSEA-1 (72.8%). Based on a comparison of these lineage markers, iVPCs appeared to be at a more differentiated state than pluripotency, close to mesoderm progenitor cells or hemangioblast progenitor cells.
Epigenetic Status of iVPCs
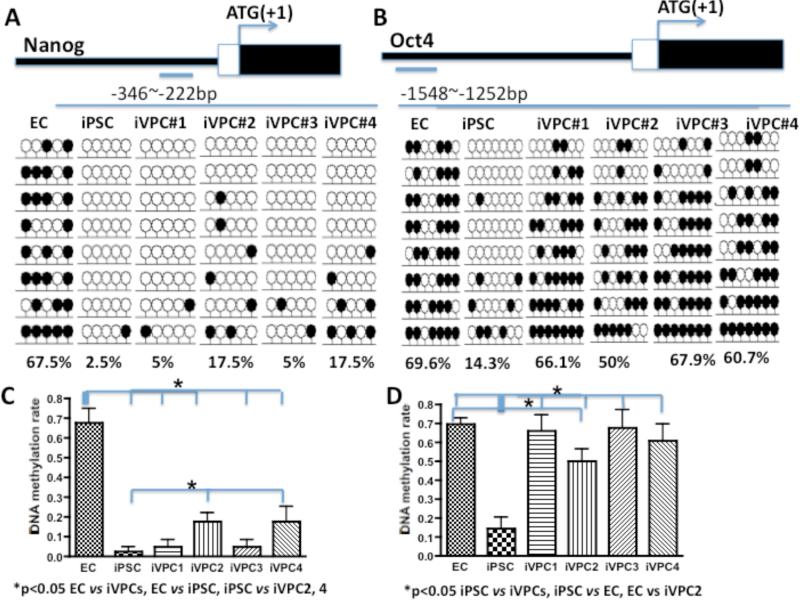
To address the mechanism of reprogramming to full pluripotency versus to a progenitor cell type, we investigated the DNA methylation status of the Nanog and Oct4 promoter region of ECs, iPSCs and iVPCs by bisulfite genomic sequencing analysis. In the promoter region of the rat Nanog gene (-346 to -222 bp upstream of the transcription start site), 67.5% of CpG was methylated in ECs; whereas in iPSCs, CpG dinucleotides in the same region were only 2.5% methylated (Figure 3A). CpG dinucleotides from four clones of iVPCs were 5% and 17.5% methylated (Figure 3A). Similarly, 69.6% of CpG in the promoter region of the rat Oct4 gene (-2142 to -1550 bp upstream of transcription start site) was methylated in ECs; whereas in iPSCs, CpG dinucleotides in the same region were 14.3% methylated (Figure 3B). CpG dinucleotides from the four clones of iVPCs were 66.1%, 50%, 67.9% and 60.1% methylated (Figure 3B). These data suggested that the DNA methylation status of the Nanog and Oct4 promoter regions in iVPCs, iPSCs, and ECs were different. The Nanog and Oct4 promoter region in iVPCs were more methylated than those of fully reprogrammed iPSCs; Nanog and Oct4 promoter regions in some iPVC clones are less methylated than those of native ECs. Since eight rows of lollipops in the Figure 3 represent eight sequences, the statistical analysis showed that in Figure 3C, DNA methylation of the Nanog promoter in ECs was significantly different from iPSCs and iVPCs; iVPC 1 and iVPC 4 were significantly different from iPSCs. Similarily, DNA methylation of the Oct4 promoter in iPSCs was significantly different from ECs and iVPCs; iVPC 2 was significantly different from ECs.
Figure 3.
DNA methylation status (bisulfite sequencing) of the Nanog (A) and Oct4 (B) promoter regions of endothelial cells (ECs), iPSCs from fully reprogrammed fibroblasts, and 4 randomly selected clones of induced vascular progenitor cells (iVPCs). Open and closed lollipops indicate unmethylated and methylated CpGs, respectively. The top panel shows the promoter region of Nanog and Oct4 relative to the translation start site. The bottom numbers indicate the methylation percentage of CpG in the region. C and D show the statistical summary for A and B.
DNA methylation of the eNOS promoter in iVPCs was different from that in iPSCs and ECs as shown in supplementary Figure I. This is consistent with our other data suggesting that iVPCs are neither ECs nor iPSCs after being reprogrammed.
Angiogenic Potential and Differentiation of iVPCs
When iVPCs were selected and expanded on feeder cells, some of these cells spontaneously formed a “tube-like” network (Figure 4A) suggesting a greater angiogenic potential than native endothelial cells that form a confluent monolayer in two dimensions. Accordingly, we performed in vitro angiogenesis assays on Matrigel to compare native ECs to iVPCs. In growth factor reduced (GFR) Matrigel, ECs (Figure 4B) and iVPCs (Figure 4C) formed tube-like networks in medium with serum and VEGF. In the absence of serum and VEGF, ECs did not form tubes in the GFR Matrigel (Figure 4D). In contrast, iVPCs formed an extensive tube network in the GFR Matrigel without serum and VEGF (Figure 4E).
Figure 4.
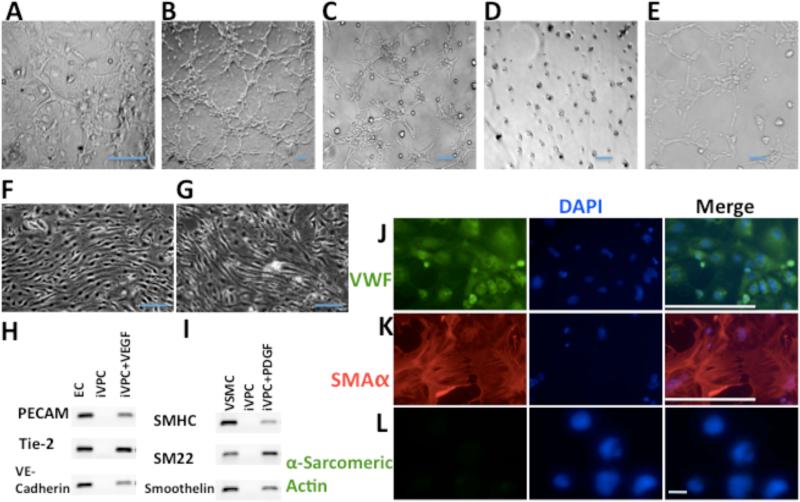
Angiogenic potential of iVPCs. (A) iVPCs spontaneously formed a “tube”-like network on feeder cells. In GFR Matrigel, ECs (B) and iVPCs (C) formed tube-like network in medium with serum and VEGF (50ng/mL). In the absence of serum and VEGF, ECs did not form tubes in the GFR Matrigel (D). In contrast, iVPCs formed an extensive tube-like network under same conditions (E). When exposed to shear stress for 72 hr, ECs (F) and differentiated iVPCs (G) aligned parallel to shear stress. iVPCs could differentiate into ECs with induction of VEGF (50ng/mL) shown in RT-PCR analysis (H) and immunostained with the EC marker VWF (J, with VWF stained in green and DAPI stained in blue. iVPCs could differentiate into VSMCs with induction of PDGF (50ng/mL) shown in RT-PCR analysis (I) and immunostained with the VSMC marker α-SMA (K, α-SMA stained in red and DAPI stained in blue). But iVPCs could not differentiate into cardiomyocytes either with BMP4 (10ng/mL), Activin A (10ng/mL) and bFGF (10ng/mL) or with 3 μM 5-azacytidine shown by negative staining of α-Sarcomeric Actin (L). Scale bar equals 100μm in A-J, and scale bar in L equals to 10μm.
When exposed to shear stress for 72 hours, iVPCs responded by aligning parallel to flow (Figure 4G), which is similar to the response of native ECs (Figure 4F). Treatment of iVPCs with VEGF (50 ng/mL) induced differentiation into ECs. RT-PCR results show that differentiated iVPCs expressed EC marker PECAM, Tie2 and VE-cadherin (Figure 4H) that was confirmed by immunostaining of Von Willebrand Factor (VWF) (Figure 4J). iVPCs could also differentiate into VSMCs after treatment with PDGF (20 ng/mL). RT-PCR results showed that differentiated iVPCs expressed smooth muscle marker smooth muscle heavy chain (SMHC), Smooth muscle 22 α and Smoothelin (Figure 4I). This was confirmed by immunostaining of α smooth muscle actin (α-SMA) (Figure 4K). These data show iVPCs had angiogenic potential and could differentiate into ECs and VSMCs in vitro. In contrast, the iVPCs did not differentiate into cardiomyocytes when treated either with BMP4 (10ng/mL), activin A (10ng/mL) and bFGF (10ng/mL) or with 3 μM 5-azacytidine, shown by negative staining of the cardiomyocyte marker α-sarcomeric actin (Figure 4L).
Embryo Body and Teratoma Formation
Rat iPSCs formed embryo bodies in vitro and teratomas in vivo consistent with previous results.21 In contrast, partially reprogrammed iVPCs did not form embryo bodies or teratomas. We should mention one exception (clone #9), which produced teratomas. This clone, out of 36 clones of iPVCs, appeared to transit from partial to full reprogramming during the passaging, and thus, we termed it (iVPC/iPSC) because of their distinct morphology versus iVPCs (Figure 8A). When iVPCs and iPSCs were seeded on non-coating low attachment petri dishes in the differentiated medium, iPSCs grew in suspension and aggregated to form embryo bodies. In contrast, iVPCs attached to the plates, spread, but did not from embryo bodies (Figure 8B). In vivo, iPSCs formed teratomas 100% of the time (Figure 8D). In contrast, iVPCs produced no teratomas (Figure 8D). The iVPCs that underwent further de-differentiation (iVPC/iPSC) also produced teratomas. Supplemental Figures II B, C shows that colony #9 and iPSCs formed teratomas and could differentiate into three germ layers. It is interesting to note that the teratoma formed from colony #9 was smaller and contained more blood vessels than typical terotomas from iPSCs. Since embryo body formation is the first step of ES cell differentiation into the three germ layers, this is likely a reason why the iPSCs produced teratomas, whereas the iVPCs did not.
iVPCs Engraftment into Blood Vessels after Implantation into Ischemic Rat Myocardium
To test if iVPCs could augment collateral growth, we implanted the cells in the rat left ventricle in a model of coronary collateral growth as shown in Figure 5A.
First, we studied cell survival and migration after injection. iVPCs were labeled with tdTomato before implantation and visualized by red fluorescence in Figure 5B. This allowed us to assess outcome of the injected cells after the protocol. We mixed FITC labeled microspheres with tdTomato iVPCs and injected this mixture in the rat myocardium. At day 2 and day 10, myocardium was harvested for imaging. In Supplemental Figure II, both the labeled iVPCs and the microspheres were found in the collateral dependent zone 1 (near injection site) but only iVPCs were visualized in the collateral dependent zone 2 (remote from injection site) suggesting that the cells migrated within the collateral dependent zone. Importantly, iVPCs were observed at both day 2 and day 10, which supports the idea that iVPCs survived after transplantation.
Second, we studied the co-localization of iVPCs with cell specific markers. Again, iVPCs were labeled with tdTomato (Figure 5B). FITC-isolectin B4 (endothelial marker) was administered intravenously to visualize ECs. Figure 5C shows tdTomato labeled iVPCs co-localized with isolectin-B4 in the rat myocardium after 10 days of the RI protocol. Note that there were no tdTomato cells present in the shams suggesting the fluorescence was specific. We also found integration of iVPCs in blood vessel using multiphoton microscopy (Supplemental Movie I). Collagen in the arterial wall was shown in green by second-harmonic generation.
Third, we compared the capability of iVPCs and iPSCs to engraft into blood vessels in the heart. For these experiments iVPCs were not labeled with tdTomato, but rather were visualized (with iPSCs) by immunostaining for ES cell marker SSEA-1 (Figure 6). ECs and VSMCs were visualized by immunostaining for VWF and α-SMA, respectively. iVPCs co-localized with vascular ECs expressing VWF (red) (Figure 6A and Supplemental Figure III). In the sham control group (without cell injection), there was no obvious SSEA-1 expression. We also observed co-localization of SSEA-1 with α-SMA (red, Figure 6B), suggesting that iVPCs also expressed smooth muscle actin. These data suggested that the implanted iVPCs became components of blood vessels. Interestingly, there were some injected iVPCs that appeared to stimulate the formation of multiple lumens within a vessel, suggesting intussusceptive angiogenesis (Figure 6A, bottom column). The iPSCs expressed SSEA-1, which also co-localized with VWF, but there was no co-localization with α-SMA. To examine if iVPCs differentiated into non-vascular cell types, we determined if SSEA-1 would co-localize with the cardiomyocyte marker α-sarcomeric actin. In the iVPC implantation group, no co-localization with α-sarcomeric actin was observed; however, iPSCs were observed to co-localize with cardiomyocytes (Figure 6C).
Figure 6.
iVPCs engrafted into blood vessels after implantation into rat ischemic myocardium. Images are from immunostaining of rat myocardium in control group and iVPC- and iPSC-implanted group after 10 days of the RI protocol. DAPI staining reveals cell nuclei. (A) Immunostaining of rat myocardium with ES cell marker SSEA-1 (green) and endothelial cell marker VWF (red). In control group, there was no obvious SSEA-1 expression. The iVPC- and iPSC-implanted groups highly expressed SSEA-1 and co-localized with vascular ECs. In the bottom panel, iVPCs branched and split from one big vessel into several small vessels, which is one type of angiogenesis (intussusceptive angiogenesis). (B) Immunostaining of rat myocardium with ES cell marker SSEA-1 (green) and vascular smooth cell marker αSMA (red). In the control group, again there was no obvious SSEA-1 expression. In the iVPC-implanted group, iVPCs expressing SSEA-1 co-localized with VSMCs. However, no co-localization of iPSCs with VSMCs was observed. (C) Immunostaining of rat myocardium with cardiomyocytes marker α-sarcomeric actin. In the iVPC delievered group, there was no co-localization of iVPCs with cardiomyocytes, but in the iPSC-implanted group, co-localization of iPSCs with cardiomyocytes was observed. Scale bar equals 100μm in A and B and 10μm in C.
iVPCs Improved Coronary Collateral Flow and Cardiac Function
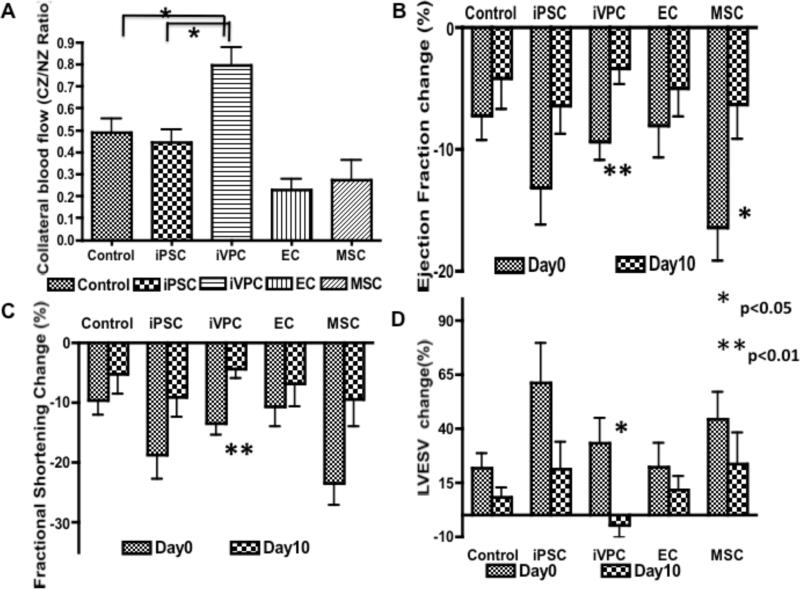
To compare the capabilities of different cell types to improve coronary collateral flow, iVPCs, iPSCs, ECs and MSCs were injected into rat myocardium. After 10 days RI protocol, coronary blood flow was measure by microsphere. Figure 7A shows that iVPCs enhanced coronary collateral growth (increase in CZ/NZ flow ratio) better than any of the other groups. Moreover, this increase in flow translated to an improvement in cardiac function, in terms of an improvement in ejection fraction (Figure 7B), fractional shortening (Figure 7C) and systolic volume (Figure 7D). The improvement in function was greatest and most apparent in the rats treated with iVPCs. These data suggested that iVPCs augment coronary collateral growth in rat myocardium better than either other cell types including iPSCs, MSCs, and ECs or just repetitive episodes of ischemia.
Figure 7.
iVPCs improved coronary collateral flow and heart function in vivo after implantation into rat myocardium. Compared to control (n=10), iPSC (n=7), EC (n=6), and MSC (n=8) implantation groups, iVPCs (n=8) augmented coronary collateral blood flow measured by microspheres. Collateral flow is expressed as a ratio of flows to the collateral and normal zones (CZ/NZ) (A). Ejection fraction (EF%), fractional shortening (FS%) and left ventricular end systolic volume (LVESV) were measured by echocardiography at day 0 and day 10 of RI protocol, and before and after balloon inflation (to produce ischemia). The percentage changes of each parameter before and after balloon inflation were calculated at day 0 and day 10. The improvement in ejection fraction (B), fractional shortening (C) and systolic volume (D) was most striking in the iVPC-treated group (n=8) between day 0 and day 10, compared to the other groups (control, n=10, iPSC, n=9, EC, n=5, and MSC (n=9). These data suggest heart function of the iVPC group improved significantly.
Discussion
In this study, we generated a vascular progenitor type cell that we have termed a “induced vascular progenitor cell” (iVPC) by reprogramming rat vascular ECs with the transcription factors Oct4, Klf4, Sox2 and c-Myc and selecting for markers consistent with hemangioblasts. The iVPCs expressed ES cell markers SSEA-1, Oct4, Rex1 and alkaline phosphatase (AP), but not the EC marker PECAM. The iVPCs showed the ability to stimulate coronary collateral growth and the capability to differentiate into either smooth muscle or endothelial cells. These cells appeared to commit to a vascular lineage, because they did not differentiate into cardiomyocytes. Although the reprogramming strategy we employed with the OKSM genes has led to the production of induced pluripotent cells (iPSCs) in mouse and rat fibroblasts, it is not surprising that we did not obtain iPSCs from rat ECs because a growing concept in reprogramming somatic cells into iPSC is that various cell types are converted to a pluripotency with varying efficiencies. 23, 25 This suggests that reprogramming is “context”-dependent and the cell type affects the capability to become an iPSC. Also, reprogramming efficiency varies depending on the origin of cell type because more differentiated cells are harder to reprogram than precursor or progenitor cells.23, 26, 27 Additionally, adult somatic cells are more difficult to reprogram than embryonic somatic cells. Even using the same protocol, some cell types could be reprogrammed but others could not. Studies have shown that successful reprogramming of rat somatic cells is also dependent on the original cell type and that the reprogramming conditions might need to be optimized for each different cell type, e.g., for rat liver progenitor cells using retrovirus expressing Oct4, Klf4, Sox2 plus “cocktail” 3i (containing inhibitors of MEK, GSK3β, and ALK5),28 for rat bone marrow cells and fibroblasts with lentivirus expressing Oct4, Klf4, Sox2, c-Myc,21 and for rat neural precursor and embryonic fibroblasts with retrovirus expressing Oct4, Klf4, Sox2 plus rat MEF feeder cells and 2i (containing inhibitors of MEK, GSK3β).29
A critical issue is to understand the differences between iVPCs and iPSCs. Our data provide insights into this distinction. First, we have shown that there was no endogenous expression of Nanog in all iVPCs colonies and not all iVPCs colonies endogenously expressed Sox2. Nanog and Sox2 are both important to maintaining the pluripotent status of ES cells and iPSCs. Expression of Nanog is essential for reprogramming, especially during late steps. It has been reported that missing expression of Nanog protein resulted in partially reprogrammed cells.30-32 Our data suggest it might be the case with iVPCs too. Expression of Sox2 at some “optimal” level is critical in that lower or higher expression will cause ES cells to differentiate into different cell lineages. Moreover, regulating the amount of Sox2 expression induced alternative cell fate during reprogramming.33, 34 Second, exogenous expression of transcription factors OKSM were not fully inactivated. In fully reprogrammed iPSCs, exogenous expression of transgenic transcription factors was fully silenced and the endogenous expression of pluripotent genes was activated.22, 23 Third, E-cadherin expression levels were lower in iVPCs than in iPSCs and ES cells, which may explain why iVPCs were only partially reprogrammed. Within this context, E-cadherin seems to be critical for a pluripotent phenotype.24 Fourth, Nanog and Oct4 promoter regions were more methylated in iVPCs compared to fully reprogrammed iPSC.23, 35, 36 Epigenetic status is important for the reprogramming and successful generation of the iPSCs. In mammals, 70~80% of CpG islands of the genome are methylated. For ES cells and iPSCs, Nanog and Oct4 promoters are typically robustly demethylated.30 In the iVPCs, DNA methylation status in the Nanog and Oct4 promoter region is different from that in parental ECs and fully reprogrammed iPSCs. From this observation we suggest that high degree of Oct4 promoter methylation may underscore the reason why ECs were not fully reprogrammed to iPSCs. This may also suggest that the epigenetic status of ECs may make them more difficult to fully reprogram into iPSCs. It is worth noting that DNA methylation analysis of Nanog and Oct4 promoters did not completely corroborate the RT-PCR results, in which exogenous Oct4 expression was activated and Nanog expression not activated. Regulation of gene expression by transcription factors can be affected by other events, including DNA methylation as well as histone modification, which we believe may be the cause of the apparent discrepancy between the RT-PCR data and the methylation analysis.
Teratoma formation is one critical standard for pluripotency. When randomly picked colonies from the generated iVPCs were injected into nude mice, teratomas did not form. A teratoma only formed from colony #9 (we named this clone iVPC/iPSC), which had ES-like cells appearing in iVPCs during late passage, implying transition from partial reprogramming to full reprogramming. It is interesting to note that the teratoma formed from #9 was smaller and contained more blood vessels than a typical teratoma from iPSCs. This is consistent with observations that partially reprogrammed iPSCs can lose their epigenetic memory during passage and revert to an iPSC.32, 37 In one of our experiments, iVPCs were implanted into rat myocardium, and no tumor or hemangioma was observed for 3 months. Importantly these data suggest iVPCs have a lower probability (0.46%) to form tumors than fully reprogrammed iPSCs, which form teratomas 100% of the time. This is in line with our goal to generate multipotent vascular progenitor cells instead of iPSCs to stimulate coronary collateral growth without formation of tumors.
Another observation suggesting that the iPSCs are very different from iVPCs pertained to expression of specific lineage markers such as RUNX2, GATA4 and GATA6. RUNX2 is a mesoderm lineage marker and is essential for osteoblastic differentiation, but it has also been suggested to be involved in angiogenesis.38 GATA4 and GATA6 are endoderm and mesoderm lineage markers and are important for embryo development including the heart and arterial systems.39, 40 These data suggested that iVPCs had been trapped in a differentiated state. Expression of these markers in iVPCs was significantly higher than iPSCs. Interestingly, a majority of the iVPC expressed pluripotent markers SSEA1 and Oct4, but some of the iVPC expressed the hemangioblast markers Flk1+, CD133 and c-kit. These data are consistent with epigenetic data, which suggest that ECs have strong epigenetic memory and thus reprogramming them resulted in the development of induced vascular progenitor cells rather than iPSCs.
An important characterization of these iVPCs is their angiogenic potential. In vitro studies showed they could spontaneously form a capillary-tube like network on feeder cells in the absence of VEGF. Typically endothelial cells do not form tubes on a 2D culture—only when cultured on Matrigel, ECs form tubes. Moreover, iVPCs could form a tube network in GFR Matrigel without serum and VEGF whereas EC could not form tubes under these conditions. This suggests greater angiogenic potential of these iVPCs compared to native ECs. When implanted in myocardium, iVPCs engrafted into blood vessels, increased coronary collateral blood flow and improved heart function during coronary occlusion—again suggesting that iVPCs enhanced vascular growth in the heart. Consistent with these data, iVPCs could differentiate into ECs and VSMCs, which are two important components of blood vessels. Traditionally, ECs and SMCs are regarded to come from different progenitor cells because during embryogenesis, endothelial cells come from hemangioblasts and smooth muscle cells arise from mesenchyme and the neural crest. However, Yamashita et al. reported that embryonic vascular progenitor cells (Flk1+) could differentiate into both ECs and SMCs.41 But postnatally, the residence of such common vascular progenitor cells has been controversial.41-43 Our result is the first report of generating iVPCs and applying them to induce coronary collateral growth. However, iVPCs may be applied more broadly for all needs requiring vascular growth, e.g., peripheral vascular disease, wound healing, diabetic neuropathy, etc.
Compared to fully reprogrammed iPSCs, MSCs and ECs, iVPCs augmented coronary collateral blood flow and significantly improved heart function. This result is consistent with our hypothesis that iVPCs may be more specific for vessel growth than other cell types. Another highlight of this study is choosing vascular cells as the starting cell type for reprogramming. Recent reports suggest that iPSCs might retain an “epigenetic memory” of their cell type from which they originate and thereby favor differentiation along that lineage. Thus, the cell origin might be important for the fate of the iPSCs as a way of committing to a fate of differentiation into vascular cells. The benefit of iVPCs in this study demonstrated that reprogrammed ECs remain committed to a vascular lineage because of their epigenetic memory and as such, become perfect building blocks for new and/or growing blood vessels in the heart.
Supplementary Material
Novelty and Significance.
What Is Known?
Somatic cells (e.g. rat fibroblasts) can be reprogrammed into induced pluripotent stem cells (iPSCs)
Stem cells (e.g. iPSCs, mesenchymal stem cells [MSC], cardiac stem cells [CSC]) are being used for many types of regenerative therapies
iPSCs have tumorigenic potential and can form teratomas
Repetitive Ischemia (RI) can induce coronary collateral growth
What New Information Does this Article Contribute?
Due to epigenetic memory, reprogramming of vascular endothelial cells (ECs) can produce induced vascular progenitor cells (iVPCs)
iVPCs remain committed to a vascular differentiation program becoming smooth muscle or endothelium, but not cardiomyocytes
Partially reprogrammed iVPCs have a much lower risk of tumorigenesis and teratoma formation
iVPCs better augment coronary collateral growth than native ECs, iPSCs, or MSCs in a rat RI model
Traditionally, cell-based therapies in ischemic heart disease focus on regenerating the myocardium. Other approaches aim to stimulate angiogenesis in the peri-infarct region. Our goal is to use cell-based therapy to stimulate coronary collateral growth to prevent myocardial infarction. Accordingly, we partially reprogrammed vascular ECs into induced vascular progenitor cells (iVPCs), instead of fully reprogramming cells into induced pluripotent stem cells (iPSCs). The risk of tumorigenesis is much lower with iVPCs compared to iPSCs because they remain committed to a vascular differentiation program. The vascular commitment of iVPCs is related to the epigenetic memory of ECs, which engenders them as cellular components of growing blood vessels in the heart. When iVPCs were implanted into myocardium, they engrafted in blood vessels and increased coronary collateral flow better than iPSCs, MSCs, or native ECs. We conclude that iVPCs are a a useful cell type to stimulate coronary collateral growth as a regenerative therapy. We also believe that partial reprogramming is an effective strategy to generate specific lineage progenitor cells, while avoiding tumorigenesis seen with other types of stem cells. However, the critical, but unresolved issue, pertains to regulating reprogramming to arrive at the partially programmed state.
Acknowledgement
We thank Dr. Lei Xiao (from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for his generosity in providing us with fully reprogrammed rat iPSCs.
Sources of Funding
This study was supported by NIH grant RHL100828Z, HL32788, R01 83366 (WMC), and Collaborative Research and Development Projects from Austen BioInnovation Institute in Akron, OH (LY).
List of non-standard abbreviations
- AP
alkaline phosphatase
- CHD
coronary heart disease
- CZ
collateral-dependent zone
- ECs
endothelial cells
- ES
embryonic stem
- iPSCs
induced pluripotent stem cells
- iVPCs
induced vascular progenitor cells
- LAD
left anterior descending coronary artery
- LVESV
left ventricular end systolic volume
- LV
left ventricle
- LVAW;d
left ventricle diastolic anterior wall
- LVAW;s
left ventricle systolic anterior wall
- LVID;d
left ventricle end-diastolic diameter
- LVID;s
left ventricle end-systolic diameter
- LVPW;d
left ventricle diastolic posterior wall
- LVPW;s
left ventricle systolic posterior wall
- NZ
normal zone
- PDGF
Platelet derived growth factor
- PECAM
Platelet endothelial cell adhesion molecule
- RI
repetitive ischemia
- SD rats
Sprague Dawley rats
- α-SMA
smooth muscle α actin
- SSEA-1
Specific Stage Embryonic Antigen-1
- VEGF
vascular endothelial growth factor
- VSMCs
vascular smooth muscle cells
- VWF
Von Willebrand Factor
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N, Komuro I. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119:2204–2217. doi: 10.1172/JCI37456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–377. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Marban E, Cheng K. Heart to heart: The elusive mechanism of cell therapy. Circulation. 2010;121:1981–1984. doi: 10.1161/CIRCULATIONAHA.110.952580. [DOI] [PubMed] [Google Scholar]
- 4.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suuronen EJ, Hazra S, Zhang P, Vincent R, Kumarathasan P, Zhang Y, Price J, Chan V, Sellke FW, Mesana TG, Veinot JP, Ruel M. Impairment of human cell-based vasculogenesis in rats by hypercholesterolemia-induced endothelial dysfunction and rescue with L-arginine supplementation. J Thorac Cardiovasc Surg. 2010;139:209–216. e202. doi: 10.1016/j.jtcvs.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Wong S, Lafleche J, Crowe S, Mesana TG, Suuronen EJ, Ruel M. In vitro functional comparison of therapeutically relevant human vasculogenic progenitor cells used for cardiac cell therapy. J Thorac Cardiovasc Surg. 2010;140:216–224. e214. doi: 10.1016/j.jtcvs.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Sellke FW, Laham R, Suuronen EJ, Ruel M. Angiogenesis for the treatment of inoperable coronary disease: the future. Semin Cardiothorac Vasc Anesth. 2006;10:184–188. doi: 10.1177/1089253206288994. [DOI] [PubMed] [Google Scholar]
- 8.Kuraitis D, Suuronen EJ, Sellke FW, Ruel M. The future of regenerating the myocardium. Curr Opin Cardiol. 2010;25:575–582. doi: 10.1097/HCO.0b013e32833f0318. [DOI] [PubMed] [Google Scholar]
- 9.Kong CW, Akar FG, Li RA. Translational potential of human embryonic and induced pluripotent stem cells for myocardial repair: insights from experimental models. Thromb Haemost. 2010;104:30–38. doi: 10.1160/TH10-03-0189. [DOI] [PubMed] [Google Scholar]
- 10.Templin C, Luscher TF, Landmesser U. Cell-based cardiovascular repair and regeneration in acute myocardial infarction and chronic ischemic cardiomyopathy current status and future developments. Int J Dev Biol. 2011;55:407–17. doi: 10.1387/ijdb.103219ct. [DOI] [PubMed] [Google Scholar]
- 11.Yu J, Vodyanik MA, He P, Slukvin, Thomson JA. Human embryonic stem cells reprogram myeloid precursors following cell-cell fusion. Stem Cells. 2006;24:168–176. doi: 10.1634/stemcells.2005-0292. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita JK. ES and iPS cell research for cardiovascular regeneration. Exp Cell Res. 2010;316:2555–9. doi: 10.1016/j.yexcr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Duinsbergen D, Salvatori D, Eriksson M, Mikkers H. Tumors originating from induced pluripotent stem cells and methods for their prevention. Ann N Y Acad Sci. 2009;1176:197–204. doi: 10.1111/j.1749-6632.2009.04563.x. [DOI] [PubMed] [Google Scholar]
- 16.Gersh BJ, Simari RD, Behfar A, Terzic CM, Terzic A. Cardiac cell repair therapy: a clinical perspective. Mayo Clin Proc. 2009;84:876–892. doi: 10.4065/84.10.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyota E, Warltier DC, Brock T, Ritman E, Kolz C, O'Malley P, Rocic P, Focardi M, Chilian WM. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 2005;112:2108–2113. doi: 10.1161/CIRCULATIONAHA.104.526954. [DOI] [PubMed] [Google Scholar]
- 18.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, Natesan S, Wagers AJ, Melnick A, Evans T, Hochedlinger K. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin L, Ma H, Ge X, Edwards PA, Zhang Y. Hepatic hepatocyte nuclear factor 4alpha is essential for maintaining triglyceride and cholesterol homeostasis. Arterioscler Thromb Vasc Biol. 2011;31:328–336. doi: 10.1161/ATVBAHA.110.217828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao J, Cui C, Chen S, Ren J, Chen J, Gao Y, Li H, Jia N, Cheng L, Xiao H, Xiao L. Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell. 2009;4:11–15. doi: 10.1016/j.stem.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redmer T, Diecke S, Grigoryan T, Quiroga-Negreira A, Birchmeier W, Besser D. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep. 2011;12:720–726. doi: 10.1038/embor.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bronckers AL, Sasaguri K, Cavender AC, D'Souza RN, Engelse MA. Expression of Runx2/Cbfa1/Pebp2alphaA during angiogenesis in postnatal rodent and fetal human orofacial tissues. J Bone Miner Res. 2005;20:428–437. doi: 10.1359/JBMR.041118. [DOI] [PubMed] [Google Scholar]
- 26.Rojas A, De Val S, Heidt AB, Xu SM, Bristow J, Black BL. Gata4 expression in lateral mesoderm is downstream of BMP4 and is activated directly by Forkhead and GATA transcription factors through a distal enhancer element. Development. 2005;132:3405–3417. doi: 10.1242/dev.01913. [DOI] [PubMed] [Google Scholar]
- 27.Morrisey EE, Ip HS, Lu MM, Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996;177:309–322. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- 28.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, Lengner CJ, Dausman JA, Jaenisch R. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Arauzo-Bravo MJ, Ruau D, Han DW, Zenke M, Scholer HR. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 30.Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, van Oudenaarden A, Jaenisch R. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Ding S. Generation of novel rat and human pluripotent stem cells by reprogramming and chemical approaches. Methods Mol Biol. 636:293–300. doi: 10.1007/978-1-60761-691-7_18. [DOI] [PubMed] [Google Scholar]
- 32.Chang MY, Kim D, Kim CH, Kang HC, Yang E, Moon JI, Ko S, Park J, Park KS, Lee KA, Hwang DY, Chung Y, Lanza R, Kim KS. Direct reprogramming of rat neural precursor cells and fibroblasts into pluripotent stem cells. PLoS One. 2011;5:e9838. doi: 10.1371/journal.pone.0009838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papp B, Plath K. Reprogramming to pluripotency: stepwise resetting of the epigenetic landscape. Cell Res. 2011;21:486–501. doi: 10.1038/cr.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sridharan R, Tchieu J, Mason MJ, Yachechko R, Kuoy E, Horvath S, Zhou Q, Plath K. Role of the murine reprogramming factors in the induction of pluripotency. Cell. 2009;136:364–377. doi: 10.1016/j.cell.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adachi K, Suemori H, Yasuda SY, Nakatsuji N, Kawase E. Role of SOX2 in maintaining pluripotency of human embryonic stem cells. Genes Cells. 2010;15:455–470. doi: 10.1111/j.1365-2443.2010.01400.x. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi S, Hirano K, Nagata S, Tada T. Sox2 expression effects on direct reprogramming efficiency as determined by alternative somatic cell fate. Stem Cell Res. 2011;6:177–86. doi: 10.1016/j.scr.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, Zhang X, Bernstein BE, Nusbaum C, Jaffe DB, Gnirke A, Jaenisch R, Lander ES. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, Bernstein BE, Jaenisch R, Lander ES, Meissner A. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meissner A, Eminli S, Jaenisch R. Derivation and manipulation of murine embryonic stem cells. Methods Mol Biol. 2009;482:3–19. doi: 10.1007/978-1-59745-060-7_1. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 42.Psaltis PJ, Harbuzariu A, Delacroix S, Holroyd EW, Simari RD. Resident vascular progenitor cells--diverse origins, phenotype, and function. J Cardiovasc Transl Res. 2011;4:161–176. doi: 10.1007/s12265-010-9248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torsney E, Xu Q. Resident vascular progenitor cells. J Mol Cell Cardiol. 2011;50:304–311. doi: 10.1016/j.yjmcc.2010.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.