Abstract
Specimens of the hard tick Amblyomma triste were found infected with Rickettsia parkeri in an area of Argentina (General Lavalle, Buenos Aires Province) where cases of human illness attributed to this microorganism have been reported. Molecular detection of R. parkeri was based on polymerase chain reactions that amplify a ca. 400-bp fragment of the 23S-5S intergenic spacer and a ca. 500-bp fragment of the gene encoding a 190-kDa outer membrane protein. Three (6.97%) of 43 A. triste ticks were determined to be positive for R. parkeri. These results provide strong evidence that A. triste is the vector of R. parkeri in the study area. The findings of this work have epidemiological relevance because human parasitism by A. triste ticks has been frequently recorded in some riparian areas of Argentina and Uruguay and new cases of R. parkeri rickettsiosis might arise in the South American localities where humans are exposed to the bites of this tick species.
Keywords: Rickettsia parkeri, Amblyomma triste, Argentina
Bacteria of the genus Rickettsia (Rickettsiales: Rickettsiaceae) are obligate intracellular parasites of eukaryotic cells. Currently, Rickettsia species are classified into a variety of groups, including the spotted fever group (SFG), typhus group, transitional group (TRG) and ancestral groups (Gillespie et al. 2007, Weinert et al. 2009). In Latin America, three species of SFG rickettsiae (Rickettsia rickettsii, Rickettsia parkeri and Rickettsia massili-ae) were recognised as agents of human disease and hard ticks of the genera Amblyomma and Rhipicepha-lus are the vectors of these pathogenic microorganisms (Labruna et al. 2011). In Argentina, human rickettsiosis caused by R. rickettsii was diagnosed in Jujuy Province (Paddock et al. 2008) and cases of human illness attributed to R. parkeri have been reported in the provinces of Buenos Aires, Entre Rios and Chaco (Romer et al. 2011). The vectors involved in the transmission of R. rickettsii and R. parkeri in Argentina are the ticks Amblyomma cajennense and Amblyomma triste, respectively (Nava et al. 2008, Paddock et al. 2008, Labruna et al. 2011, Romer et al. 2011). Additionally, a recent report documented human infection with R. massiliae in Argentina (García-García et al. 2010), although new data are needed to confirm the epidemiological relevance of this case.
Although the cases of R. parkeri infection in humans were diagnosed in different localities within Argentina (Romer et al. 2011), no data exist regarding the potential vectors in these areas. The only exception is the finding of Nava et al. (2008) in the Paraná Delta region of Buenos Aires Province, where A. triste ticks were found infected with R. parkeri. The other reports of R. parkeri rickettsiosis in Buenos Aires Province correspond to sites located approximately 350 km to the south of the Paraná Delta in the Bahia Samborombón Region (General Lavalle and Verónica); however, descriptions of clinical cases constitute the only available information (Romer et al. 2011). Therefore, a survey of ticks was conducted during August and September of 2011 in General Lavalle (36º22'S 56º21'W), Buenos Aires Province. The study area is located on the western coast of the Rio de la Plata estuary and is characterised by the presence of intertidal mudflats and creeks, freshwater lagoons and marshes and slow-flowing streams. The vegetation in this area is dominated by grasslands, small groups of trees (principally Celtis tala) and shrubs. Human activities related to recreation and livestock production are common at this site.
Questing adult ticks were collected from vegetation by dragging and were subsequently preserved in 96% ethanol. A total of 51 specimens were collected and determined to be adults of A. triste based on the methods of Estrada-Peña et al. (2005); of these 51 specimens, 43 were individually tested for Rickettsia spp infection by polymerase chain reaction (PCR). DNA was extracted using the AxyPrep Multisource Genomic DNA Miniprep kit (Axygen Biosciences, USA) according to the manufacturer's instructions and eluted in a final volume of 100 µL. Detection of Rickettsia spp was based on a PCR that amplifies a ca. 400-bp fragment of the 23S-5S intergenic spacer using the primers RCK/23-5-F: GATAGGTCRGRTGTGGAAGCAC and RCK/23-5-R: TCGGGAYGGGATCGTGTGTTTC (Jado et al. 2006). PCR-positive samples were used to amplify a ca. 500-bp fragment of the 190-kDa outer membrane protein gene (ompA) with the primers Rr190.70p: ATGGCGAATATTTCTCCAAAA and Rr190.602n: AGTGCAGCATTCGCTCCCCCT (Regnery et al. 1991). All amplicons were purified and sequenced. Sequencing reactions were performed using an ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit and an Applied Biosystems 373A gene sequencer. The sequences were aligned with each other and with the corresponding sequences of the Rickettsia species available in GenBank. A phylogenetic analysis was performed using the neighbour-joining (NJ) method with the program Mega 4.0 (Tamura et al. 2007). The NJ tree was generated using the Tamura-Nei model, gaps were excluded in the pairwise comparison and support for the topology was tested using 1,000 bootstrap replications.
Amplicons were obtained from the DNA extracts of three (6.97%) of the 43 A. triste ticks. The samples that were positive for the 23S-5S intergenic spacer were used to obtain sequences of the ompA gene fragment (sequences submitted to GenBank: JX534933, JX534932). The three sequences were identical with each other and with the ompA sequences of R. parkeri from the United States (U43802) and from Paraná Delta in Buenos Aires Province (FJ172358). The NJ tree obtained with partial ompA sequences of Rickettsia species is illustrated in the Figure.
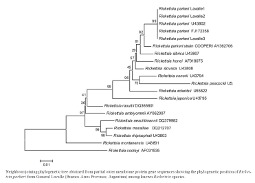
This work provides strong evidence that A. triste is the vector of R. parkeri in the study area. These results are not unexpected because A. triste is known to be the principal vector of R. parkeri in Brazil (Silveira et al. 2007, Labruna et al. 2011), Uruguay (Venzal et al. 2004, Pacheco et al. 2006) and other areas of Argentina in localities belonging to the lower Paraná River Delta region in northeastern Buenos Aires Province (Nava et al. 2008, Romer et al. 2011). The prevalence of A. triste ticks infected with R. parkeri in General Lavalle was similar to that recorded in previous studies performed in Argentina (Nava et al. 2008), Brazil (Silveira et al. 2007) and Uruguay (Pacheco et la. 2006), where the values ranged from 2.6-9.7%.
A. triste has a one-year life cycle in the Southern Cone of South America; immature stages are principally active in the summer, whereas adults, which frequently bite humans, are more abundant from late winter to mid-spring (Venzal et al. 2008, Nava et al. 2011). Therefore, the risk of human infestation by A. triste ticks increases during the part of the year when adults reach their peak abundance. Thus, most of the cases of disease in humans that are attributed to infection with R. parkeri are reported in spring and early summer (Venzal et al. 2004, Seijo et al. 2007, Conti-Díaz et al. 2009).
Information from previous studies in Argentina, Brazil and Uruguay (Venzal et al. 2004, Pacheco et al. 2006, Silveira et al. 2007, Nava et al. 2008, Widmer et al. 2011) along with the results obtained in this study suggest that the infection of A. triste ticks with R. parkeri is a ubiquitous phenomenon. Particularly in Argentina, A. triste is present in wetlands and environments that are prone to flooding, as demonstrated by records in the administrative provinces of Buenos Aires, Corrientes, Entre Rios and Formosa (Nava et al. 2011). Because human parasitism by A. triste adults is frequent in some riparian areas where this tick is present (Nava et al. 2011), new cases of R. parkeri rickettsiosis might arise in the localities where humans are exposed to the bites of A. triste ticks.
Acknowledgments
To Mario Beade, for contribution during field work.
REFERENCES
- Conti-Díaz IA, Moraes-Filho J, Pacheco RC, Labruna MB. Serological evidence of Rickettsia parkeri as etiological agent of rickettsiosis in Uruguay. Rev Inst Med Trop Sao Paulo. 2009;51:337–339. doi: 10.1590/s0036-46652009000600005. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A, Venzal JM, Mangold AJ, Cafrune MM, Guglielmone AA. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae: Amblyomminae) tick group: diagnostic characters, description of the larva of A. parvitarsum Neumann, 1901, 16S rDNA sequences, distribution and hosts. Syst Parasitol. 2005;60:99–112. doi: 10.1007/s11230-004-1382-9. [DOI] [PubMed] [Google Scholar]
- García-García JC, Portillo A, Núñez MJ, Santibáñez S, Castro B, Oteo JA. Case report: a patient from Argentina infected with Ricket-tsia massiliae . Am J Trop Med Hyg. 2010;82:691–692. doi: 10.4269/ajtmh.2010.09-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JJ, Beier MS, Rahman MS, Ammerman NC, Shallom JM, Purkayastha A, Sobral BS, Azad AF. Plasmids and rickettsial evolution: insight from Rickettsia felis . PLoS ONE. 2007;2: doi: 10.1371/journal.pone.0000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jado I, Escudero R, Gil H, Jiménez-Alonso MI, Sousa R, García-Pérez AL, Rodríguez-Vargas M, Lobo B, Anda P. Molecular method for identification of Rickettsia species in clinical and environmental samples. J Clin Microbiol. 2006;44:4572–4576. doi: 10.1128/JCM.01227-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna MB, Mattar S, Nava S, Bermudez S, Venzal JM, Dolz G, Abarca K, Romero L, de Sousa R, Oteo J, Zavala-Castro J. Rickettsioses in Latin America, Caribbean, Spain and Portugal. Rev MVZ Cordoba. 2011;16:2435–2457. [Google Scholar]
- Nava S, Elshenawy Y, Eremeeva ME, Sumner JW, Mastropaolo M, Paddock CD. Rickettsia parkeri in Argentina. Emerg Infect Dis. 2008;14:1894–1897. doi: 10.3201/eid1412.080860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava S, Mangold AJ, Mastropaolo M, Venzal JM, Fracassi N, Guglielmone AA. Seasonal dynamics and hosts of Amblyomma triste (Acari: Ixodidae) in Argentina. Vet Parasitol. 2011;181:301–308. doi: 10.1016/j.vetpar.2011.03.054. [DOI] [PubMed] [Google Scholar]
- Pacheco RC, Venzal JM, Richtzenhain LJ, Labruna MB. Ricket-tsia parkeri in Uruguay. Emerg Infect Dis. 2006;12:1804–1805. doi: 10.3201/eid1211.060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Fernández S, Echenique GA, Summer JW, Reeves WK, Zaki SR, Remondegui CE. Rocky Mountain spotted fever in Argentina. Am J Trop Med Hyg. 2008;78:687–692. [PubMed] [Google Scholar]
- Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer Y, Seijo AC, Crudo F, Nicholson WL, Varela-Stokes A, Lash RR, Paddock CD. Rickettsia parkeri rickettsiosis, Argentina. Emerg Infect Dis. 2011;17:1169–1173. doi: 10.3201/eid1707.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seijo A, Picollo M, Nicholson WL, Paddock CD. Medicina. Vol. 67. Buenos Aires: 2007. Fiebre manchada por rickettsias en el delta del Paraná. Una enfermedad emergente; pp. 723–726. [PubMed] [Google Scholar]
- Silveira I, Pacheco RC, Szabó MPJ, Ramos HGC, Labruna MB. Rickettsia parkeri in Brazil. Emerg Infect Dis. 2007;13:1111–1113. doi: 10.3201/eid1307.061397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Venzal JM, Portillo A, Estrada-Peña A, Castro O, Cabrera PA, Oteo JA. Rickettsia parkeri in Amblyomma triste from Uruguay. Emerg Infect Dis. 2004;10:1493–1495. doi: 10.3201/eid1008.030999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venzal JM, Estrada-Peña A, Castro O, de Souza CG, Félix ML, Nava S, Guglielmone AA. Amblyomma triste Koch, 1844 (Acari: Ixodidae): hosts and seasonality of the vector of Rickettsia par-keri in Uruguay. Vet Parasitol. 2008;155:104–109. doi: 10.1016/j.vetpar.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Weinert LA, Werren JH, Aebi A, Stone GN, Jiggins FM. Evolution and diversity of Rickettsia bacteria. BMC Biol. 2009;7:6–6. doi: 10.1186/1741-7007-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer CE, Azevedo FCC, Almeida AP, Ferreira F, Labruna MB. Tick-borne bacteria in free-living jaguars (Panthera onca) in Pantanal, Brazil. Vector Borne Zoonotic Dis. 2011;11:1001–1005. doi: 10.1089/vbz.2011.0619. [DOI] [PubMed] [Google Scholar]


